Abstract
The aye-aye is a rare lemur from Madagascar that uses its highly specialized middle digit for percussive foraging. This acoustic behavior, also termed tap-scanning, produces dominant frequencies between 6 and 15 kHz. An enhanced auditory sensitivity to these frequencies raises the possibility that the acoustic and auditory specializations of aye-ayes have imposed constraints on the evolution of their vocal signals, especially their primary long-distance vocalization, the screech. Here we explore this concept, termed receiver bias, and suggest that the dominant frequency of the screech call (~2.7 kHz) represents an evolutionary compromise between the opposing adaptive advantages of long-distance sound propagation and enhanced detection by conspecific receivers.
Keywords: Daubentonia madagascariensis, lemur, audiogram, auditory sensitivity, receiver bias, vocalization, sensory trap
The aye-aye (Daubentonia madagascariensis) is a nocturnal primate endemic to Madagascar. It is an enduring source of fascination, both because of its many unique features and because it is the only survivor of a lineage with an origin ~70 million years ago.1 As a result, Daubentonia is allocated to its own family (Daubentoniidae) and infraorder (Chiromyiformes). The aye-aye is perhaps best known for its acoustic foraging behaviors, termed percussive foraging or tap-scanning,2-5 and suite of unusual anatomical specializations, particularly in the hand, skull, and central nervous system.6-15 For example, aye-ayes have elongated hands with long, thin middle fingers that have been described as villiform, filamentous, gracile, or grotesquely attenuated.6-9 This singular digit is highly mobile10 due to a unique ball-and-socket metacarpophalangeal joint.12 Such morphology enables rapid tapping and the detection, localization, and extraction of embedded foods such as the wood-boring larvae of cerambycid beetles.13
For aye-ayes, the importance of percussive foraging (5–41% of foraging time13) and the functional demands of integrating two sensory modalities -haptic touch and audition- appear to be linked with the evolution of large and elaborate ear structures16-19 and the expansion of cerebral cortical regions associated with auditory processing, such as the inferior colliculus.11 As a result, aye-ayes are relatively encephalized and reported to have high levels of sensorimotor intelligence.20 Such attributes suggest that aye-ayes might also have exceptional hearing abilities, yet the auditory sensitivities of strepsirrhine primates are relatively unstudied.
Aye-ayes as auditory specialists
Recently, Ramsier et al.21 used the auditory brainstem response (ABR) method to generate audiograms from 11 strepsirrhine primates, and they confirmed that aye-ayes have relatively enhanced auditory sensitivity between 2.8 and 22.6 kHz, with 2.8–15.9 kHz being the 10-dB bandwidth (the bandwidth across which thresholds are within 10 dB of the threshold of the frequency of best sensitivity).22 Although ABR-derived thresholds are sometimes elevated in comparison with behavioral tests of primates, especially for frequencies ≤ 2.0 kHz, the two methods produce audiograms with similar shapes, high-frequency limits, frequencies of best sensitivity, and upper-frequencies of the 10-dB bandwidth.22 Our estimate for the low-frequency end of the 10-dB bandwidth of aye-ayes appears robust given the close agreement between two individuals for all low-frequency thresholds (0.2–1.6 dB difference, depending on frequency), and the very steep incline for frequencies ≤ 1 kHz (already > 30 dB above the threshold of best sensitivity at 1 kHz).21
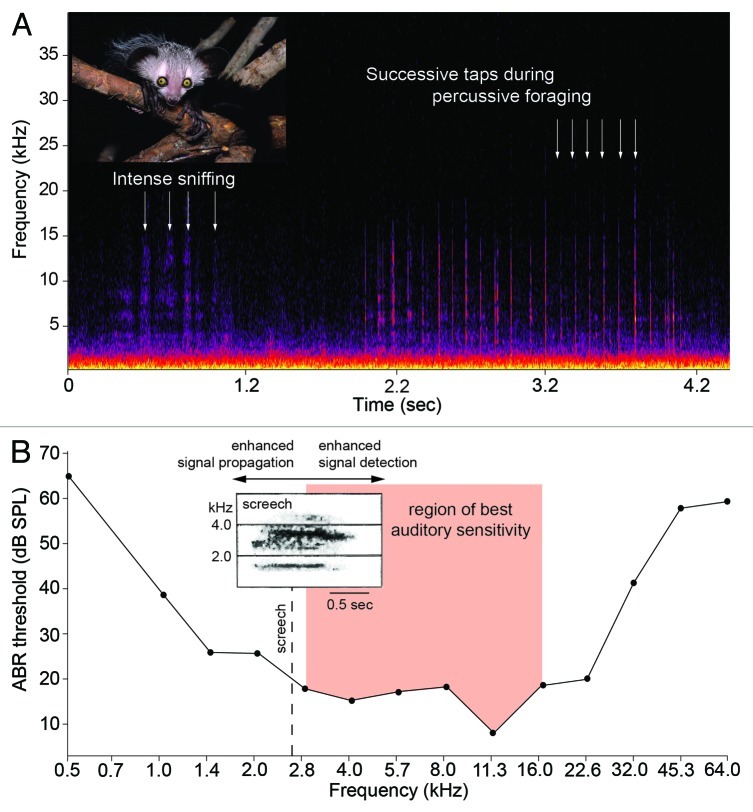
If we accept this region of best auditory sensitivity, we must now ask if it corresponds with the acoustic properties of percussive foraging. To explore this premise, we used a studio condenser microphone (Sennheiser, Old Lyme, Connecticut; frequency response 0.03–50 kHz) and a solid-state recorder (PMD-671, Marantz, Mahwah, New Jersey; sampling frequency 96 kHz, 24-bit) to analyze the percussive foraging of an adult male aye-aye, Merlin, housed at the Duke Lemur Center. We recorded tap-scanning on a typical stimulus used for enrichment purposes: 2x4-inch (5.1x10.2-cm) boards of Eastern white pine (Pinus strobus) permeated with food rewards. The rate of tapping was consistent across recordings (97.7 ± 19.9 ms) and each tap had a dominant energy of 6–15 kHz contained between 2 and 27 kHz (Fig. 1A). The percussive tapping of aye-ayes is thus a broadband sound that corresponds well to their frequency region of best auditory sensitivity (Fig. 1B); however, the acoustic attributes of a temperate softwood should be interpreted with caution. In the wild, extractive foraging is most strongly associated with the decaying stumps of trees, but aye-ayes do sometimes excavate living tissues (e.g., branches of Protorhus sp.; trees of Anthocleista spp.).3 The acoustics of percussive foraging on these woods are unknown.
Figure 1. (A) Adult aye-aye and spectrogram of percussive foraging behavior. Each tap of the third digit is discernable with a dominant energy of 6–15 kHz. (B) Mean audiogram of two aye-ayes and the region of best auditory sensitivity (modified from Ramsier et al.21). Insert: spectrogram of the aye-aye’s primary long-distance vocalization, the screech (‘aack’ variant), with a dominant frequency of 2.66 kHz (modified from Figure 1J in Stanger and Macedonia30). Photograph of aye-aye by D.M. Haring, reproduced with permission.
Receiver bias and the vocal ecology of aye-ayes
Receiver bias, or preexisting bias, is a model of animal communication that emphasizes bias in the sensory systems of signal receivers.23 For aye-ayes, the auditory demands associated with percussive foraging might drive, or bias, the evolution of their vocal signals. According to this model, the dominant frequencies of aye-aye vocalizations are predicted to fall in the range of best auditory sensitivity, ca. 2.8–15.9 kHz (Fig. 1B). Yet aye-ayes are solitary foragers with extensive home ranges (females: 30–40 ha; males: 120–215 ha),13 and population densities are sparse.24 Thus vocal signals must propagate through relatively vast expanses of forest. Under these conditions, environmental noise can exert a significant selective pressure on the acoustic structure of vocal signals,25 including those of primates.26 In fact, most primates have evolved long-distance calls with dominant frequencies < 1.5 kHz,27 probably because they propagate farther and are less susceptible to masking by forest noise.
The primary long-distance vocalization, or contact call, between aye-ayes has been described onomatopoetically (creee or nee-a) and prosaically (screech).28-30 The screech is a variable signal with a dominant frequency of 2.66 kHz and a peak frequency of 8.45 kHz.30 In addition, two types of alarm call, or screams, have dominant frequencies of 2.80 and 3.25 kHz and peak frequencies of 8.58 and 9.20 kHz.30 The similar acoustic properties of these calls is puzzling given their different functions. Long-distance calls normally have low dominant frequencies,27 whereas alarm calls have much higher dominant frequencies.31 For aye-ayes, it is plausible that a dominant frequency close to ~2.7 kHz represents an evolutionary tradeoff between the opposing adaptive advantages of long-distance sound propagation and enhanced detection by conspecific receivers (Fig. 1B). Yet the elegance of this potential compromise is deceptive in part because it raises the possibility that aye-ayes are caught in a sensory trap.23
Sensory traps and the reproductive ecology of aye-ayes
Sensory traps are part of a broader concept, the evolutionary trap,32 which holds that animals can experience reduced fitness, or become ‘trapped’, by their own sensory adaptations.23 Here we hypothesize that the auditory demands of percussive foraging resulted in a receiver bias that precluded the evolution of lower-frequency contact calls. This constraint is expected to become increasingly suboptimal (i.e., contact calls will be increasingly inaudible) as aye-ayes become more widely dispersed as a result of habitat fragmentation. Indeed, the exceedingly low genetic diversity of aye-ayes is puzzling given their large geographic distribution.33 These recent findings suggest that aye-ayes are quite vulnerable to extinction, not least, perhaps, because they have a limited ability to communicate over large distances.
In sum, we suggest that the unique acoustic ecology and auditory adaptations of aye-ayes have partly contributed to their low genetic diversity. Although speculative, this concept of a sensory trap invites testing; if true, it has profound conservation implications for a unique and highly endangered primate.
Acknowledgments
We thank D.M. Brewer, A.J. Cunningham, E. Ehmke, D.M. Haring, A.D. Melin, G.L. Moritz, G.H. Perry, A.D. Yoder, and S. Zehr for their comments and contributions to this research, and the David and Lucile Packard Foundation for funding (Fellowship no. 2007–31754). Data were collected at the Duke Lemur Center (Durham, USA) under protocols approved by the Institutional Animal Care and Use Committees of Dartmouth College, Duke University, and the Duke Lemur Center (approval nos. 10–11–02, A209–08–08, and M-5–08–1, respectively). This is Duke Lemur Center publication no. 1229.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/21509
References
- 1.Perry GH, Reeves D, Melsted P, Ratan A, Miller W, Michelini K, et al. A genome sequence resource for the aye-aye (Daubentonia madagascariensis), a nocturnal lemur from Madagascar. Genome Biol Evol. 2012;4:126–35. doi: 10.1093/gbe/evr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson CJ. Percussive foraging in the aye-aye, Daubentonia madagascariensis. Anim Behav. 1991;41:793–801. doi: 10.1016/S0003-3472(05)80346-X. [DOI] [Google Scholar]
- 3.Erickson CJ. Feeding sites for extractive foraging by the aye-aye, Daubentonia madagascariensis. Am J Primatol. 1995;35:235–40. doi: 10.1002/ajp.1350350306. [DOI] [PubMed] [Google Scholar]
- 4.Erickson CJ. Cues for prey location by aye-ayes (Daubentonia madagascariensis) Folia Primatol (Basel) 1998;69:35–40. doi: 10.1159/000052697. [DOI] [Google Scholar]
- 5.Erickson CJ, Nowicki S, Dollar L, Goehring N. Percussive foraging: Stimuli for prey location by aye-ayes (Daubentonia madagascariensis) Int J Primatol. 1998;19:111–22. doi: 10.1023/A:1020363128240. [DOI] [Google Scholar]
- 6.Owen R. Monograph on the aye-aye (Chiromys madagascariensis, Cuvier). London: Taylor and Francis, 1863. [Google Scholar]
- 7.Cartmill M. Daubentonia, Dactylopsila, woodpeckers and klinorhynchy. In: Martin RD, Doyle GA, Walker AC, eds. Prosimian Biology. Gloucester: Duckworth, 1974:655-70. [Google Scholar]
- 8.Jouffroy FK. Osteology and myology of the lemuriform postcranial skeleton. In: Tattersall I, Sussman RW, eds. Lemur Biology. New York: Plenum Press, 1975:149-92. [Google Scholar]
- 9.Oxnard CE. The uniqueness of Daubentonia. Am J Phys Anthropol. 1981;54:1–21. doi: 10.1002/ajpa.1330540102. [DOI] [Google Scholar]
- 10.Milliken GW, Ward JP, Erickson CJ. Independent digit control in foraging by the aye-aye (Daubentonia madagascariensis) Folia Primatol (Basel) 1991;56:219–24. doi: 10.1159/000156551. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JA, Ahrens ET, Laidlaw DH, Zhang S, Allman JM. Anatomical analysis of an aye-aye brain (Daubentonia madagascariensis, primates: Prosimii) combining histology, structural magnetic resonance imaging, and diffusion-tensor imaging. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1026–37. doi: 10.1002/ar.a.20264. [DOI] [PubMed] [Google Scholar]
- 12.Soligo C. Anatomy of the hand and arm in Daubentonia madagascariensis : a functional and phylogenetic outlook. Folia Primatol (Basel) 2005;76:262–300. doi: 10.1159/000088034. [DOI] [PubMed] [Google Scholar]
- 13.Sterling EJ, McCreless EE. Adaptations in the aye-aye: A review. In: Gould L, Sauther ML, eds. Lemurs: Ecology and adaptation. New York: Springer, 2006:159-84. [Google Scholar]
- 14.Melin AD, Moritz GL, Fosbury RAE, Kawamura S, Dominy NJ. Why aye-ayes see blue. Am J Primatol. 2012;74:185–92. doi: 10.1002/ajp.21996. [DOI] [PubMed] [Google Scholar]
- 15.Moritz GL, Dominy NJ. Thermal imaging of aye-ayes (Daubentonia madagascariensis) reveals a dynamic vascular supply during haptic sensation. Int J Primatol. 2012 doi: 10.1007/s10764-011-9575-y. [DOI] [Google Scholar]
- 16.Coleman MN, Ross CF. Primate auditory diversity and its influence on hearing performance. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1123–37. doi: 10.1002/ar.a.20118. [DOI] [PubMed] [Google Scholar]
- 17.Masali M, Cremasco MM. Hoc alterum auditus organi ossiculum est: Ear ossicles in physical anthropology. Hum Evol. 2006;21:1–17. doi: 10.1007/s11598-006-9000-2. [DOI] [Google Scholar]
- 18.Kirk EC, Gosselin-Ildari AD. Cochlear labyrinth volume and hearing abilities in primates. Anat Rec A Discov Mol Cell Evol Biol. 2009;292:765–76. doi: 10.1002/ar.20907. [DOI] [PubMed] [Google Scholar]
- 19.Lebrun R, de León MP, Tafforeau P, Zollikofer C. Deep evolutionary roots of strepsirrhine primate labyrinthine morphology. J Anat. 2010;216:368–80. doi: 10.1111/j.1469-7580.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling EJ, Povinelli DJ. Tool use, aye-ayes, and sensorimotor intelligence. Folia Primatol (Basel) 1999;70:8–16. doi: 10.1159/000021669. [DOI] [PubMed] [Google Scholar]
- 21.Ramsier MA, Cunningham AJ, Finneran JJ, Dominy NJ. Social drive and the evolution of primate hearing. Philos Trans R Soc Lond B Biol Sci. 2012;367:1860–8. doi: 10.1098/rstb.2011.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsier MA, Dominy NJ. A comparison of auditory brainstem responses and behavioral estimates of hearing sensitivity in Lemur catta and Nycticebus coucang. Am J Primatol. 2010;72:217–33. doi: 10.1002/ajp.20780. [DOI] [PubMed] [Google Scholar]
- 23.Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol. 1998;13:415–20. doi: 10.1016/S0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- 24.Mittermeier R, Hawkins F, Louis EE. Lemurs of Madagascar, 3rd Edition. Arlington: Conservation International, 2010. [Google Scholar]
- 25.Brumm H, Slabbekoorn H. Acoustic communication in noise. Adv Stud Behav. 2005;35:151–209. doi: 10.1016/S0065-3454(05)35004-2. [DOI] [Google Scholar]
- 26.Brown CH, Gomez R, Waser PM. Old world monkey vocalizations: Adaptation to the local habitat? Anim Behav. 1995;50:945–61. doi: 10.1016/0003-3472(95)80096-4. [DOI] [Google Scholar]
- 27.Mitani J, Stuht J. The evolution of nonhuman primate loud calls: Acoustic adaptation for long-distance transmission. Primates. 1998;39:171–82. doi: 10.1007/BF02557729. [DOI] [Google Scholar]
- 28.Petter J-J. The aye-aye. In: HSH Prince Rainier III of Monaco, Bourne GH, eds. Primate Conservation. New York: Academic Press, 1977:37-57. [Google Scholar]
- 29.Iwano T. An ecological and behavioral study of the aye-aye (Daubentonia madagascariensis) Afr Study Monogr. 1991;12:19–42. [Google Scholar]
- 30.Stanger KF, Macedonia JM. Vocalizations of aye-ayes (Daubentonia madagascariensis) in captivity. Folia Primatol (Basel) 1994;62:160–9. doi: 10.1159/000156773. [DOI] [PubMed] [Google Scholar]
- 31.Caro T. Antipredator defenses in birds and mammals. Chicago: University of Chicago Press, 2005. [Google Scholar]
- 32.Schlaepfer MA, Runge MC, Sherman PW. Ecological and evolutionary traps. Trends Ecol Evol. 2002;17:474–80. doi: 10.1016/S0169-5347(02)02580-6. [DOI] [Google Scholar]
- 33.Perry GH, Melsted P, Marioni JC, Wang Y, Bainer R, Pickrell JK, et al. Comparative RNA sequencing reveals substantial genetic variation in endangered primates. Genome Res. 2012;22:602–10. doi: 10.1101/gr.130468.111. [DOI] [PMC free article] [PubMed] [Google Scholar]