Abstract
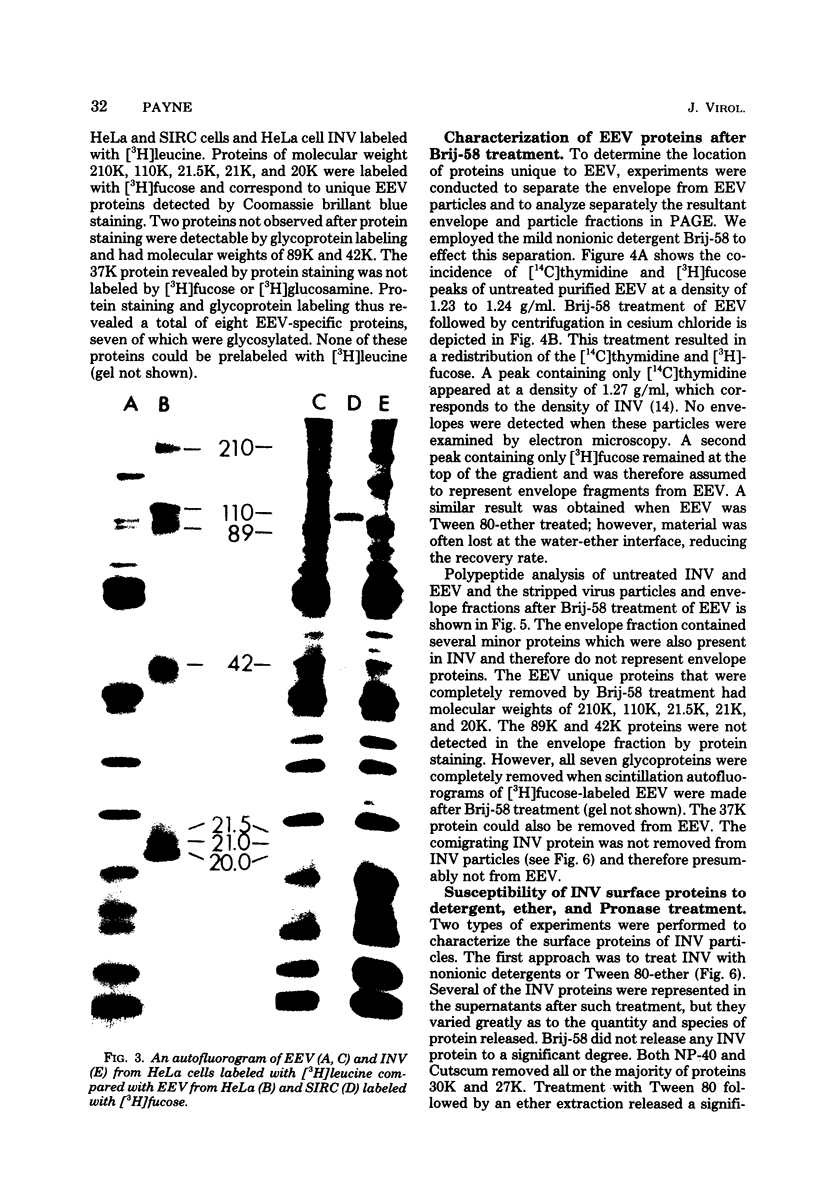
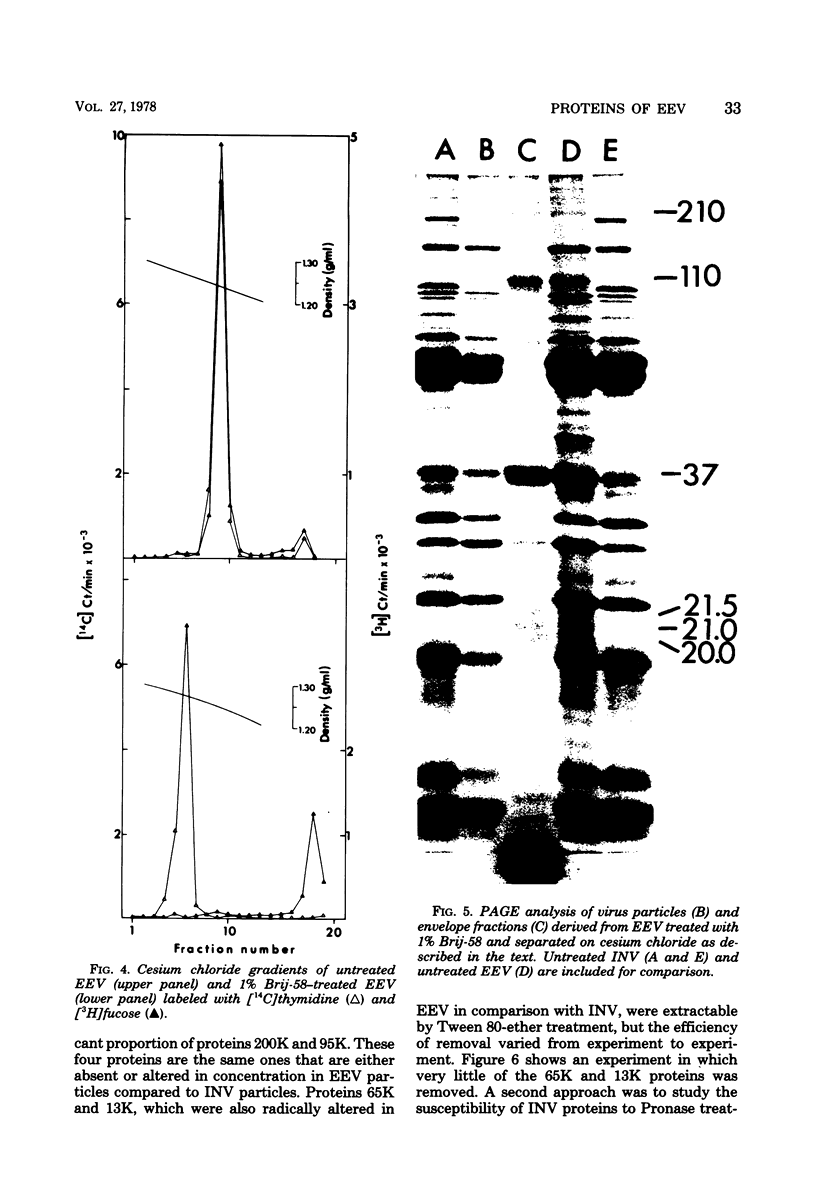
Extracellular enveloped vaccinia (EEV) virus grown in SIRC and in HeLa cells was purified by consecutive equilibrium centrifugations in sucrose and cesium chloride gradients. A higher degree of purity was obtained with virus material prepared in SIRC cells. The polypeptides of purified EEV and INV (intracellular naked vaccinia) virus were compared in polyacrylamide slab gel electrophoresis. Three proteins (200,000 molecular weight [200K], 95K, and 13K) detected in HeLa-derived INV were absent in EEV. In addition, two INV proteins (65K and 30K) occurred in reduced concentrations in EEV, white another INV protein (27K) was increased in EEV. INV from SIRC cells showed similar alterations of these proteins (with the exception of the 30K and 13K proteins). Detergent treatment, ether extraction, and Pronase treatment showed that these six proteins are located at the surface of INV and are not cecessary for infectivity. Eight proteins (210K, 110K, 89K, 42K, 37K, 21.5K, 21K, and 20K) were detected in EEV that were absent from inv. Brij-58 treatment was employed to remove the envelope from EEV, resulting in the formation of naked particles and an envelope fraction which were separated on cesium chloride gradients. The envelope fractions contained all eight proteins. Seven of the eight proteins were glycoproteins, with the 37K protein being the only unglycosylated protein. It is concluded that a processing of surface INV particle proteins occurs during evelopment. The resultant EEV particle is comprised of an INV particle with a modified surface composition enclosed in an envelope containing virus-specific proteins unique to EEV.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Hapel A. J., Boulter E. A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971 Oct;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. [PMC free article] [PubMed] [Google Scholar]
- Holowczak J. A. Glycopeptides of vaccinia virus. I. Preliminary characterization and hexosamine content. Virology. 1970 Sep;42(1):87–99. doi: 10.1016/0042-6822(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Szilágyi J. F. Preparation and characterisation of a subviral particle of vaccinia virus containing the DNA-dependent RNA polymerase activity. Virology. 1975 Nov;68(1):234–244. doi: 10.1016/0042-6822(75)90164-6. [DOI] [PubMed] [Google Scholar]
- NORRBY E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc Soc Exp Biol Med. 1962 Dec;111:814–818. doi: 10.3181/00379727-111-27930. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Palmer E. L., Gafford L. G., Randall C. C. Polyacrylamide gel electrophoresis of fowlpox and vaccinia virus proteins. Virology. 1973 Feb;51(2):512–516. doi: 10.1016/0042-6822(73)90454-6. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Prakash V. J., Norrby E., Payne L. Single radial immunodiffusion test for detecting antibodies against surface antigens of intracellular and extracellular vaccinia virus. J Gen Virol. 1977 Jun;35(3):465–472. doi: 10.1099/0022-1317-35-3-463. [DOI] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Characterization of intermediates in the uncoating of vaccinia virus DNA. Virology. 1972 Nov;50(2):593–602. doi: 10.1016/0042-6822(72)90410-2. [DOI] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: isolation and characterization of a surface component that elicits antibody suppressing infectivity and cell-cell fusion. Virology. 1976 Nov;75(1):232–241. doi: 10.1016/0042-6822(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: relationship of the envelope to virus assembly. Virology. 1976 Nov;75(1):242–255. doi: 10.1016/0042-6822(76)90023-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Squires E. J. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J Gen Virol. 1971 Oct;13(1):19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dales S. Biogenesis of poxviruses: genetically controlled modifications of structural and functional components of the plasma membrane. Virology. 1974 Jul;60(1):96–127. doi: 10.1016/0042-6822(74)90369-9. [DOI] [PubMed] [Google Scholar]








