Abstract
The presence of selfish genetic elements can have fatal consequences for populations that harbor them. In the well known t haplotype in wild house mice, large proportions of the population die from t/t recessive lethal effects. Due to strong advantages at the gamete level (drive), t haplotypes nevertheless occur at substantial frequencies. The stable presence of a lethal is not the only effect of the t. It also distorts the fate of mutations that differentially affect male and female survival and reproduction (such as in sexual conflict), by giving male selective effects a strong advantage over female selective effects. In a recent study, we proposed polyandry as a potential counterstrategy against t deleterious effects. Here, we show that (1) the efficiency of polyandry in reducing the t frequency strongly depends on the selective context and (2) polyandry helps to reduce male-biased leverage in sex dependent selection.
Keywords: intragenomic conflict, overdominance, segregation distortion, sexually antagonistic effects, t haplotype
The ‘fair’ Mendelian 50:50 ratio of chromosomal segregation during meiosis is an important ingredient to our understanding of evolution. Yet an increasing number of selfish genetic elements are described that systematically deviate from Mendelian expectations.1 By distorting Mendelian inheritance ratios in their favor, selfish genetic elements spread in populations despite fatal fitness consequences for their hosts. One of the best known selfish genetic elements is the t haplotype in house mice (Mus domesticus). The t haplotype is a variant of mouse chromosome 17 and comprises a whole complex of genes that is protected from recombination through inversions.2 Male +/t heterozygotes transmit it to up to 90% of their progeny.2 This deviation from the typical 50% inheritance ratio is called drive (τ). The strong advantage on a gamete level is opposed by negative fitness effects on an individual level: most t haplotype variants carry recessive lethal alleles. As a result, t/t homozygotes die early during embryogenesis.3
Drive is exclusive to one sex in most known cases. In the t haplotype, it is exclusive to males. It follows that a mutation changing the reproductive value of a male will be selected more strongly than a mutation with identical effects in females.1,3
Recently, we studied the effects of polyandry on expected distorter frequencies, allowing viability selection to differ between males and females.4 We showed that polyandry can lead to substantial reductions in expected mean t frequencies, as t carrying males do typically worse in sperm competition.5 Here, we investigate more systematically under which combinations of viability polyandry is most effective in reducing the frequency of the t. Parameter choices were inspired by measurements from our wild house mouse population (i.e., scenario II).
Sex Dependent Selection without Drive and Polyandry
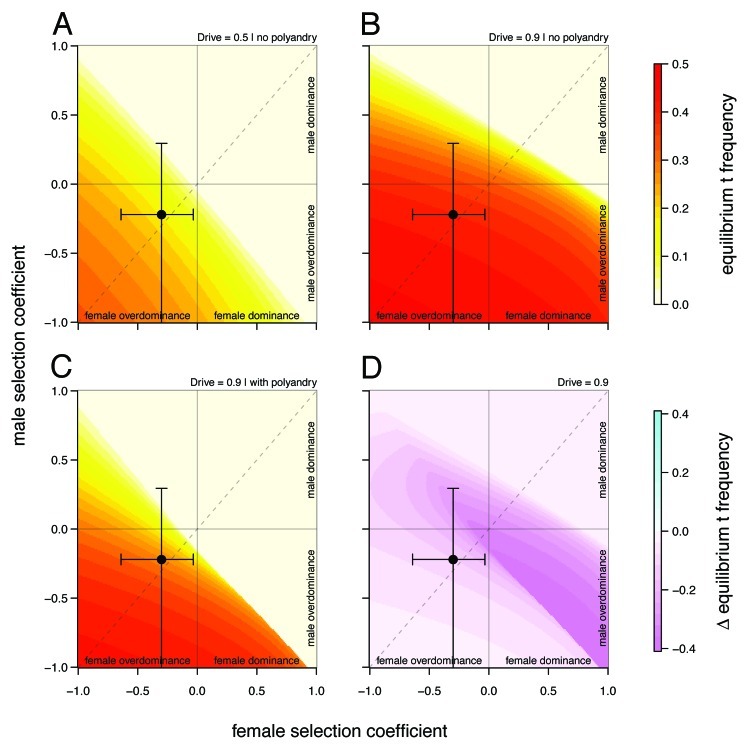
Figures 1A–D illustrate the influence of sex dependent selection, drive and polyandry on equilibrium t frequencies in an infinite, well-mixed population. These figures are based on the model presented in Manser et al.4 The results in Figure 1A and B are identical to the analytical solutions of Hartl.3 Sex dependent selection coefficients 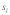 , where
, where  defines sex, denote relative viability differences between +/+ and +/t individuals. Relative viability of +/t individuals is therefore
defines sex, denote relative viability differences between +/+ and +/t individuals. Relative viability of +/t individuals is therefore 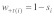 relative to a value of one for +/+ homozygotes (
relative to a value of one for +/+ homozygotes (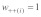 ). t/t homozygotes are lethal in both sexes (i.e.,
). t/t homozygotes are lethal in both sexes (i.e., 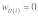 ). Without drive (
). Without drive (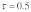 , see Fig. 1A) t haplotypes only occur at equilibrium when overdominant at least in one sex. Note that without drive, equilibrium t frequencies
, see Fig. 1A) t haplotypes only occur at equilibrium when overdominant at least in one sex. Note that without drive, equilibrium t frequencies 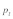 are symmetrical with respect to the diagonal (dashed line), indicating that selection has the same effect on
are symmetrical with respect to the diagonal (dashed line), indicating that selection has the same effect on 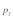 , irrespective of whether it occurs in males or in females.
, irrespective of whether it occurs in males or in females.
Figure 1. Equilibrium t frequencies 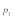 as a function of male and female relative selection coefficient
as a function of male and female relative selection coefficient 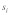 (A) without drive and polyandry (
(A) without drive and polyandry (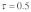 ), (B) with drive without polyandry (
), (B) with drive without polyandry (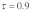 ) and (C) with drive and polyandry (
) and (C) with drive and polyandry (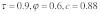 ). Figure (D) shows the difference in equilibrium frequency between (B) and (C)
). Figure (D) shows the difference in equilibrium frequency between (B) and (C) 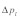 The upper right quadrant represents cases of incomplete dominance, the lower left quadrant cases of overdominance in both sexes. The upper left and lower right quadrants capture sexually antagonistic alleles. Black circles indicate selection coefficients observed in our study population (including 95% CI bands).
The upper right quadrant represents cases of incomplete dominance, the lower left quadrant cases of overdominance in both sexes. The upper left and lower right quadrants capture sexually antagonistic alleles. Black circles indicate selection coefficients observed in our study population (including 95% CI bands).
Sex Dependent Selection with Drive
Figure 1B shows the same relationship for the drive levels observed in our study population (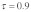 ). There are two important changes: (1) As expected, drive leads to a general increase
). There are two important changes: (1) As expected, drive leads to a general increase 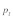 for given selection levels. (2) Equilibrium t frequencies are no longer symmetrical with respect to the diagonal (dashed line). Now, a mutation with a given set of selection coefficients
for given selection levels. (2) Equilibrium t frequencies are no longer symmetrical with respect to the diagonal (dashed line). Now, a mutation with a given set of selection coefficients 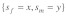 typically results in a different than a mutation with opposite effects in the sexes
typically results in a different than a mutation with opposite effects in the sexes 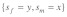 More precisely, male selective effects dominate the evolutionary outcome, as they are enforced by drive. This asymmetry has interesting implications for sexual conflict (see below).
More precisely, male selective effects dominate the evolutionary outcome, as they are enforced by drive. This asymmetry has interesting implications for sexual conflict (see below).
Adding Polyandry
Figure 1C analyzes the effects of polyandry on 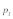 Parameter settings are based on the intermediate polyandry scenario II of the original publication, which assumes a fraction
Parameter settings are based on the intermediate polyandry scenario II of the original publication, which assumes a fraction 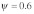 of females mating twice, and +/t heterozygote sperm competitiveness levels of
of females mating twice, and +/t heterozygote sperm competitiveness levels of 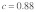 . In this model, +/t males are less likely to successfully fertilize eggs in sperm competition with +/+ males due to reductions in sperm quantity (as a consequence of drive τ) and sperm quality (described by the sperm competitiveness parameter c). The difference in equilibrium t frequency to the model without female multiple mating is shown in Figure 1D. Clearly, the decrease in t frequency due to polyandry is not uniformly distributed on the continuum of selection coefficients
. In this model, +/t males are less likely to successfully fertilize eggs in sperm competition with +/+ males due to reductions in sperm quantity (as a consequence of drive τ) and sperm quality (described by the sperm competitiveness parameter c). The difference in equilibrium t frequency to the model without female multiple mating is shown in Figure 1D. Clearly, the decrease in t frequency due to polyandry is not uniformly distributed on the continuum of selection coefficients 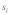 In cases of female dominance and male overdominance, multiple mating is more efficient in removing the t allele from the population than in others. We think the main reason for this is the fact that polyandry is a frequency dependent process. Sperm competition can only play a role in cases where a female mates with a male of each genotype (+/+ and +/t). The probability for such a mating combination is highest if about half of the male population carry a t (hence
In cases of female dominance and male overdominance, multiple mating is more efficient in removing the t allele from the population than in others. We think the main reason for this is the fact that polyandry is a frequency dependent process. Sperm competition can only play a role in cases where a female mates with a male of each genotype (+/+ and +/t). The probability for such a mating combination is highest if about half of the male population carry a t (hence 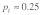 ). Consequently, the combination of male overdominance, which keeps male t frequency at these optimal high levels, and female dominance, which reduces t frequency independent of sperm competition, creates the strongest impact on
). Consequently, the combination of male overdominance, which keeps male t frequency at these optimal high levels, and female dominance, which reduces t frequency independent of sperm competition, creates the strongest impact on 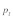 .
.
Empirical Data
Data on the sex specific fitness consequences from wild house mouse populations, especially on the +/t heterozygote fitness effects of the t haplotype, are still scarce and contradictory (e.g., Dunn et al.6 and Carroll et al.7). In Manser et al.,4 we quantified the effect of the t haplotype on male and female survival into adulthood based on a free-living house mouse population near Zurich. In agreement with Dunn et al.,6 we found a survival advantage of +/t heterozygotes in both sexes. The estimated selection coefficients were within a range where polyandry is not particularly efficient against the t (see also Fig. 1): 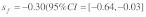 and
and 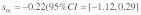 Because of the low confidence in the male estimate, we used
Because of the low confidence in the male estimate, we used 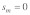 in the original paper.
in the original paper.
Drive and Sexual Conflict
Males and females have distinctive roles in reproduction. A situation where a trait expressed in both sexes has different fitness optima in males and females is referred to as intralocus sexual conflict or sexually antagonistic selection.8 It has been argued that drive, because it is usually sex-biased, can have consequences for intralocus sexual conflict.1 A sexually antagonistic gene giving males a selection advantage (e.g., 10%) and females a disadvantage (e.g., 15%) may be positively selected with the help of drive, even if the net effect over both sexes is negative. We did not find any indication of sexual conflict in the trait measured here (survival to sexual maturity). However, Chippindale et al.9 suggested that early developmental effects are unlikely to be sex biased, as the fitness objectives for both sexes are likely to coincide. For future studies, it would thus be interesting to investigate whether we find signs of intralocus conflict in adult traits such as fertility or reproductive success, where gender roles diverge. Our model suggests that polyandry considerably reduces the asymmetry induced by drive (Fig. 1C). Polyandry may therefore not only be a successful female mating strategy against the lethal effects of the t, but thereby also help to reduce a male-biased leverage in sexual conflict.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Forschungskredit fund of the University of Zurich and the Swiss National Science Foundation (SNF: 3100A0–120444/1) for financial support.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21955
References
- 1.Burt A, Trivers R. Genes in conflict: the biology of selfish genetic elements. Cambridge, US: Belknap Press, 2006. [Google Scholar]
- 2.Silver LM. The peculiar journey of a selfish chromosome: mouse t haplotypes and meiotic drive. Trends Genet. 1993;9:250–4. doi: 10.1016/0168-9525(93)90090-5. [DOI] [PubMed] [Google Scholar]
- 3.Hartl DL. A mathematical model for recessive lethal segregation distorters with differential viabilities in the sexes. Genetics. 1970;66:147–63. doi: 10.1093/genetics/66.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manser A, Lindholm AK, König B, Bagheri HC. Polyandry and the decrease of a selfish genetic element in a wild house mouse population. Evolution. 2011;65:2435–47. doi: 10.1111/j.1558-5646.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haig D, Bergstrom C. Multiple mating, sperm competition and meiotic drive. J Evol Biol. 1995;8:265–82. doi: 10.1046/j.1420-9101.1995.8030265.x. [DOI] [Google Scholar]
- 6.Dunn L, Beasley AB, Tinker H. Relative fitness of wild house mice heterozygous for a lethal allele. Am Nat. 1958;92:215–20. doi: 10.1086/282029. [DOI] [Google Scholar]
- 7.Carroll LS, Meagher S, Morrison L, Penn DJ, Potts WK. Fitness effects of a selfish gene (the Mus t complex) are revealed in an ecological context. Evolution. 2004;58:1318–28. doi: 10.1554/03-544. [DOI] [PubMed] [Google Scholar]
- 8.Rice WR. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–42. doi: 10.2307/2408385. [DOI] [PubMed] [Google Scholar]
- 9.Chippindale AK, Gibson JR, Rice WR. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc Natl Acad Sci U S A. 2001;98:1671–5. doi: 10.1073/pnas.98.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]