Abstract
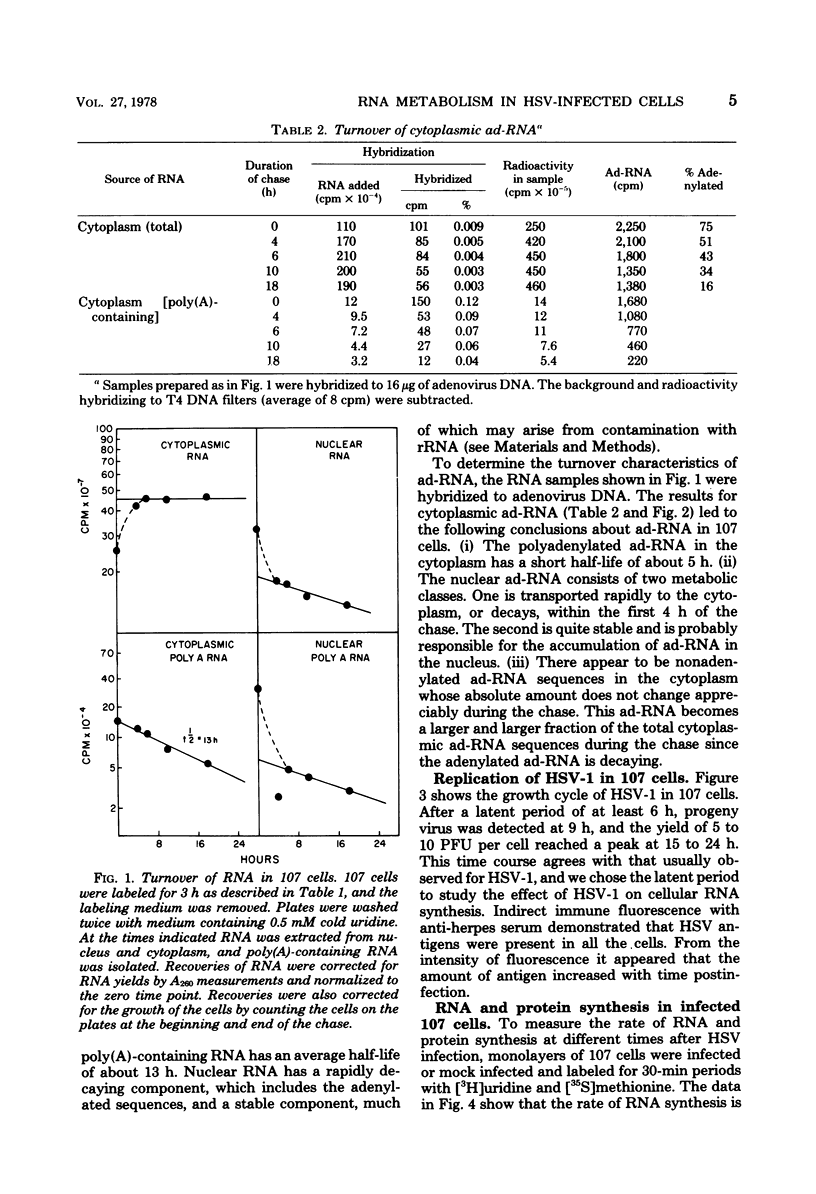
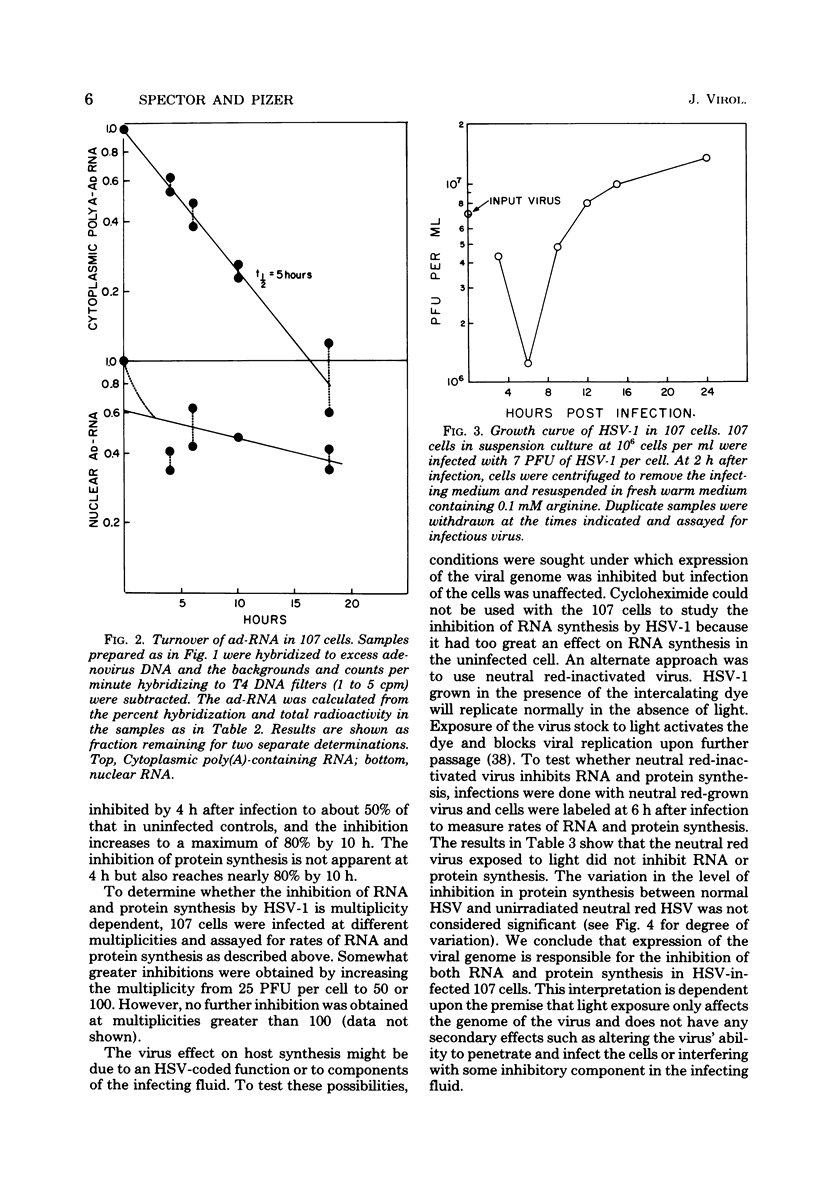
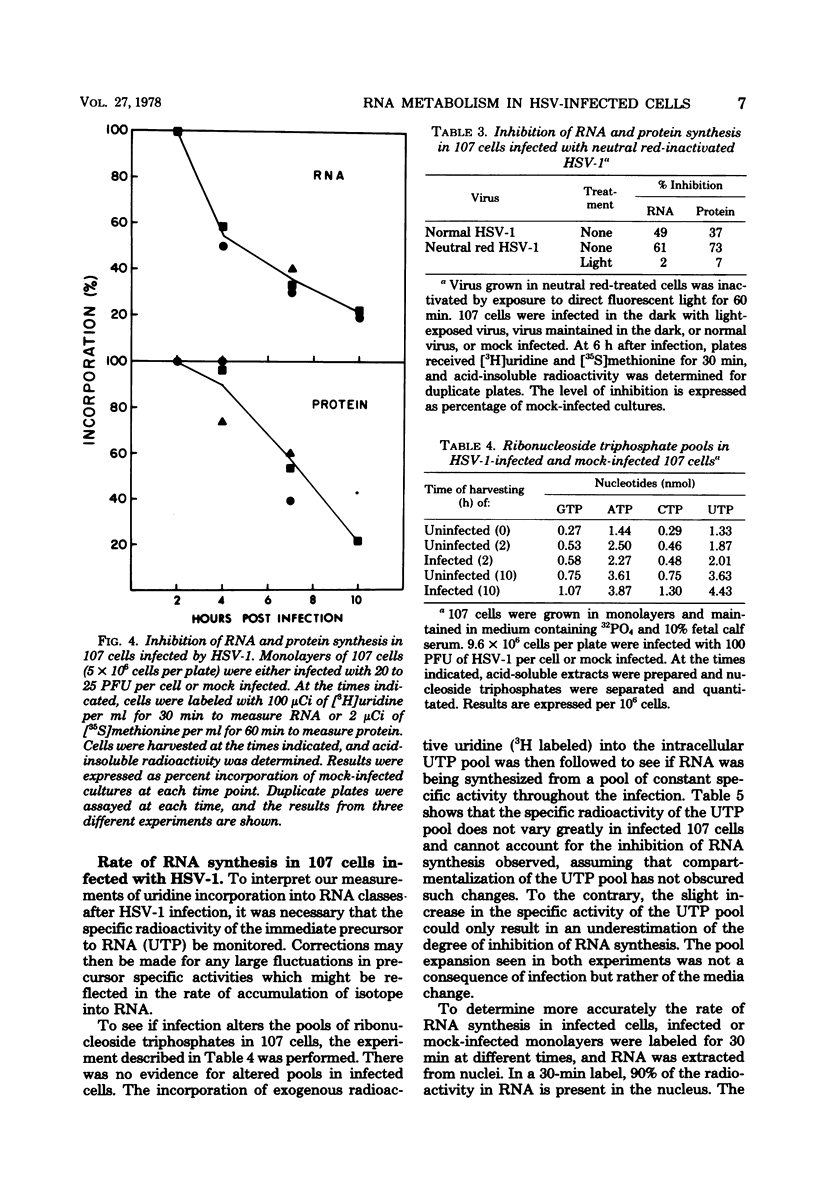
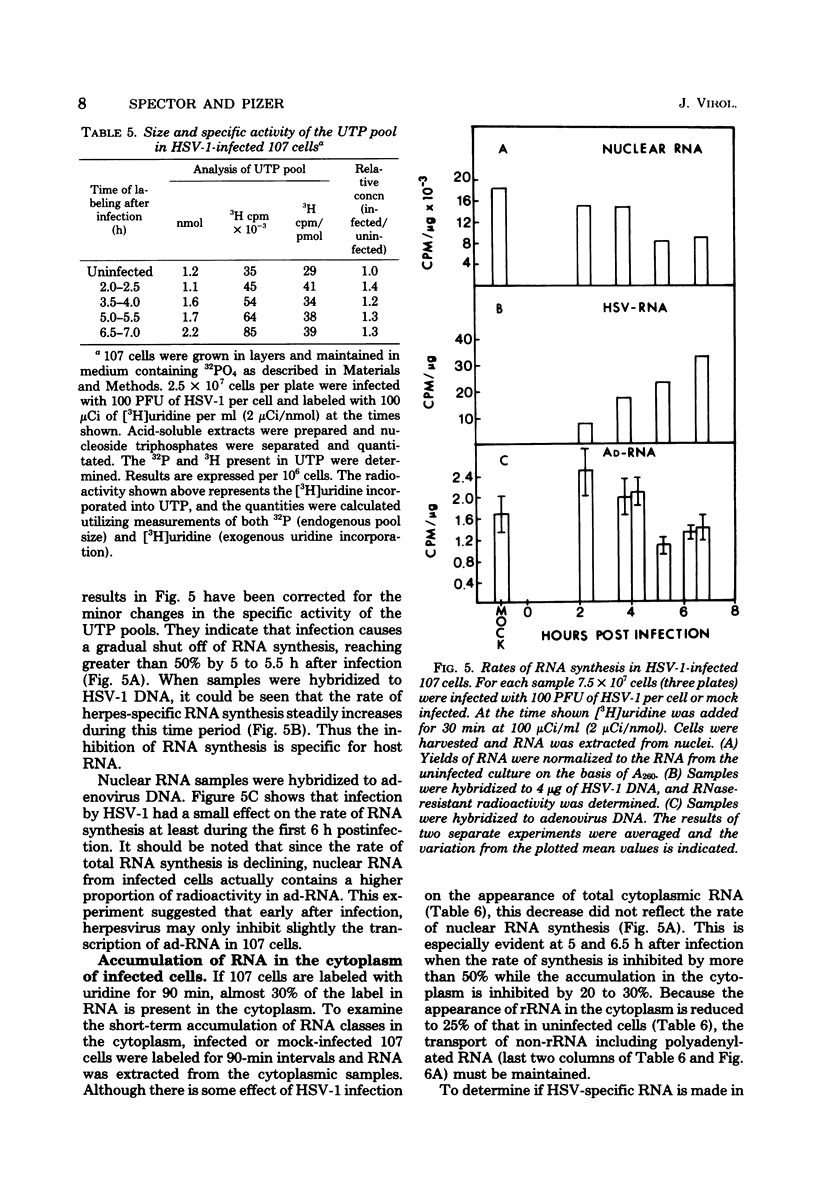
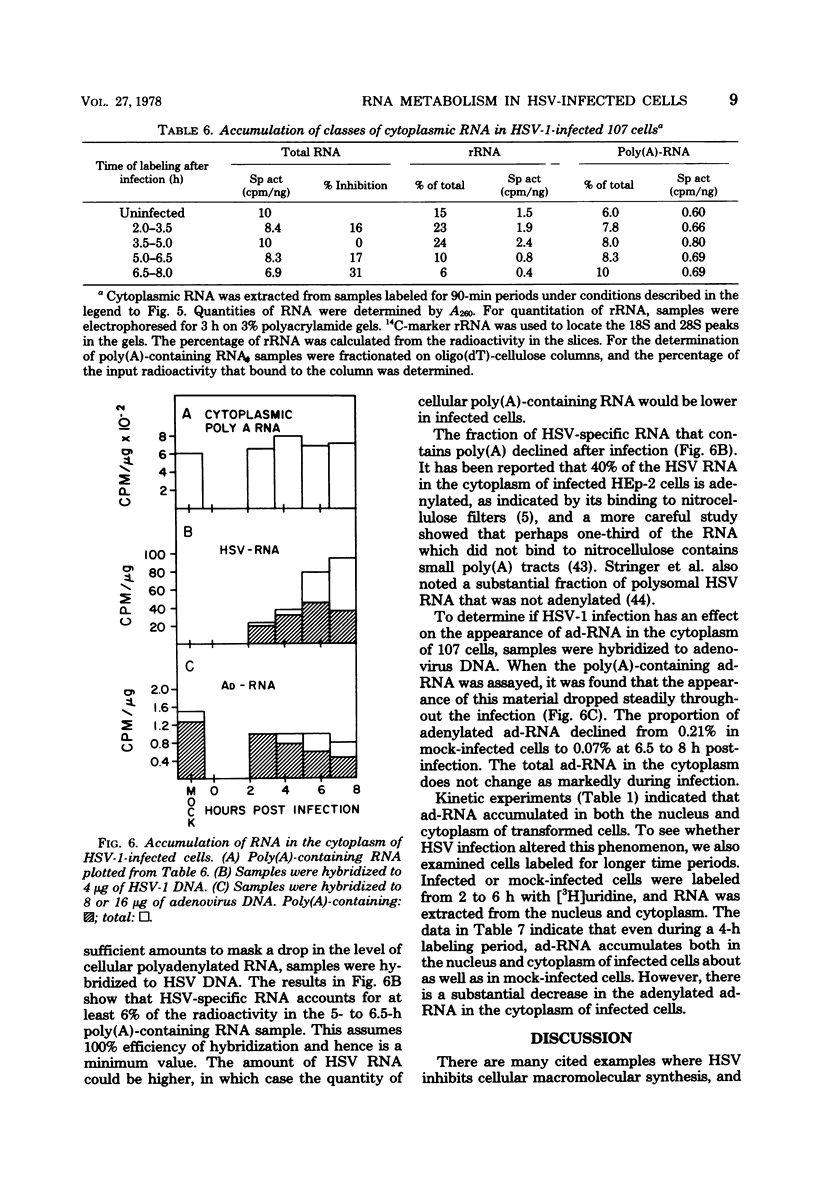
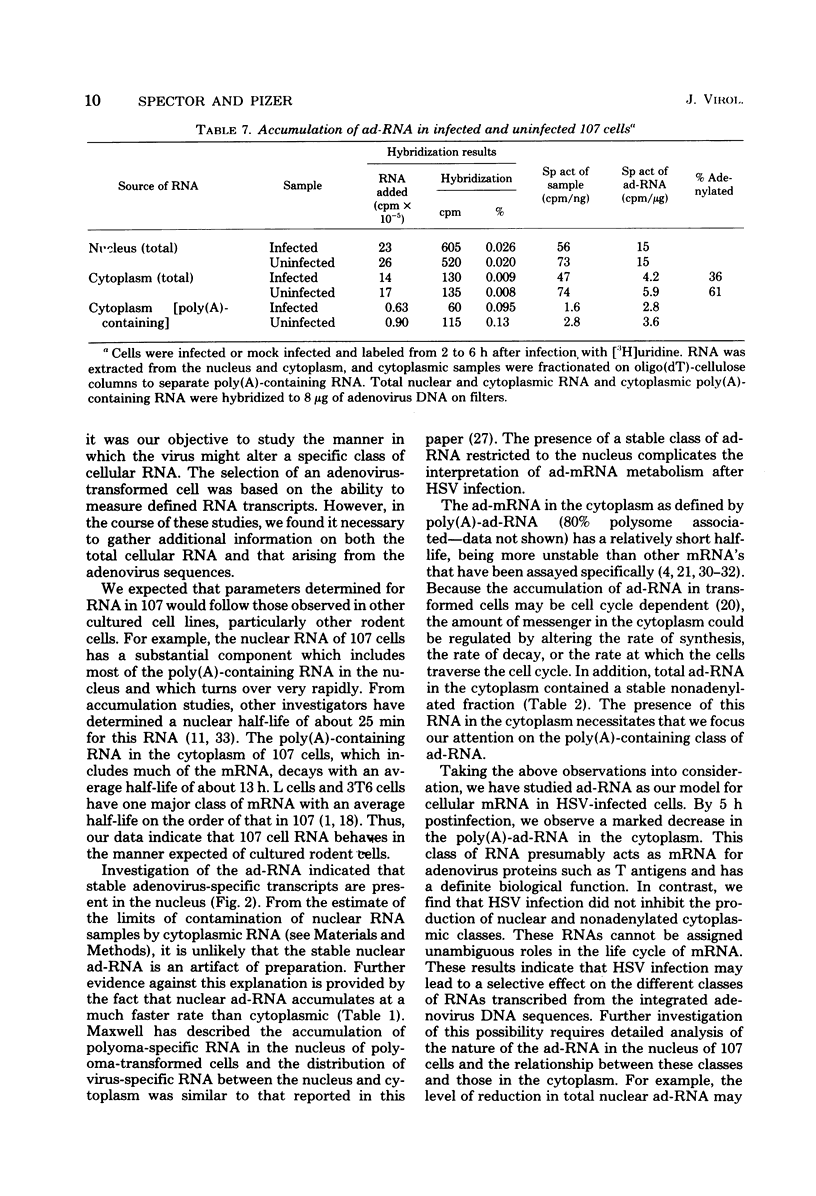
The effect of herpes simplex virus (HSV) infection of mRNA metabolism was examined in a system where the fate of specific RNA sequence can be assayed. Adenovirus type 5-transformed rat embryo cell line 107 synthesizes adenovirus-specific RNA (ad-RNA), which functions in the cytoplasm as mRNA. We have utilized ad-RNA as a model for mRNA metabolism, and in a preliminiary study we characterized ad-RNA in the nucleus and cytoplasm by hybridization to filter-bound adenovirus DNA. The results indicated the as-RNA accumulates in the nucleus and that cytoplasmic polyadenylic acid [poly(A)]-containing ad-RNA turns over with a half-life of a few hours. Pulse-chase experiments confirmed these observations and a half-life of about h was determined for the poly(A)-containing cytoplasmic ad-RNA. A second class of ad-RNA remains in the nucleus, where it turns over with a longer hlaf-life (about 24 h). The infection of 107 cells by HSV was restricted at 37 degree C, giving a burst size of 5 PFU per cell and allowing continued host DNA synthesis. Protein synthesis was inhibited greater than 50% by 7 h after infection, and total RNA synthesis was 50% inhibited by 4 h after infection. During the first 8 h after infection, HSV has little effect on the rate of synthesis of ad-RNA as determined by hybridization of nuclear RNA samples, but,during the same period, HSV inhibits the accumulation of poly(A)-containing ad-RNA in the cytoplasm. The degree of this inhibition increases steadily throughout this period and reaches 60% by 6.5 to 8 h after infection. Nosignificant effect was seen on the accumulation of total cellular poly(A)-containing RNA. It was concluded from these experiments that HSV infection alters the metabolism of ad-RNA so as to prevent the normal appearance of the poly(A)-containing mRNA in the cytoplasm. The result for ad-RNA may not represent the behavior of total cellular poly(A)-containing RNA under conditions where infection is restricted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bachenheimer S. L., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. VI. Polyadenylic acid sequences in viral messenger ribonucleic acid. J Virol. 1972 Oct;10(4):875–879. doi: 10.1128/jvi.10.4.875-879.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard P., Acheson N. H., Maxwell I. H. Strand-specific transcription of polyoma virus DNA-early in productive infection and in transformed cells. J Virol. 1975 Jan;17(1):20–26. doi: 10.1128/jvi.17.1.20-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Buetti E. Characterization of late polyoma mRNA. J Virol. 1974 Aug;14(2):249–260. doi: 10.1128/jvi.14.2.249-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. F. Virus-specific ribonucleic acid synthesis in KB cells infected with herpes simplex virus. J Virol. 1967 Jun;1(3):583–590. doi: 10.1128/jvi.1.3.583-590.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J., Reeson D. E. Rapid screening of tissue culture cells for mycoplasmal contaminants. J Biol Stand. 1975;3(2):181–184. doi: 10.1016/0092-1157(75)90045-1. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hay J., Koteles G. J., Keir H. M., Subak Sharpe H. Herpes virus specified ribonucleic acids. Nature. 1966 Apr 23;210(5034):387–390. doi: 10.1038/210387b0. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. R., Darnell J. E., Jr Differential accumulation of virus-specific RNA during the cell cycle of adenovirus-transformed rat embyro cells. J Virol. 1975 Apr;15(4):806–811. doi: 10.1128/jvi.15.4.806-811.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. A. Rate of synthesis and half-life of globin messenger ribonucleic acid. Rate of synthesis of globin messenger ribonucleic acid calculated from data of cell haemoglobin content. Biochem J. 1974 Mar;138(3):499–510. doi: 10.1042/bj1380499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. The incorporation of C14-labeled nucleosides into rabbit kidney cells infected with pseudorabies virus. Virology. 1960 May;11:12–27. doi: 10.1016/0042-6822(60)90053-2. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell I. H. Intracellular distribution and sedimentation properties of virus-specific RNA in two clones of BHK cells transformed by polyoma virus. J Virol. 1976 May;18(2):461–472. doi: 10.1128/jvi.18.2.461-472.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Beard P. The effect of herpes virus infection on mRNA in polyoma virus transformed cells. Virology. 1976 Dec;75(2):477–480. doi: 10.1016/0042-6822(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Rapp F., Li J. L., Jerkofsky M. Transformation of mammalian cells by DNA-containing viruses following photodynamic inactivation. Virology. 1973 Oct;55(2):339–346. doi: 10.1016/0042-6822(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Roizman B., Borman G. S., Rousta M. K. Macromolecular synthesis in cells infected with herpes simplex virus. Nature. 1965 Jun 26;206(991):1374–1375. doi: 10.1038/2061374a0. [DOI] [PubMed] [Google Scholar]
- Roller B., Cohen G. H. Deoxyribonucleoside triphosphate pools in synchronized human cells infected with herpes simplex virus types 1 and 2. J Virol. 1976 Apr;18(1):58–64. doi: 10.1128/jvi.18.1.58-64.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Fujinaga K., Hama S., Sekikawa K., Ito Y. Virus-specific ribonucleic acid in the nucleus and cytoplasm of rat embryo cells transformed by adenovirus type 2. J Virol. 1972 Oct;10(4):648–652. doi: 10.1128/jvi.10.4.648-652.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertien S., Millette R., Jones P., Roizman B. RNA synthesis in cells infected with herpes simplex virus. XII. Sequence complexity and properties of RNA differing in extent of adenylation. J Virol. 1976 Jun;18(3):977–991. doi: 10.1128/jvi.18.3.977-991.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R., Holland L. E., Swanstrom R. I., Pivo K., Wagner E. K. Quantitation of herpes simplex virus type 1 RNA in infected HeLa cells. J Virol. 1977 Mar;21(3):889–901. doi: 10.1128/jvi.21.3.889-901.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- Thiel J. F., Smith K. O. Fluorescent focus assay of viruses on cell monolayers in plastic Petri plates. Proc Soc Exp Biol Med. 1967 Jul;125(3):892–895. doi: 10.3181/00379727-125-32232. [DOI] [PubMed] [Google Scholar]
- Tsuei D., Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: RNA transcripts containing viral and highly reiterated cellular base sequences in adenovirus-transformed cells (DNA-RNA hybridization-viral-cell mRNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):427–430. doi: 10.1073/pnas.69.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELOCK E. F., TAMM I. Enumeration of cell-infecting particles of Newcastle disease virus by the fluorescent antibody technique. J Exp Med. 1961 Feb 1;113:301–316. doi: 10.1084/jem.113.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


