Abstract
The proteasome inhibitor bortezomib has shown remarkable clinical success in the treatment of multiple myeloma. However, the efficacy and mechanism of action of bortezomib in solid tumor malignancies is less well understood. In addition, the use of this first-in-class proteasome inhibitor is limited by several factors, including off-target effects that lead to adverse toxicities. We recently reported the impact and mechanisms of carfilzomib and oprozomib, second-in-class proteasome inhibitors with higher specificities and reduced toxicities, against head and neck squamous cell carcinoma (HNSCC). Carfilzomib and oprozomib potently inhibit HNSCC cell survival and the growth of HNSCC tumors. Both compounds promote upregulation of proapoptotic BIK and antiapoptotic MCL1, which serves to mediate and attenuate, respectively, the killing activities of these proteasome inhibitors. Both compounds also induce complete autophagic flux that is partially dependent on activation of the unfolded protein response (UPR) and upregulation of ATF4. Carfilzomib- and oprozomib-induced autophagy acts to promote HNSCC cell survival. Our study indicates that the therapeutic benefit of these promising proteasome inhibitors may be improved by inhibiting MCL1 expression or autophagy.
Keywords: ATF4, ONX 0912, apoptosis, autophagy, carfilzomib, oprozomib, proteasome inhibitor, unfolded protein response
The first-in-class proteasome inhibitor bortezomib (Velcade) has demonstrated considerable success in the treatment of multiple myeloma and mantle cell lymphoma. Bortezomib acts to reversibly inhibit the chymotrypsin-like (CT-L) and caspase-like (C-L) activities of the 20S proteasome core, and also inhibits the activities of several serine proteases. Bortezomib induces autophagy in multiple cell types. In some cell types bortezomib-induced autophagy promotes cell death, while in other cell types it promotes cell survival.
Less is known about the therapeutic impact of proteasome inhibitors on solid tumors. The application of bortezomib to the treatment of solid tumors has been hindered by multiple factors, including inherent and acquired resistance, adverse toxicities due to off-target effects, the need for frequent dosing, and the lack of oral bioavailability. In view of these limitations, considerable effort has been expended to develop next generation proteasome inhibitors. Carfilzomib and oprozomib (also called ONX 0912) are leading, second-in-class inhibitors of the proteasome. Oprozomib represents an orally bioavailable derivative of carfilzomib. Both compounds irreversibly inhibit the proteasome, resulting in a longer duration of inhibition compared with bortezomib. Moreover, both are highly selective for the CT-L activity of the proteasome, and exhibit reduced off-target effects and corresponding adverse toxicities, such as peripheral neuropathy.
Our recent work demonstrated that carfilzomib and oprozomib potently kill HNSCC cells in vitro, and inhibit the growth of HNSCC xenograft tumors in vivo following subcutaneous (carfilzomib) or oral (oprozomib) delivery. Treatment of HNSCC cells with carfilzomib or oprozomib activates both apoptosis and autophagy (Fig. 1). Similar to what has been shown with bortezomib, both carfilzomib and oprozomib upregulate expression of proapoptotic BIK and antiapoptotic MCL1. On the one hand, suppression of BIK upregulation using siRNA demonstrated that carfilzomib- and oprozomib-induced apoptosis is mediated via this “BH3 domain-only” member of the BCL2 protein family. On the other hand, carfilzomib- and oprozomib-induced upregulation of MCL1 attenuates the abilities of these agents to kill HNSCC cells.
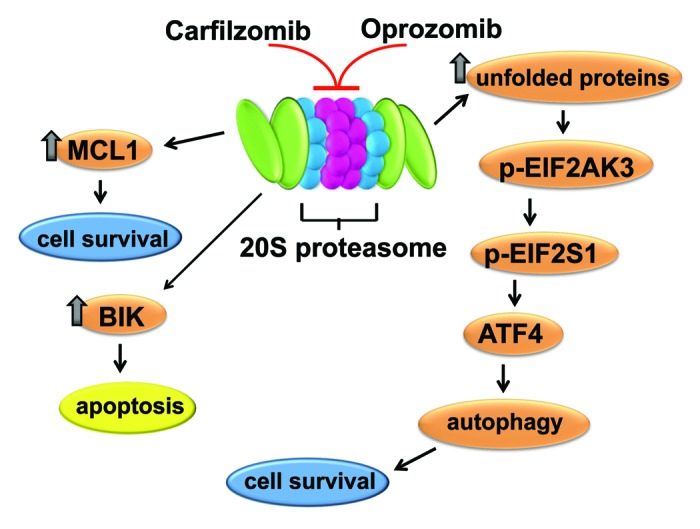
Figure 1. The impact of carfilzomib and oprozomib on HNSCC cells. Treatment of HNSCC cells with carfilzomib or oprozomib leads to induction of BIK, which mediates apoptosis induction. Carfilzomib and oprozomib also induce MCL1, which attenuates apoptosis by these compounds. Inhibition of the proteasome by carfilzomib and oprozomib also leads to activation of the unfolded protein response, resulting in upregulation of ATF4. Induction of ATF4 stimulates autophagy in HNSCC cells, which acts to promote cell survival.
Our study also demonstrated potent induction of autophagy by carfilzomib and oprozomib. Treatment of HNSCC cells with either compound leads to modest upregulation of BECN1 and the ATG12-ATG5 conjugate, and marked upregulation of LC3-II. In HNSCC cells stably expressing GFP-LC3, treatment with carfilzomib or oprozomib results in a roughly 10-fold increase in the average number of puncta/cell. Because proteasome inhibitors have the potential to inhibit fusion of the autophagosome with autolysosomes, or another step in the autophagic process, it was important to determine whether complete autophagic flux was occurring. In cells undergoing complete autophagic flux, the induced LC3-II protein is eventually degraded in autolysosomes by lysosomal proteases. Inclusion of the lysosomal protease inhibitors E64d, pepstatin A, and leupeptin in carfilzomib- and oprozomib-treated cells leads to even further upregulation of LC3-II, indicating the occurrence of complete autophagic flux.
To determine whether carfilzomib and oprozomib activate the UPR, our study examined components of this pathway. One branch of the UPR involves phosphorylation of the endoplasmic reticulum-resident kinase EIF2AK3/PERK, leading to phosphorylation and inhibition of the translation initiation factor EIF2S1/eIF2α. While this leads to a general reduction in mRNA translation, certain mRNAs are preferentially translated when EIF2S1 is inhibited, including the mRNA encoding ATF4 transcription factor. We observed that carfilzomib and oprozomib treatment leads to phosphorylation of EIF2AK3 and EIF2S1, and dramatic upregulation of ATF4, indicating activation of the UPR.
In time course analyses, induction of ATF4 by carfilzomib and oprozomib precedes upregulation of LC3-II, raising the question whether the UPR-ATF4 plays a role in autophagy induction by these inhibitors. Suppression of ATF4 upregulation leads to a partial reduction in the levels of induced LC3-II, as well as a very marked reduction in the average number of puncta/cell. Thus, carfilzomib- and oprozomib-induced autophagy is partially dependent on ATF4 induction.
The impact of carfilzomib- and oprozomib-induced autophagy on the survival of HNSCC cells was determined by inhibiting autophagy with chloroquine. Inhibition of autophagy enhances cell death induction by both compounds, indicating that autophagy promotes cell survival in HNSCC cells treated with these agents. These findings are supported by experiments using siRNA to inhibit ATF4 expression. Suppression of ATF4 expression, and ATF4-dependent autophagy, results in a modest enhancement of HNSCC cell apoptosis.
In summary, our recent report demonstrates that the second-in-class proteasome inhibitors carfilzomib and oprozomib promote both apoptosis and autophagy in HNSCC. Apoptosis induction is mediated by BIK upregulation and attenuated by MCL1 upregulation. Complete autophagic flux is observed in carfilzomib- and oprozomib-treated cells, and autophagy induction is mediated, in part, via upregulation of ATF4. Using in vivo models, subcutaneous or oral delivery of these agents was found to inhibit the growth of HNSCC tumors. It is notable that the therapeutic efficacies of these promising proteasome inhibitors are likely to be further improved by co-administration with inhibitors of MCL1 or autophagy.
Acknowledgments
This work was supported by NIH grants R01 CA137260 and P50 CA097190, and, in part, by P30CA047904. E.T.C. and C.J.K. are employed by Onyx Pharmaceuticals, Inc. C.J.K. has ownership interest (including patents) in Onyx. The authors thank Matthew E. Johnson for providing the figure.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/22185


