Abstract
Neurons compensate for changes in network activity by altering the sensitivity of transmission across collections of synapses by up- or downregulating the number of synaptic AMPA receptors. We recently reported that, in parallel to increasing AMPA receptor surface expression, suppression of network activity with TTX increases protein SUMOylation by decreasing levels of the deSUMOylating enzyme SENP1. SUMOylation of the immediate early gene product Arc is required for synaptic scaling. These results reveal a previously unsuspected role for protein SUMOylation in activity-dependent AMPA receptor trafficking and the regulation of neuronal network activity, processes which play important roles in neurodegenerative disease.
Keywords: SUMO, posttranslational modification, synaptic transmission, synapse, plasticity, synaptic scaling
Synaptic scaling is a type of homeostatic plasticity by which a neuron alters the sensitivity of groups of excitatory synapses in response to network activity by adjusting the number and composition of synaptic AMPARs over hours or days.1 Distinct from long-term potentiation (LTP) and long-term depression (LTD), which cause the rapid insertion or removal of AMPARs at individual synapses in an input-specific manner,2 homeostatic plasticity collectively regulates AMPARs at groups of synapses over a much longer timeframe. This allows neurons to tune synaptic gain and stabilize firing, while preserving the differences in the relative strengths between individual synapses.3 Thus, LTP and LTD in combination with synaptic scaling allow constant adjustment and refinement of the processes that underlie learning and memory at both the synaptic and the network level.
The synergistic properties of LTP/LTD and scaling, combined with the fact that the potentiation status of individual synapses in relation to their neighbors is retained after scaling, suggest different regulatory mechanisms for AMPAR trafficking. Consistent with this, knockdown of the immediate early gene product Arc/Arg3.1 (Arc) reduces basal AMPAR internalisation but has no effects on NMDAR-dependent AMPAR LTD.4 Arc has been extensively studied in the context of synaptic scaling because its activity-dependent local protein synthesis enhances basal AMPAR endocytosis.5 Although the mechanisms are not fully understood, a current model is that Arc promotes AMPAR internalisation through its interactions with the endocytic proteins endophilin-3 and dynamin-2.6,7
A widely used protocol for scaling up synaptic AMPARs is sustained incubation of dispersed neuronal cultures with the sodium channel blocker TTX, which prevents action potentials and suppresses network activity8,3. Our results confirmed that sustained TTX robustly increases GluA1 and GluA2 AMPAR subunit surface expression. In addition, we discovered that TTX also selectively increased the levels of protein modification by one isoform of Small Ubiquitin-like MOdifier (SUMO-1).9
SUMOylation is an important regulator of neuronal function and dysfunction.10-12 SUMO is attached to lysine residues in target proteins by a three-enzyme pathway analogous to, but distinct from, ubiquinitation. A major difference is that unlike for ubiquitin, where there are many E2 ligases, Ubc9 is the only E2 for SUMOylation. There are three validated SUMO isoforms in mammals, designated SUMO-1–3 although SUMO-2 and -3 differ by only three amino acids, and are thus collectively known as SUMO-2/3. SUMO is removed from substrates via the actions of a family of SUMO-specific deconjugating enzymes, SENPs.13
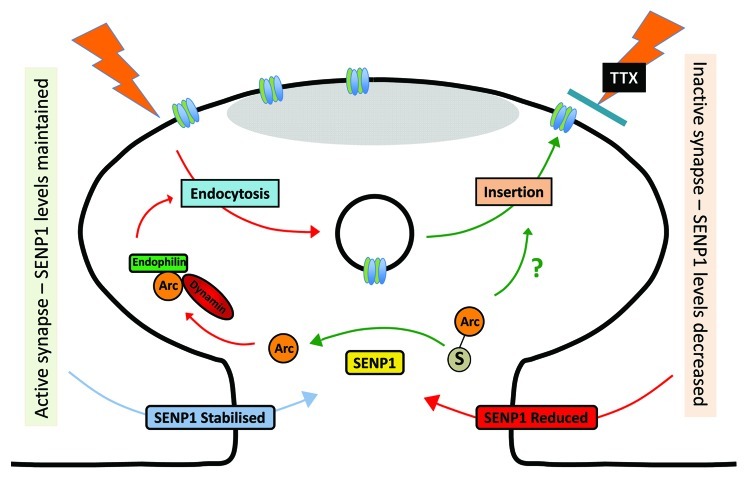
We found that, in parallel to the increase in AMPAR surface expression, TTX caused a reduction in levels of the deSUMOylating enzyme SENP1 (Fig. 1). Further, we showed that overexpression of the constitutively active catalytic domain of SENP1 prevents the TTX-induced increase in surface AMPARs but had no effect on AMPAR surface expression in the absence of TTX treatment.9 To confirm the scaling effects we observed by confocal imaging and surface biotinylation assays, we used hippocampal slice electrophysiology to measure AMPAR-mediated excitatory postsynaptic responses (EPSCs) in CA1 neurons.9 Sindbis virus overexpression of the catalytic domain of SENP1 decreased AMPAR EPSCs only in cells treated with TTX. In contrast, the control catalytically inactive SENP1 mutant had no effect on AMPAR EPSCs in control slices or those treated with TTX. These functional data indicate that the TTX-induced increase in AMPAR surface expression is synaptic. Thus, we conclude that SUMOylation of one or more protein targets is required for the forward trafficking of AMPAR to synapses under conditions of activity suppression.
Figure 1. Schematic of how synaptic activity can control SENP1 stability, which in turn regulates the SUMOylation status of Arc. In this model blockade of synaptic activity reduces SENP1 levels, which in turn prevents deSUMOylation of Arc. SUMO conjugation inhibits Arc binding to interacting proteins involved in endocytosis such as endophilin and dynamin, to reduce AMPAR internalisation and therefore upregulate AMPAR surface expression under conditions of reduced synaptic activity.
What might these SUMO targets be?
Arc contains two consensus SUMOylation sites at lysine residues K110 and K268, which are located close to the binding sites for endophilin-3 and dynamin-2 respectively. Mutation of these sites disrupts Arc localization in dendrites and this has been interpreted to suggest that Arc SUMOylation plays a role in structural changes required for some forms of LTP consolidation.5 Thus, SUMOylation of K110 and/or K268 might disrupt the interaction of Arc with endophilin-3 and dynamin-2 to inhibit AMPAR endocytosis, which could explain the dependence of synaptic scaling on SUMOylation. We therefore compared the affects of expressing wild-type and a non-SUMOylatable double lysine mutant of Arc (Arc-ΔKK). In contrast to control cells, neurons expressing Arc-ΔKK did not exhibit any TTX-induced scaling of AMPAR surface expression. While other, as yet unidentified, SUMO substrates are almost certainly involved, these biochemical results suggest that decreased SENP1, which leads to increased SUMOylated Arc, is permissive for synaptic scaling and reveal a previously unsuspected regulatory mechanism for the control of AMPAR trafficking, long-term homeostatic plasticity and cell responsiveness.
The discovery that SUMOylation can regulate Arc function may have far reaching implications for the understanding of AMPAR trafficking and mechanisms of plasticity. Further, the roles of Arc, SUMO and synaptic scaling in the development and expression of neurological and neurodegenerative diseases hold exciting potential. For example, synaptic scaling is impaired in a FMRP mouse model of fragile X syndrome14 and defective synaptic scaling has been strongly implicated in Alzheimer disease.15,16 Intriguingly, Arc is required for activity-dependent generation of β-amyloid (Aβ) and binds to amyloid precursor protein (APP), β-site APP cleaving enzyme 1 (BACE1) and presenilin1 to regulate activity dependent γ-secretase.17 Arc deletion reduces Aβ in mouse models of Alzheimer disease and can be present in abnormally high levels in human Alzheimer patients.17 APP is also a SUMO substrate and SUMOylation of APP reduces Aβ production whereas mutagenesis of the SUMO target lysines in APP enhances Aβ production.18-20 Thus, we anticipate that SUMOylation will prove to be a fundamental factor in several neurological disorders and may provide a therapeutic target for drug development.
In summary, we believe that the activity-dependent regulation of SENP1 levels, which in turn regulates SUMOylation of Arc is a novel and exciting observation. We expect future work will reveal in greater detail how these regulatory mechanisms control AMPAR trafficking, homeostatic plasticity and network activity in normal and diseased brain.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We are grateful to the MRC, BBSRC, and ERC for financial support and to all of the authors on the JBC paper for their invaluable contributions. We are also grateful to Keri Hildick for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21712
References
- 1.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–89. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–84. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–40. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–7. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anggono V, Clem RL, Huganir RL. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci. 2011;31:2188–96. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig TJ, Jaafari N, Petrovic MM, Rubin PP, Mellor JR, Henley JM. Homeostatic synaptic scaling is regulated by protein SUMOylation. J Biol Chem. 2012;287:22781–8. doi: 10.1074/jbc.M112.356337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8:948–59. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig TJ, Henley JM. Protein SUMOylation in spine structure and function. Curr Opin Neurobiol. 2012;22:480–7. doi: 10.1016/j.conb.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–45. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–21. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14:1112–4. doi: 10.1038/nn.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savioz A, Leuba G, Vallet PG, Walzer C. Contribution of neural networks to Alzheimer disease’s progression. Brain Res Bull. 2009;80:309–14. doi: 10.1016/j.brainresbull.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, et al. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent β-amyloid generation. Cell. 2011;147:615–28. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gocke CB, Yu H, Kang J. Systematic identification and analysis of mammalian small ubiquitin-like modifier substrates. J Biol Chem. 2005;280:5004–12. doi: 10.1074/jbc.M411718200. [DOI] [PubMed] [Google Scholar]
- 19.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem. 2006;281:9919–24. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YQ, Sarge KD. Sumoylation of amyloid precursor protein negatively regulates Abeta aggregate levels. Biochem Biophys Res Commun. 2008;374:673–8. doi: 10.1016/j.bbrc.2008.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]