Abstract
Several reports in fly, nematode and mammalian cells have revealed that the inactivation of endosomal sorting complexes required for transport (ESCRT) blocks the endosomal maturation but also leads to the increased number of autophagosomal structures. In this review we compare these data and conclude that the way ESCRT mutations affect the relationships between autophagosomes and endosomes cannot be generalized but depends on the studied species. We propose that the effect of ESCRT mutations on autophagy is directly dependent of the level of interaction between autophagosomes and endosomes. In particular, the formation of amphisomes during autophagosomal maturation could be the key point to explain the differences observed between species. These observations highlight the importance of multiple model organisms to decipher the complexity of relationships between such dynamic vesicles.
Keywords: autophagosomes, endosomes, amphisomes, VPSE proteins, ATG8/LC3/LGG-1, C. elegans
Of Autophagosomes and Endosomes
Eukaryotic cells contain a highly dynamic vesicle-mediated transport system which selects and delivers proteins and lipids to different subcellular organelles. The degradation and recycling of the cellular material is essential for the cell to maintain its homeostasis. Autophagosomes and endosomes, involved in the process of autophagy and endocytosis respectively, are the main players for vesicular degradation within the cell, both have the fate to fuse with lysosome to deliver their cargo for degradation (Fig. 1A). Autophagy, which usually refers to macroautophagy, allows the degradation of cytoplasmic constituents, long-lived proteins and organelles.1,2 Briefly, the autophagy activation relies on inducing stimuli, such as starvation, which triggers the nucleation and the elongation of a flat isolation mambrane also called phagophore which could originate from various membrane reservoir.3-7 The complete sequestration of cytoplasmic constituents is achieved by the closure of the phagophore resulting in the double-membrane autophagosome. In the next step, the autophagosomes fuse with lysosomes to form the autophagolysosomes, where the inner membrane and the cytoplasmic content are degraded.8 Elongation of autophagosomal membranes requires the recruitment of the ubiquitin-like protein Atg8p/LC3 (Fig. 1A). Atg8p/LC3 is present on autophagosome membranes as a phosphatidylethanolamine conjugated form (named LC3 II) and is widely used as a marker of autophagosomes.9-11
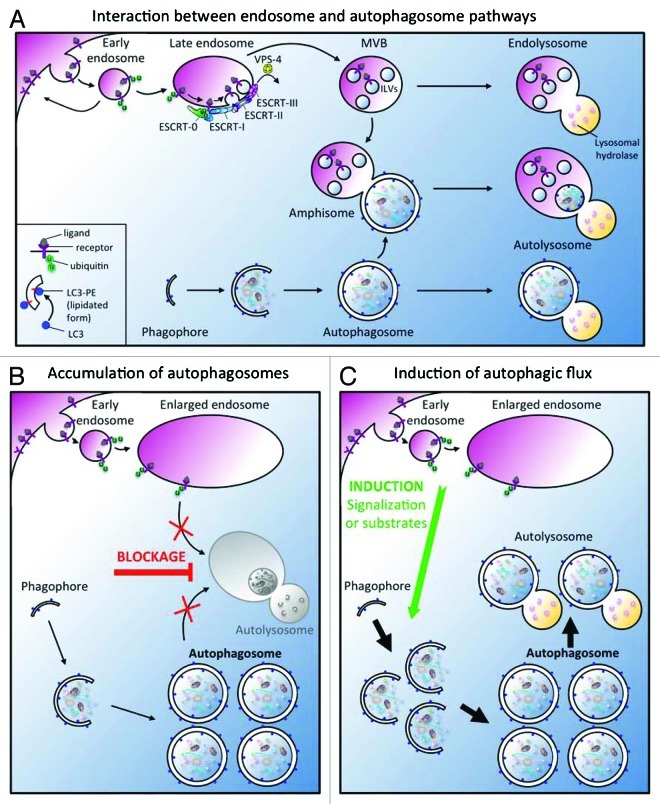
Figure 1. (A) Interactions between endocytic and autophagic pathways. The endosomal system allows the sorting of membrane lipids and proteins to the lysosome for degradation.47 The degradation of membrane proteins which is triggered by ubiquitylation and the subsequent delivery of cargoes to the intralumenal vesicles (ILV) of a late endosomal compartment, called multi-vesicular body (MVB), required the ESCRT machinery. Ultimately, ILVs are degraded after fusion with the lysosome. Induction of the autophagic degradative pathway drives the expansion of a small flat membrane bag, named the phagophore, which sequesters cytoplasmic cargoes. After completion and closure, a double-membrane autophagosome is formed, which then fuses with the lysosome to forme an autolysosome. Alternatively, autophagosomes can fuse with early endosomes and MVBs to generate amphisomes containing both cytoplasmic cargo and endocytosed materials which finally fuse with lysosomes. (B-C). Main models to explain the increase of autophagosomes in ESCRT mutants. (B) The inactivation of ESCRT machinery leads to the accumulation of autophagosomes because the endosomes are abnormal. A blockage (red bar) of autophagosome-lysosome fusion is then responsible for the increase of autophagosomes but the autophagic degradation is inhibited. (C) The inactivation of ESCRT machinery promotes the induction of a functional autophagic flux (green arrow) in response to homeostasis defect. A defect in signaling or in the “feeding status” due to the presence of abnormal endosomes alters cell homeostasis. To correct this imbalance, the cell generates a signal which triggers a functional autophagic flux increase. Note that these two models are not mutually exclusive.
The endosomal compartment functions as a central sorting site for both the endocytic and biosynthetic pathways and is therefore involved in the regulation of many signaling pathways. During receptor-mediated endocytosis, ubiquitylated ligand/receptor complexes are transported to early endosomes and are either delivered to lysosomes for degradation or recycled back to the membrane (Fig. 1A). Endosomal maturation is characterized by a Rab5/Rab7 GTPases switch and the formation of a late compartment called the multi-vesicular body (MVB), defined by the presence of intralumenal vesicles (ILV).12,13 Electron microscopy and biochemical studies in mammals have documented that fusion events could occur between endosomes and autophagosomes, to generate intermediate vesicles named amphisomes which also finally fuse with the lysosome (Fig. 1A).14,15
ESCRT, the Keystone of MVB Formation
The ESCRT machinery which is essential for the sorting of ubiquitylated membrane proteins and the formation of ILVs in the MVB compartment, is composed of four hetero-multimeric complexes, ESCRT-0 to III. In addition, a number of accessory proteins are associated to the ESCRT complexes. The current model for the coordinated function of the ESCRT complexes proposes that ESCRT-0, formed by Vps27p/HRS and Hse-1, is dedicated to the formation of cargoes of ubiquitylated proteins at the endosomal membrane. Subsequently, ESCRT-0 recruits ESCRT-I via a direct interaction between Vps27p/HRS and Vps23p/TSG10116-18 and ESCRT-II interacts with ESCRT-I through the binding of Vps36p/EAP45 to Vps28p.19 Recent data, obtained using giant uni-lamellar vesicles, suggests that ESCRT-I and II are necessary to generate inward budding vesicles at the endosomal membrane.20 The oligomerisation of the coiled-coil proteins forming the ESCRT-III complex is then required for the membrane scission to release the ILVs. During the addressing of cargoes to the ILVs, ubiquitin is removed by the deubiquitinase Doa4. Finally, the ESCRT machinery is dissociated from the endosomal membrane by the ATPase Vps4p.21,22 However, if the ESCRTs machinery is well conserved in all eukaryotes, its complexity has arisen during the evolution of multi-cellular organisms. The duplication of several genes in mammals23 raises the possibility of alternative roles for ESCRT components.24
Several reports in nematode, fly and mammals showed that in addition to the characteristic endosomal maturation defect, mutations in ESCRT components lead to an increase in the number of autophagosomes (Table 1).25-29 Specific studies have tried to understand why autophagy is affected in ESCRT mutants according with two main hypotheses presented in Figure 1: the accumulation of autophagosomes is due to the lack of autophagosome-lysosome fusion (Fig. 1B) or there is an induction of autophagic flux (Fig. 1C).
Table 1. Effects of ESCRT mutants on autophagosome.
| Species | Cell type | Effect on autophagy pathway | ESCRT complex investigated | Mutant analyzed* | Technical approach | Reference |
|---|---|---|---|---|---|---|
|
C.e. |
Embryos and Larvae epidermis |
induction |
0, I, III |
vps-27, vps-37, vps-32 |
IF, WB |
Djeddi et al. 2012 Roudier et al. 2005 |
|
H.s. |
HeLa cells HEK 293 |
blockage |
0, I, II, III |
VPS27/hrs, VPS23/tsg101, vps22, vps24, VPS32/mSnf7–2, VPS2/chmp2B |
Immuno-EM, IF, WB |
Filimonenko et al. 2008 Tamai et al. 2007 Lee et al. 2007 |
|
D.m. |
Fat body Follicle cells Eye |
blockage |
I, II, III |
vps28, vps25, VPS32/shrub, VPS2/chmp2B |
EM, IF |
Rusten et al. 2007 Lee et al. 2007 |
| M.m. | Embryonic fibroblasts Neurons | blockage | III | VPS27/hrs, VPS32/mSfn7–2, VPS2/chmp2B | EM, IF, WB | Tamai et al. 2007 Lee et al. 2007 |
C.e., Caenorhabditis elegans; H.s., Homo sapiens; D.m., Drosophila melanogaster; M.m., Mus musculus; *Mutants or RNAi or Dominant negative
Historically, the first observations linking autophagy and endosome maturation defects in ESCRT mutants came from models of neurodegenerative disorders in which autophagy is clearly involved for the clearance of toxic proteins and aggregates.
From Cell Culture…
Mutations in the ESCRT-III component VPS2/chmp2B has been found to be associated with neurodegenerative diseases, such as frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS), which present proteins aggregates positive both for ubiquitin moiety and the p62 autophagic adaptor protein.30-32 From these observations, Filimonenko and collaborators explored the link between ESCRT depletions and autophagy defects using mammalian cell culture experiments.26 By combining siRNA approaches against VPS23/tsg101 (ESCRT-I) or VPS24/chmp3 (ESCRT-III), colocalization experiments, western blot analyses and electron microscopy, they characterized the nature of enlarged vesicular structures. They observed the apparition of large ubiquitin positive structures associated with endosomal markers but also with the autophagic proteins p62, Alfy (autophagy-linked FYVE protein) and GFP-Atg8p/LC3. By electron microscopy they observed the presence of autophagosomes, amphisomes but not autolysosomes in VPS23/tsg101 and VPS24 depleted cells. The analyses of both Atg8p/LC3II, the lipidated form associated to the autophagic membranes, and the elegant tandem fusion mCherry-GFP-Atg8p/LC3, support the idea that in ESCRT mutants the fusion with lysosomal acidic compartments was inhibited. Interestingly, similar results were obtained by overexpressing mutant forms of VPS2/chmp2B (ESCRT-III), previously identified in ALS or FTD patients. Together these data strongly suggest that the depletion of ESCRT subunits inhibit autophagic maturation, leading to accumulation of ubiquitin-positive aggregates in autophagosomes and amphisomes.
Tamai et al. reached a similar conclusion using a pathogenic group A Streptococcus (GAS) which infects cells through early endosomes but is nevertheless efficiently degraded by autophagosomes.29 Using HeLa cells and mouse embryonic fibroblasts in nutrient restriction condition, they observed a colocalization between endo-lysosomal and autophagic marker respectively Vps27p/HRS, Lamp-1 and GFP-Atg8p/LC3 (Fig. 2). In cells deficient for the ESCRT-0 protein VPS27/hrs, degradation of GAS by autophagy was strongly diminished whereas both Atg8p/LC3I and II were strongly increased. From these data the authors also concluded that the maturation of autophagosomes to form autophagolysosomes is impaired by VPS27/hrs depletion.
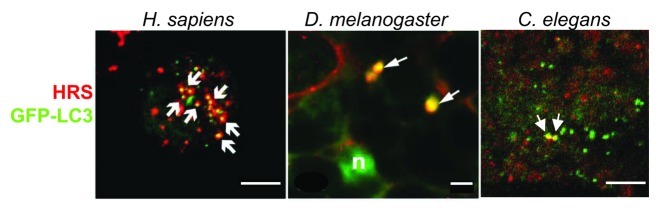
Figure 2. Visualization of amphisomes in D. melanogaster, H. sapiens and C. elegans by colocalization between the autophagic protein Atg8p/LC3 and the endosomal protein Vps27p/HRS. Left: Vps27p/HRS localizes to Atg8p/LC3-positive autophagosomes. Confocal microscopic images of native Vps27p/HRS (red) and Atg8/pLC3-positive vesicles (green) in HeLa cells that stably expressed GFP-Atg8p/LC3, grown under nutrient-starvation conditions. Arrows indicate co-localization of both stainings. Scale bar is 10 µm. (From ref-29, with permission). Middle: Colocalization in wild-type cells was investigated in the fat body of D. melanogaster (L3 larval stage). A subset of GFP-Atg8a (green) structures in mid L3 fat body colocalizes with Vps27p/HRS (red, arrows). Scale bar is 5µm. (From ref-33, with permission). Right: Confocal images of VPS-27 (red) and GFP-Atg8p/LGG-1 (green) in C. elegans embryo. Because amphisomes are very rare in wild-type animals, a rab-7(RNAi) animal is shown, where amphisomes are easier to visualize. Scale bar is 10 µm. (From ref-25, with permission.)
…To Mouse and Drosophila
Lee et al. focused on the interaction between ESCRT-III mutant and autophagy in mouse.27 The authors generated a knockout mouse for VPS32/mSnf7–2 which died on embryonic day 7.5–8.5. Because VPS32/mSnf7–2 is highly expressed in several neuronal populations they then analyzed its function in cultured cortical neurons and showed that it is required for viability. They also demonstrated that expression of the mutant Vps2p/CHMP2B protein responsible of FTD is sufficient to cause neurodegeneration of cortical neurons. Finally, the authors showed that either the expression of the mutated VPS2/chmp2B or the depletion of VPS32/mSnf7–2 causes an accumulation of GFP-Atg8p/LC3 autophagosomes and an increase in Atg8p/LC3I and II. Electron microscopy confirmed the accumulation of multilamellar bodies and autophagosomes. Interestingly, the authors were able to reproduce a similar increase of autophagosomes in two other systems, HEK293 cells and the fly eye photoreceptor. They concluded that ESCRT-III dysfunction is likely to interfere with the fusion between autophagosomes and MVB.
Concomitantly, Rusten and colleagues have used a genetic approach to analyze in the fly D. melanogaster the relationships between endosomal maturation and autophagy in ESCRT mutants.33 Using the FLP recombinase technology they generated somatic clones of cells homozygous for null alleles of vps28 (ESCRT-I), vps25 (ESCRT-II) and vps32 (ESCRT-III). They then analyzed the autophagy induction process either in tissues where the basal level of autophagy is almost undetectable (e. g. ovarian follicular cells) or in the fat body, an adipose tissue where autophagy is rapidly induced in starvation (aminoacids) conditions. In each case, they observed that GFP-Atg8a positive structures accumulate in ESCRT mutant cells. They demonstrated a similar effect by overexpressing a dominant negative form of vps4 in the fat body.
To analyze the type of structures that accumulate, they then performed colocalization experiments between the autophagosome marker GFP-Atg8a and either the endosomal protein Vps27p/HRS or the lysosomal marker Lamp1. While in wild-type fat body, Atg8a and Vps27p/HRS positive amphisomes can be easily detected (Fig. 2), this common compartment, was absent in ESCRT mutant cells. Moreover, the formation of autolysosomes (positive for Atg8a and Lamp1) was also affected in vps25 mutant cells. These data indicate that in ESCRT mutants, autophagosomes but neither amphisome nor autolysosome accumulate, suggesting a defect of fusion between endosomes and autophagosomes. This hypothesis was confirmed by electron microscopy analyses of ESCRT mutant larvae which allows the authors to conclude that ESCRT and Vps4 are necessary for autophagosome-endolysosome fusion. Finally, using a fly model of Huntington’s disease, the authors demonstrated that ESCRT depletion affects the autophagic clearance of protein aggregates.
C. elegans, the Exception that Proves the Rule?
Since several years, our group has used the nematode C. elegans to study the interactions between autophagosomes and endosomes.25,34-37 C. elegans only possess one homolog for each ESCRT component and using knockdown and mutants in both the ESCRT machinery (ESCRT-0, I, II, III and the ATPase VPS-4) and the autophagic pathway, we analyzed in vivo, the functional links between endosomal maturation and autophagy. To investigate the autophagic pathway in wild-type or ESCRT mutant context, we first performed EM analyses37 and observed numerous abnormal vesicular structures of variable sizes containing an accumulation of membranous material and presenting similarities to late autophagic vacuoles observed in mammals.38 We then used GFP fusion proteins of Atg8p/LGG-1 and Atg8p/LGG-2, the worm homologs of the LC3 human autophagic marker, to visualize the autophagic process. We observed a strong increase of the number of autophagosomes in all ESCRT mutants analyzed and then monitored the autophagic flux by western blotting. When the autophagosome fuses with the lysosome, GFP-Atg8p/LGG-1 protein is degraded releasing a GFP fragment. The detection of this GFP fragment in ESCRT mutants indicates that autophagosomes are formed and still able to complete fusion with the lysosome. Our study also revealed that in C. elegans embryo, amphisomes can be detected (Fig. 2) but are very infrequent either in basal autophagic conditions or in ESCRT mutants.
We then performed a genetic approach to modify the level of autophagy and analyze whether it modifies the endosomal phenotype of ESCRT mutants. The impairment of autophagy (ATG8/lgg-1, ATG8/lgg-2, atg-7) leads to an increase of the endosomal defect while the increase of autophagic basal level (TOR) improves it. The use of spermidine to pharmacologicaly induce autophagy confirmed this result.25
Altogether our results led us to conclude that in C. elegans ESCRT mutants, the induction of autophagy is an adaptive response trying to promote cell survival and maintain homeostasis. However, it is surprising that in C. elegans, conversely to fly and mammals phenotype, the increase in the number of autophagosomes is not due to a blockage of fusion.
To Form or Not to Form an Amphisome, That is the Question
Depending on the species and possibly the cell type, it appears that ESCRT mutations could differentially affect the interaction between the endosomal and autophagic pathways. A blockage of autophagosomal maturation was described in ESCRT mutants in flies and mammals whereas we showed an induction of the autophagy in C. elegans. Specificities in the mechanisms of autophagosomal maturation and fusion are one possible explanation of this differential observation. In particular, the amphisome could be a key player to explain such a difference. In mammals, the convergence between endosomes and autophagosomes is a multi-step process that can generate intermediate vesicular types named amphisomes but which regulation is not well understood.14,39-41 However, in mammals, autophagosomes can also directly fuse with the lysosome.42 Finally, in fly and nematode, amphisomes can be detected (Fig. 2) but except the studies presented in this review there is almost no data on the mechanisms of their formation.
From the data discussed above, it appears that in cell types where amphisome formation is the main way for autophagosomal maturation and occurs by multiple fusion with early and late endosomes (e. g., HeLa), the depletion of ESCRT mechanically results in the accumulation of autophagosomes, and amphisomes. In fly, amphisome formation is important (fat body) but as ESCRT mutants only accumulate autophagosomes, one can hypothesize that amphisomes mainly depend on fusion with late endosomes. Our data in C. elegans suggest that a direct fusion between the autophagosome and the lysosome could be preferential to the formation of amphisomes, and revealed an increase of autophagic flux in ESCRT mutants.
One can notice that in mammals and fly no experimental data formally excludes that in addition to the fusion blockage ESCRT depletion could also trigger an upregulation of proautophagic signaling. The autophagic flux has not been quantified in these studies due to technical limitations in the context of ESCRT mutants.
Conclusion
Albeit the limited number of studies on the role of ESCRT complexes on the autophagosome maturation process, they revealed the high level of versatility and variability of interactions between autophagosomes and endosomes. More studies will be necessary first to describe the formation of amphisomes in various cell types and species and then to characterize the mechanistic aspects. Several data on autophagosome formation have identified some SNARE as important factors41,43,44 and one can postulate that they are also good candidates for amphisome formation but additional data are needed to identify the molecular partner involved in the specific fusion that gives rise to amphisome.
In the light of recent results, a higher level of complexity concerning the functions of ESCRT proteins in autophagy could be anticipated. A recent study performed with dendritic cells45 has reported that MVB could mediate a microautophagic process which is impaired when ESCRT components are depleted. Finally, a number of observations have indicated that ESCRT proteins could be involved in non-endosomal functions (for review see ref-24, 36) and present MVBs as “a signalling organelle.”46 Moreover, two recent papers raise the possibility that some ESCRT components could interact with autophagic process independently of endosomal maturation in yeast (see Box 1).
Box 1. A novel link between ESCRT and autophagy in yeast.
ESCRT mutants have been first described and intensively analyzed in yeast, but conversely to metazoan data, deficiency of autophagy in an ESCRT mutant has not been reported. Moreover, there is no evidence of amphisome in yeast in which the autophagosomes can only directly fuse with the vacuole, the lysosome counterpart. However, two papers have recently described the existence of vesicular compartments positive for both autophagosomal proteins and ESCRT components. In both cases these structures are only detected in nitrogen starvation conditions. In one case, this autophagosomal structure is positive for ESCRT-I Vps23p but not ESCRT-III Vps37p and is involved in a non conventional secretion pathway.48 This compartment has been called CUPS (compartment for autophagosome-mediated unconventional protein secretion) by the authors. Mutations of ESCRT components affect differently the formation of CUPs. Similarly, the second report describes autophagosomal structure which formation is affected in ESCRT-I VPS23 and ESCRT-0 VPS27 but no ESCRTIII VPS37 mutants.49 This compartment is involved in a vacuolar degradative pathway (more similar to macroautophagy) but colocalization between ESCRT and autophagosomal marker has not been documented for this particular vesicle. These two studies are the first evidences that interactions between autophagosomal compartment and ESCRT machinery occur in yeast, even so, these compartments are only observed in particular growth conditions.
Altogether these data raise new questions on the role of ESCRT regarding autophagic processes, and highlight the need of various models to better understand the mechanistic of autophagosomal maturation and the interaction between the endosomal and the autophagic pathway. Endocytosis and autophagy are highly dynamic processes, essential for cellular homeostasis, both involved in the responses to the variations of environmental conditions. One can anticipate that regulation of multiple cellular functions could be the result of yet unknown combinations of complexes and organelles involved in these two processes.
Acknowledgments
This work was supported by the Fondation ARC pour la Recherche sur le Cancer and MMS is a recipient of a fellowship from the Ligue Nationale contre le Cancer.
Glossary
Abbreviations:
- MVB
Multi Vesicular Body
- ILVs
Intra Luminal Vesicles
- ESCRT
Endosomal Sorting Complex Required for Transport
- VPS-E
class E Vacuolar Protein Sorting
- LGG
LC3 GABARAP GATE16 family
- CUPS
Compartment for autophagosome-mediated Unconventional Protein Secretion
Footnotes
Note About the Nomenclature
Because the nomenclature and sometimes the names of genes are different between species, we have chosen to use a double nomination with the yeast name preceding the name of the particular species homolog if they are different (e.g., Atg8p/LC3 in mammals, Atg8p/LGG-1 in the worm but Atg8a in drosophila).
Previously published online: www.landesbioscience.com/journals/cib/article/21522
References
- 1.Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453–8. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–7. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 4.Ylä-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5:1180–5. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- 5.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–67. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–57. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–5. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 9.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–92. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 10.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263–76. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–23. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 13.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–47. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg TO, Fengsrud M, Strømhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–92. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 15.Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun. 1988;151:40–7. doi: 10.1016/0006-291X(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 16.Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003;162:435–42. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol. 2003;162:413–23. doi: 10.1083/jcb.200302136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci U S A. 2003;100:7626–31. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Im YJ, Hurley JH. Integrated structural model and membrane targeting mechanism of the human ESCRT-II complex. Dev Cell. 2008;14:902–13. doi: 10.1016/j.devcel.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–9. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–98. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefani F, Zhang L, Taylor S, Donovan J, Rollinson S, Doyotte A, et al. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr Biol. 2011;21:1245–50. doi: 10.1016/j.cub.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Slagsvold T, Pattni K, Malerød L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–26. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Djeddi A, Michelet X, Culetto E, Alberti A, Barois N, Legouis R. Induction of autophagy in ESCRT mutants is an adaptive response for cell survival in C. elegans. J Cell Sci. 2012;125:685–94. doi: 10.1242/jcs.091702. [DOI] [PubMed] [Google Scholar]
- 26.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol. 2007;17:1561–7. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Rusten TE, Filimonenko M, Rodahl LM, Stenmark H, Simonsen A. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 2008;4:233–6. [Google Scholar]
- 29.Tamai K, Tanaka N, Nara A, Yamamoto A, Nakagawa I, Yoshimori T, et al. Role of Hrs in maturation of autophagosomes in mammalian cells. Biochem Biophys Res Commun. 2007;360:721–7. doi: 10.1016/j.bbrc.2007.06.105. [DOI] [PubMed] [Google Scholar]
- 30.Parkinson N, Ince PG, Smith MO, Highley R, Skibinski G, Andersen PM, et al. MRC Proteomics in ALS Study. FReJA Consortium ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–7. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- 31.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 32.Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum Mol Genet. 2006;15(Spec No 2):R182–7. doi: 10.1093/hmg/ddl202. [DOI] [PubMed] [Google Scholar]
- 33.Rusten TE, Vaccari T, Lindmo K, Rodahl LM, Nezis IP, Sem-Jacobsen C, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol. 2007;17:1817–25. doi: 10.1016/j.cub.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 34.Alberti A, Michelet X, Djeddi A, Legouis R. The autophagosomal protein LGG-2 acts synergistically with LGG-1 in dauer formation and longevity in C. elegans. Autophagy. 2010;6:622–33. doi: 10.4161/auto.6.5.12252. [DOI] [PubMed] [Google Scholar]
- 35.Michelet X, Alberti A, Benkemoun L, Roudier N, Lefebvre C, Legouis R. The ESCRT-III protein CeVPS-32 is enriched in domains distinct from CeVPS-27 and CeVPS-23 at the endosomal membrane of epithelial cells. Biol Cell. 2009;101:599–615. doi: 10.1042/BC20090025. [DOI] [PubMed] [Google Scholar]
- 36.Michelet X, Djeddi A, Legouis R. Developmental and cellular functions of the ESCRT machinery in pluricellular organisms. Biol Cell. 2010;102:191–202. doi: 10.1042/BC20090145. [DOI] [PubMed] [Google Scholar]
- 37.Roudier N, Lefebvre C, Legouis R. CeVPS-27 is an endosomal protein required for the molting and the endocytic trafficking of the low-density lipoprotein receptor-related protein 1 in Caenorhabditis elegans. Traffic. 2005;6:695–705. doi: 10.1111/j.1600-0854.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 38.Eskelinen EL. Fine structure of the autophagosome. Methods Mol Biol. 2008;445:11–28. doi: 10.1007/978-1-59745-157-4_2. [DOI] [PubMed] [Google Scholar]
- 39.Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–45. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 2009; 1793:1901-16. [DOI] [PubMed]
- 42.Dunn WA., Jr. Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990;110:1935–45. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146:303–17. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–9. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemmon SK, Traub LM. Sorting in the endosomal system in yeast and animal cells. Curr Opin Cell Biol. 2000;12:457–66. doi: 10.1016/S0955-0674(00)00117-4. [DOI] [PubMed] [Google Scholar]
- 48.Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol. 2011;195:979–92. doi: 10.1083/jcb.201106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimobayashi M, Takematsu H, Eiho K, Yamane Y, Kozutsumi Y. Identification of Ypk1 as a novel selective substrate for nitrogen starvation-triggered proteolysis requiring autophagy system and endosomal sorting complex required for transport (ESCRT) machinery components. J Biol Chem. 2010;285:36984–94. doi: 10.1074/jbc.M110.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]