Abstract
Deficiency in the retromer sorting pathway is known to be associated with the onset of Alzheimer disease (AD), and has been suggested to involve regulation of Amyloid precursor protein (APP) trafficking. Absence of the APP sorting receptor sorLA is also associated to AD, as amyloidogenic processing of APP is increased due to missorting. Reduced activity of either retromer or sorLA thus both lead to enhanced amyloidogenic APP processing, and these pathways are therefore important factors for understanding the development of AD. It is therefore key to outline the neuronal APP trafficking in order to determine the mechanisms that influence AD onset.
Keywords: sorLA, APP, retromer, retrograde trafficking
Accumulation of the amyloid β (Aβ)-peptide is widely accepted as a neurotoxic event in AD. The Aβ peptide is produced by cleavages of APP, by the β-and γ-secretases. Alternatively, APP can be cleaved byα-secretase in the non-amyloidogenic pathway predominant in secretory vesicles and at the cell surface. It is becoming evident that the intracellular trafficking of APP determines the accessibility of APP to the cleaving secretases, i.e., APP is processed differently in the endosomes than at the cell surface. Effects on APP localization change the balance between the α- and β-cleavages of APP and can eventually lead to development of AD.1 Accordingly, it is important to outline the key aspects of neuronal APP trafficking in order to understand the mechanisms that influence AD onset.2
The retromer complex is involved in sorting of cargo from the endosome to the trans-Golgi network (TGN). The complex consists of five proteins arranged in two sub complexes, i.e., a trimer composed of the Vps26, Vps29, and Vps35 and a dimer of two sorting nexin proteins Snx1 and Snx2.3-5 Proper retromer-dependent sorting is significant for correct trafficking in the endosome-Golgi pathway and subsequent function of several transmembrane proteins, where missorting impairs cargo function and may ultimately lead to disease onset.6,7 Studies have shown that deficiencies in the retromer sorting pathway can be linked to late-onset AD.8,9 Furthermore, it has been shown that expression of the retromer complex is decreased in vulnerable regions of AD brains,10 and retromer deficiency in mice and flies increase the production of Aβ, most likely by missorting of APP.11,12 Although the retromer complex is believed to be involved in AD by regulating the trafficking of APP,13-15 a direct binding between APP and the retromer complex has not yet been found, suggesting the existence of a sorting receptor that bridges APP to retromer.
SorLA (also known as SORL1 and LR11) is a determinant for APP transport, slowing down APPs exit from the TGN and thereby preventing APP from both amyloidogenic and non-amyloidogenic cleavages.16,17 SorLA is genetically associated to late-onset AD,18,19 and similar to the decrease found for retromer expression, there is also an underexpression of sorLA in vulnerable neurons from AD brains.10 Accordingly, others and we have proposed that sorLA is the molecule that bridges retromer action to APP sorting.1,20
A Direct Interaction between Retromer and sorLA
In a recent study we provided experimental evidence that sorLA indeed is the molecular link between APP and the retromer sorting complex.21 The cargo binding activity of the retromer is historically assigned to the trimer of Vps26-Vps29-Vps35, whereas the Snx subunit is responsible for membrane association (reviewed recently in refs4,22). Numerous studies have previously identified Vps35 as the receptor binding component.23,24 However, in our study we have demonstrated that Vps26 binds directly to a cargo receptor, i.e., the cytoplasmic tail of sorLA. This is in line with Vps26 containing an arrestin fold, which is a conformation found to be responsible for cargo-binding activity in other proteins.25 Also, we found that the receptor interaction with Vps26 was dependent on a phenylalanine residue located in a six amino acid FANSHY-sequence in the tail of sorLA.21 This agrees well with retromer subunits previously shown to prefer binding to aromatic sorting motifs.26
SorLA FANSHY→6A changes the localization
To study the functional relevance of the interaction between retromer and sorLA, we generated a sorLA mutant carrying six alanine residues instead of the FANSHY motif (i.e., sorLA FANSHY→6A). Using this sorLA mutant we observed a mis-targeting to the endosomal compartments of the mutant receptor in line with retromer’s role in retrograde transport.
Subsequently, we focused on the localization of the sorLA-FANSHY→6A by performing co-localization studies between non-mutated sorLA (sorLA-WT) and sorLA-FANSHY→6Awithin the same cell. These results confirmed that the mutated and the non-mutated sorLA receptors localize differently, with sorLA-WT found in the perinulear region corresponding to the TGN compartment and sorLA-FANSHY→6A found in a more distal vesicular compartment representing late/recycling endosomes and/or tubular endosomal network (TEN). This clearly shows that a lack of retromer binding alters receptor localization by impairing the retrograde trafficking from peripheral compartments (i.e., the TEN) back to the Golgi.
Based on these observations, we speculated that this mislocalization might influence the function of sorLA keeping APP from processing. This was relevant based on previous findings where we showed that sorLA exert part of its function by slowing APP exit from the Golgi into the secretory pathway whereby APP avoids cleavage by the secretases.16,27,28
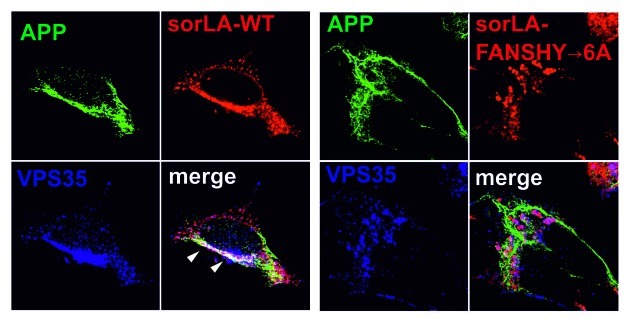
In order to analyze the exact role of sorLA in retromer-dependent APP sorting, we have now determined whether retromer is able to retrieve APP from the endosomal compartments independent of its interaction with sorLA. Accordingly, by triple immunohistochemical staining of SH-SY5Y we found a strong co-localization between APP and retromer (i.e., Vps35) in the presence of sorLA-WT (Fig. 1). In contrast, there was very little co-localization between APP and Vps35 when cells express the sorLA-FANSHY→6A mutant that is unable to associate with retromer (Fig. 1). This set of data clearly demonstrates that sorLA activity is needed to bridge retromer and APP.
Figure 1. SorLA-WT expression, but not sorLA-FANSHY→6A, mediates co-localization between retromer and APP. SH-SY5Y cells transfected with either sorLA-WT (left panel) or sorLA-FANSHY→6A (right panel) were stained with an antibody against the extracellular domain of sorLA (in red), or the endogenously expressed proteins APP (in green) and Vps35 (in blue). Co-localization of the trimeric complex between APP, Vps35 and sorLA-WT is indicated by white arrow heads.
Abnormal APP processing upon disrupting the sorLA-retromer complex
Having identified sorLA as a protein binding both APP and retromer, we next asked whether sorLA also is involved in the mechanism where retromer is reported to decrease amyloid production.
It was therefore of interest to determine how the sorLA mutant, not retrieving back to the perinuclear region, behaved in terms of APP trafficking and subsequent processing. First, to study APP transport we used live cell imaging confirming previous data, which showed that sorLA-WT significantly decreased the velocity and distance traveled by APP.21 However, upon mutation of the FANSHY motif this effect is completely abolished although the receptor mutant is still able to associate with APP in the TEN.21 Second, we also found that the APP:sorLA-FANSHY→6A complex formed in the peripheral TEN did not lead to any decrease of Aβ production compared with sorLA-WT binding APP early in the secretory pathway.
These findings lead to a model where retromer and sorLA co-operate in the retention of APP in the perinuclear region, where sorLA is the direct binding partner of APP and retromer functions in the retrieval of sorLA from the endosomal compartment back to early compartments of the secretory pathway (Fig. 2).
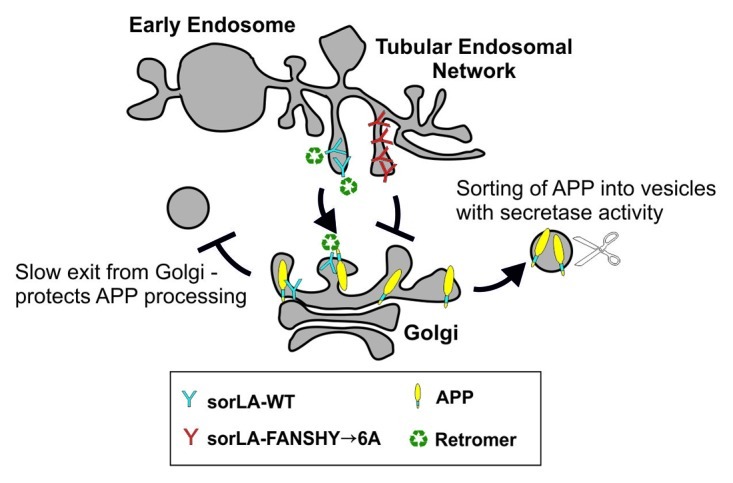
Figure 2. Schematic model of sorLA- and retromer-dependent transport of APP. After mutation of the FANSHY sequence, sorLA is no longer able to interact with retromer. By disrupting this interaction, sorLA cannot keep APP in the Golgi/TGN compartment. APP will then localize into other cellular compartments where APP is more prone to secretase cleavage. Since endosomal processing leads to production of Aβ, increasing endosomal delivery of APP increases the amyloidogenic processing. This change in localization of APP is important because sorLA can only protect against APP processing when located in the TGN. This is supported by the fact that sorLA-FANSHY→6A cannot protect against APP cleavage. When the retromer binding site is deleted sorLA is not recycled back to TGN, but rather stays in the late endosomal compartment, where sorLA has no impact on APP.
This model explains how genomic deficiency or misfunction of either sorLA or retromer can influence APP processing. These results not only shed new light on the mechanism of how retromer deficiency may lead to AD, but also provided new insight of how APP sorting receptors are key players in Aβ metabolism.
Conclusions
Based on our studies, we believe that the retromer complex is indeed the adaptor protein necessary for the retrieval of sorLA from the endosomes to the TGN, and the localization of sorLA in the TGN together with APP is of crucial importance for regulating the processing of APP. Once disrupting the retromer binding site, the cellular distribution of sorLA is changed, and sorLA is no longer able to protect against the production of Aβ.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21433
References
- 1.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sannerud R, Annaert W. Trafficking, a key player in regulated intramembrane proteolysis. Semin Cell Dev Biol. 2009;20:183–90. doi: 10.1016/j.semcdb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Collins BM. The structure and function of the retromer protein complex. Traffic. 2008;9:1811–22. doi: 10.1111/j.1600-0854.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 7.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–82. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 8.Small SA. Retromer sorting: a pathogenic pathway in late-onset Alzheimer disease. Arch Neurol. 2008;65:323–8. doi: 10.1001/archneurol.2007.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardarajan BN, Bruesegem SY, Harbour ME, George-Hyslop PS, Seaman MN, Farrer LA. Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol Aging. 2012;33:2231–, e15-30. doi: 10.1016/j.neurobiolaging.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, et al. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol. 2005;58:909–19. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- 11.Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, et al. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A. 2008;105:7327–32. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, et al. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J Cell Biol. 2011;195:765–79. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira SI, Rebelo S, Esselmann H, Wiltfang J, Lah J, Lane R, et al. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, et al. Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis. 2011;43:338–45. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol Dis. 2012;47:126–34. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen OM, et al. SorLA/LR11, a neuronal sorting receptor that regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–6. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, et al. The lipoprotein receptor LR11 regulates amyloid β production and amyloid precursor protein traffic in endosomal compartments. J Neurosci. 2006;26:1596–603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, et al. Genetic and Environmental Risk in Alzheimer Disease 1 Consortium Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68:99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen MS, Gustafsen C, Madsen P, Nyengaard JR, Hermey G, Bakke O, et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol Cell Biol. 2007;27:6842–51. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32:1467–80. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGough IJ, Cullen PJ. Recent advances in retromer biology. Traffic. 2011;12:963–71. doi: 10.1111/j.1600-0854.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 23.Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Rojas R, Bonifacino JS, Hurley JH. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol. 2006;13:540–8. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seaman MN. Endosome protein sorting: motifs and machinery. Cell Mol Life Sci. 2008;65:2842–58. doi: 10.1007/s00018-008-8354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, et al. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007;282:32956–64. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt V, Baum K, Lao A, Rateitschak K, Schmitz Y, Teichmann A, et al. Quantitative modelling of amyloidogenic processing and its influence by SORLA in Alzheimer’s disease. EMBO J. 2012;31:187–200. doi: 10.1038/emboj.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]