Abstract
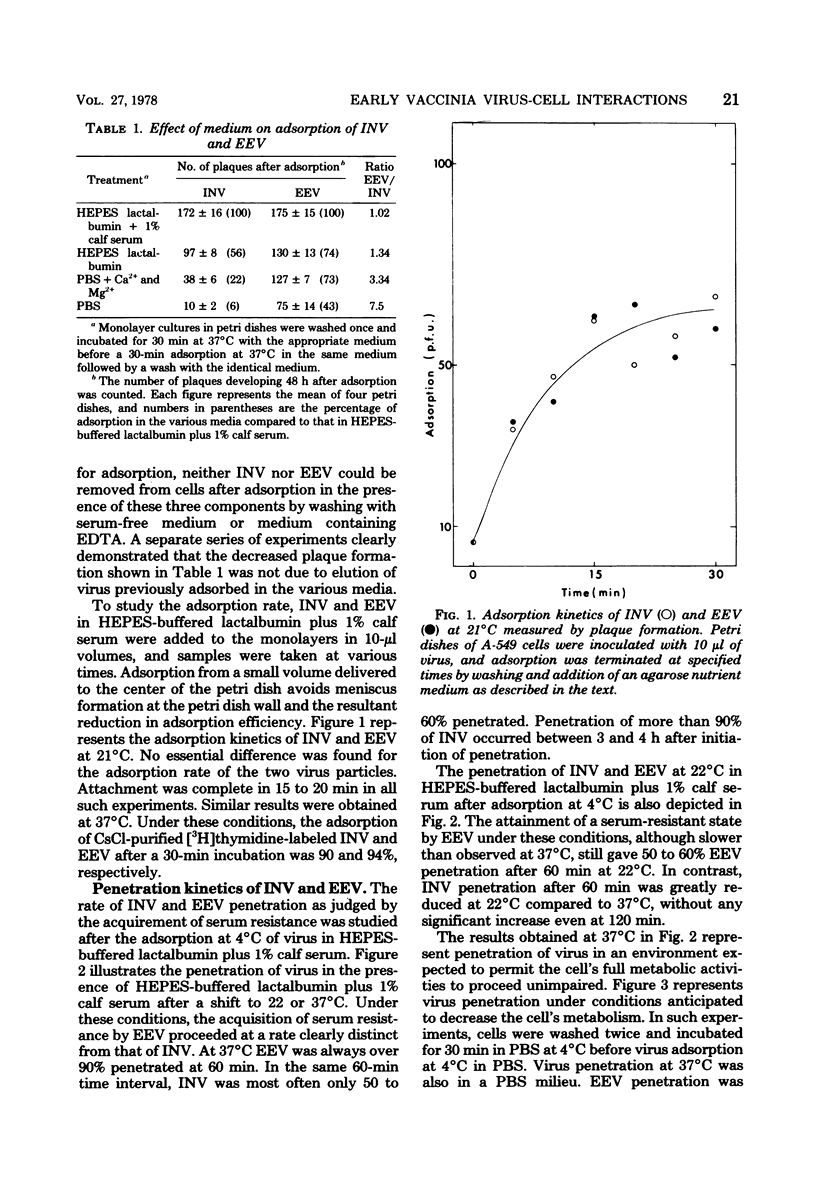
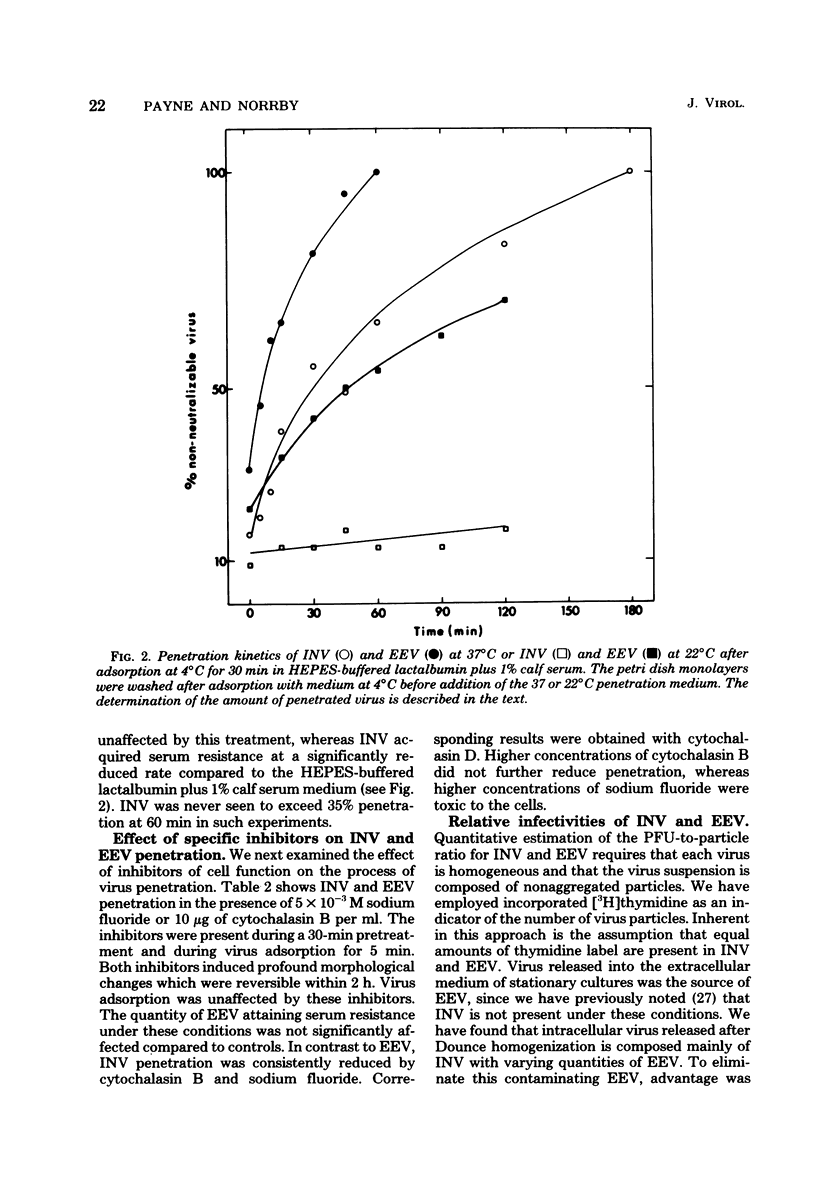
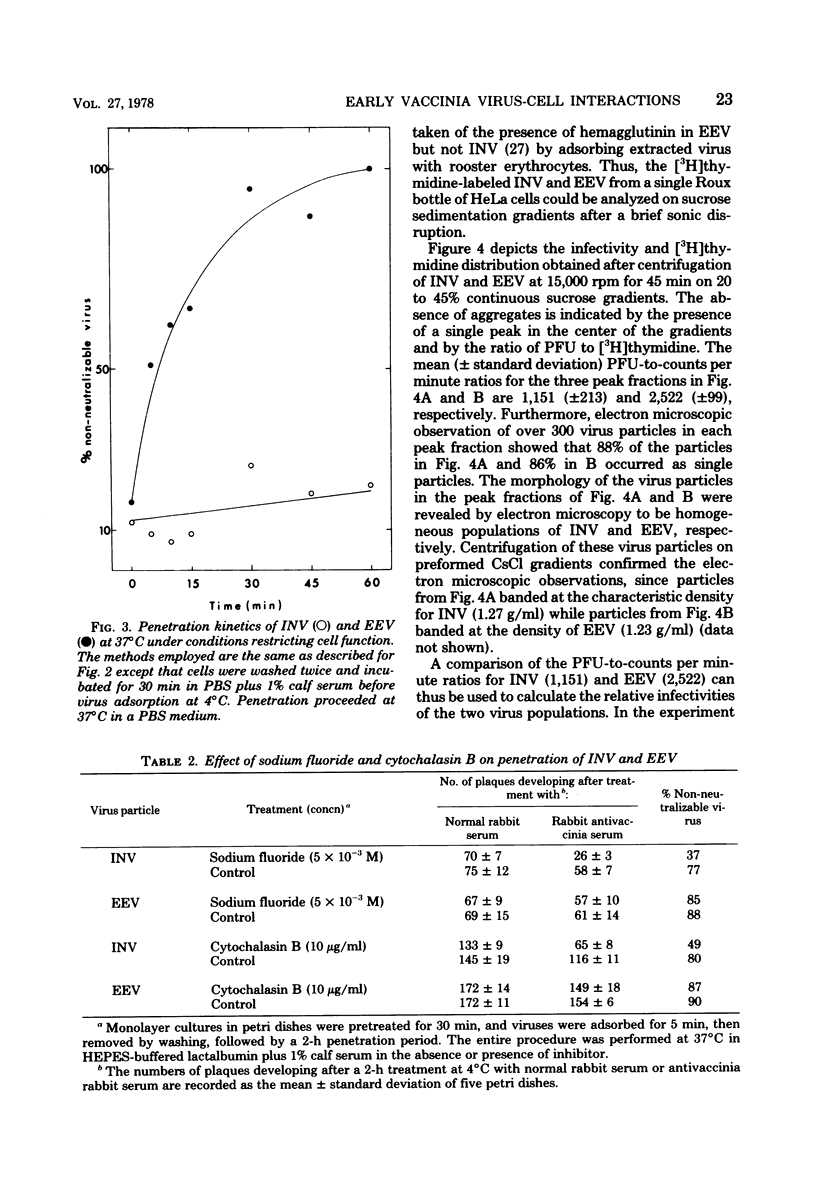
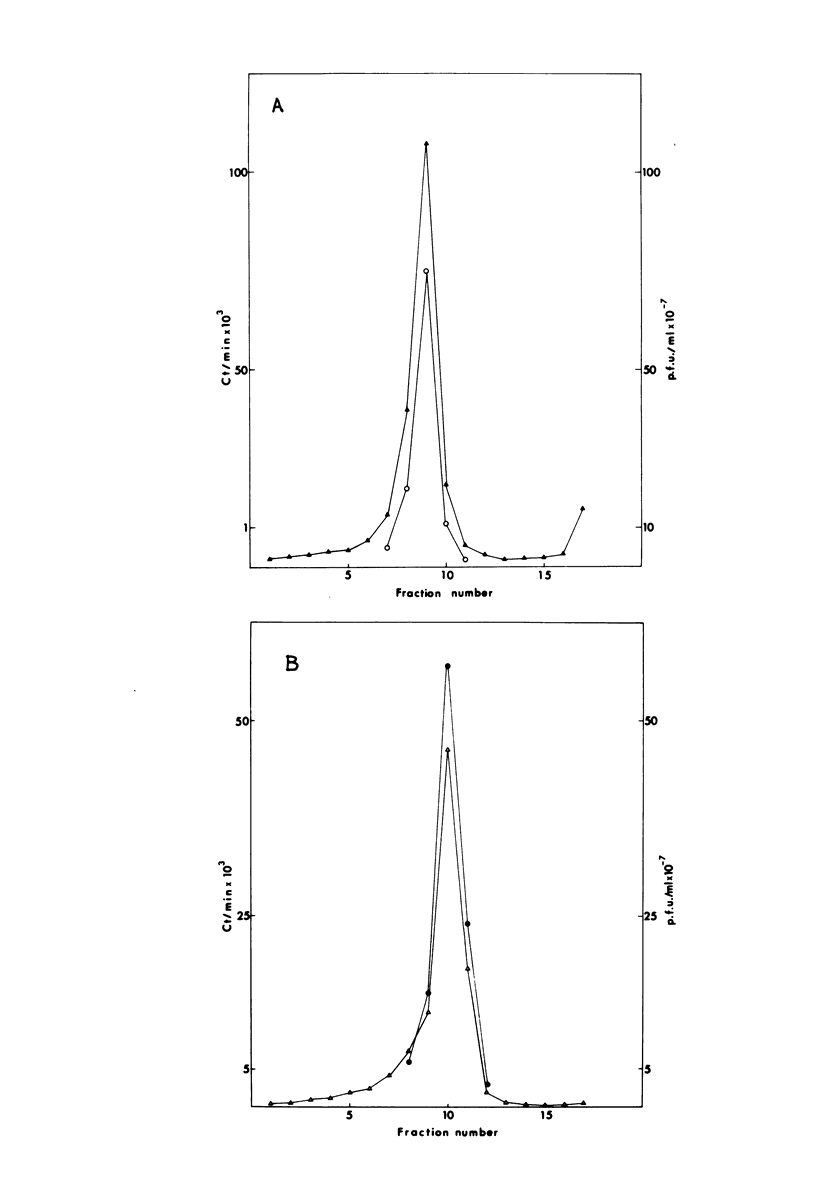
The adsorption and penetration of intracellular naked vaccinia virus (INV) and extracellular enveloped vaccinia virus (EEV) were examined. The adsorption kinetics of INV and EEV were similar, but INV adsorption was found to be more sensitive to the adsorption environment than EEV. The PFU-to-particle ratio for the two virus particles indicated that EEV was approximately two times as infectious as INV. Kinetic studies at 37 degree C showed that EEV penetrated cells more rapidly than INV. Penetration of EEV was unaffected by incubation in phsophate-buffered saline, but was somewhat reduced by incubation at 22 degree C. In contrast, INV penetration was effectively eliminated by incubation in phosphate-buffered saline or by incubation at 22 degree C. In addition, INV but not EEV pentration was sensitive to treatment with sodium fluoride and cytochalasin. B. These results are discussed with regard to the mechanism of INV and EEV penetration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD G., WESTWOOD J. C. THE GROWTH OF RABBITPOX VIRUS IN TISSUE CULTURE. J Gen Microbiol. 1964 Dec;37:391–401. doi: 10.1099/00221287-37-3-391. [DOI] [PubMed] [Google Scholar]
- Appleyard G., Hapel A. J., Boulter E. A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971 Oct;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Metz D. H., Young M. R. The mode of entry of vaccinia virus into L cells. J Gen Virol. 1973 Dec;21(3):533–537. doi: 10.1099/0022-1317-21-3-533. [DOI] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. doi: 10.1177/003591576906200349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E. A., Zwartouw H. T., Titmuss D. H., Maber H. B. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am J Epidemiol. 1971 Dec;94(6):612–620. doi: 10.1093/oxfordjournals.aje.a121360. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Grunert R. R., Korant B. D., Lonberg-Holm K., Yin F. H. Replication of rhinoviruses. Arch Virol. 1976;51(3):169–189. doi: 10.1007/BF01318022. [DOI] [PubMed] [Google Scholar]
- Chang A., Metz D. H. Further investigations on the mode of entry of vaccinia virus into cells. J Gen Virol. 1976 Aug;32(2):275–282. doi: 10.1099/0022-1317-32-2-275. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch A. D., Sawicki S. G., Godman G. C., Tanenbaum S. W. Enhancement of viral infectivity by cytochalasin. Virology. 1973 Dec;56(2):417–428. doi: 10.1016/0042-6822(73)90046-9. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Granados R. R. Entry of an insect poxvirus by fusion of the virus envelope with the host cell membrane. Virology. 1973 Mar;52(1):305–309. doi: 10.1016/0042-6822(73)90422-4. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- NORRBY E. C., FALKSVEDEN L. G. SOME GENERAL PROPERTIES OF THE MEASLES VIRUS HEMOLYSIN. Arch Gesamte Virusforsch. 1964 Mar 13;14:474–486. doi: 10.1007/BF01555079. [DOI] [PubMed] [Google Scholar]
- Okada Y. Factors in fusion of cells by HVJ. Curr Top Microbiol Immunol. 1969;48:102–128. doi: 10.1007/978-3-642-46163-7_5. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Squires E. J. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J Gen Virol. 1971 Oct;13(1):19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]



