Abstract
Recently, the biological roles of lipid peroxidation products have received a great deal of attention not only for elucidating pathological mechanisms but also for practical clinical applications as biomarkers. In the last 50 years, lipid peroxidation has been the subject of extensive studies from the viewpoints of mechanisms, dynamics, product analysis, involvement in diseases, inhibition, and biological signaling. Lipid hydroperoxides are formed as major primary products, but they are substrates for various enzymes and they also undergo various secondary reactions. During this decade, hydroxyoctadecadienoic acid from linoleates, F2-isoprostanes from arachidonates, and neuroprostanes from docosahexanoates have been proposed as biomarkers for evaluating oxidative stress in vivo and its related diseases. The implications of lipid peroxidation products in vivo will be briefly reviewed and their practical applications will be discussed.
Keywords: Lipid peroxidation, oxidative stress, HODE, oxycholesterol
Lipid Peroxidation
Lipid peroxidation has received renewed attention from the viewpoints of nutrition and medicine. Lipid peroxidation is implicated in the underlying mechanisms of several disorders and diseases such as cardiovascular diseases, cancer, neurodegenerative diseases, and even aging, with increasing evidence showing the involvement of in vivo oxidation in these conditions.(1–3) More importantly, it has been reported that specific lipid peroxidation products exert various biological functions in vivo such as regulating gene expression, signaling, activating receptors, and adaptive responses.(4–7) Researchers have focused their attention on lipid peroxidation products in order to elucidate the mechanism of lipid oxidation and its involvement in pathogenesis, and to develop specific and practical biomarkers for diagnosing diseases and evaluating therapies.
Mechanism of Lipid Peroxidation
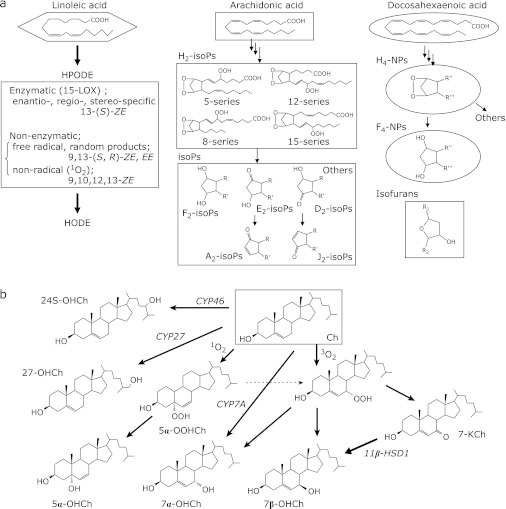
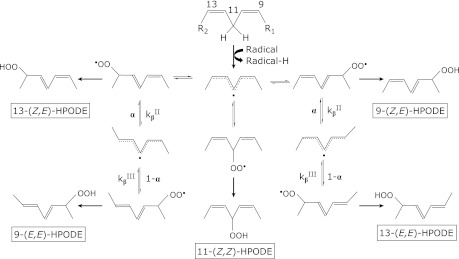
Lipid peroxidation proceeds by 3 distinct mechanisms: (1) free radical-mediated oxidation, (2) free radical independent non-enzymatic oxidation, and (3) enzymatic oxidation. Both PUFA and cholesterol are oxidized by enzymatic and non-enzymatic pathways (Fig. 1 a and b). For example, the oxidation of linoleate by lipoxygenase proceeds catalytically to produce regio-, stereo-, and enantio-specific hydroperoxy octadecadienoates (HPODEs), as shown in Fig. 1a. The specificity depends on the types of enzymes, substrates, and reaction milieu. As shown in Fig. 2, the free radical-mediated peroxidation of PUFA proceeds by 5 elementary reactions: (1) hydrogen atom transfer from PUFA to the chain initiating radical or chain carrying peroxyl radicals to produce a pentadienyl carbon-centered lipid radical, (2) reaction of the lipid radical with molecular oxygen to produce a lipid peroxyl radical, (3) fragmentation of the lipid peroxyl radical to produce oxygen and a lipid radical [a reverse reaction of the above reaction 2)], (4) rearrangement of the peroxyl radical, and (5) cyclization of the peroxyl radical.(8) Reaction (5) is important only for PUFA when it has more than 3 double bonds, and it does not take place during the oxidation of linoleates.
Fig. 1.
Biomarkers of lipids. Oxidation products of PUFA (a) and cholesterol (b).
Fig. 2.
The mechanism of free radical induced linoleate oxidation.
Lipid Peroxidation Products
Prostanes
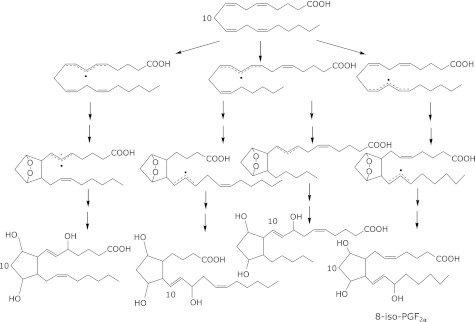
The oxidation of arachidonates by lipoxygenases and cyclooxygenases has been studied extensively.(9) During this decade, isoprostanes (IsoPs), which are prostaglandin F2-like compounds, and neuroprostanes (NPs) that are formed by the non-enzymatic, free radical-mediated oxidation of arachidonates and docosahexaenoates, respectively, are now regarded as being the ”gold standards” for assessing oxidative stress in vivo (Fig. 1a and 3).(10) Similar products that are characterized by a substituted tetrahydrofuran ring structure and are termed isofurans have also been measured and found to increase with increasing oxygen tension, in contrast to IsoPs.(10) Isoprostanes are formed in situ on phospholipids at sites of free radical generation. Once they are released from cell membranes by phospholipases, IsoPs circulate in the plasma. IsoPs have been measured in biological fluids such as urine, plasma, exhaled breath condensate, bile, cerebrospinal fluids, and normal tissues. Recently, much clinical data have been reported in terms of assessing IsoPs levels in patients (for example, see ref. 11 for cardiovascular diseases). However, as shown in Fig. 1a, IsoPs are minor oxidation products of arachidonates as there are many kinds of isomers through various reactions. Furthermore, arachidonates are not abundant in vivo, especially in human plasma. Thus, the absolute concentrations that are measured in vivo are considered to be quite low. Furthermore, artificial oxidation during sample processing, storage, and analysis is always a potential concern.
Fig. 3.
Oxidation of arachidonates and formation of 8-iso-PGF2α.
Hydroxyoctadecadienoic acid
In contrast, linoleates are the most abundant PUFAs in vivo, and their oxidation proceeds by a straightforward mechanism that yields much simpler products than arachidonates and more highly unsaturated fatty acids such as docosahexaenoates. As shown in Fig. 2, hydroperoxyoctadecadienoic acids (HODEs) that are formed by a radical-mediated oxidation mechanism consist of 4 isomers: 13-hydroperoxy-9(Z), 11(E)-octadecadienoic acid (13-(Z,E)-HPODE); 13-hydroperoxy-9(E), 11(E)-octadecadienoic acid (13-(E,E)-HPODE); 9-hydroperoxy-10(E), 12(Z)-octadecadienoic acid (9-(E,Z)-HPODE); and 9-hydroperoxy-10(E), 12(E)-octadecadienoic acid. Little 11-HPODE is formed under normal conditions as the pentadienyl radical that is formed by the abstraction of hydrogen at 11-carbon rearranges rapidly to form stable conjugated diene radicals. 9- and 13-(Z,E)-HPODE are also formed by enzymatic oxidation via lipoxygenase as enantio-, regio-, and stereo-specific products. Thus, 9- and 13-(E,E)-HPODE are specific products of radical-mediated oxidation. On the other hand, singlet oxygen and ozone oxidize linoleic acids by non-radical oxidation to form 13-hydroperoxy-9(Z), 11(E)-octadecadienoic acid (13-(Z,E)-HPODE), 10-hydroperoxy-8(E), 12(Z)-octadecadienoic acid (10-(E,Z)-HPODE), 12-hydroperoxy-9(Z), 13(E)-octadecadienoic acid (12-(Z,E)-HPODE), 9-hydroperoxy-10(E), and 12(Z)-octadecadienoic acid (9-(E,Z)-HPODE). In this case, 10- and 12-(Z,E)-HPODEs are specific oxidation products of singlet oxygen.
The absolute concentrations of lipid hydroperoxides in vivo are considered to be minimal since they are substrates of many enzymes such as glutathione peroxidases and phospholipases. In such cases, the stable oxidation products are HODEs. Linoleates are more stable than arachidonates and docosahexaenoates in terms of free radical-mediated oxidation.
Oxysterols
Cholesterol oxidation products, which are commonly referred to as oxysterols, have received increasing attention as diagnostic biomarkers of oxidative stress, as intermediates in bile acid biosynthesis, and as messengers for cell signaling and cholesterol transport.(12) Cholesterol is also oxidized by both enzymatic and non-enzymatic mechanisms. The free radical-mediated oxidation of cholesterol yields 7α- and 7β-hydroperoxycholesterol (7α-OOHCh and 7β-OOHCh), 7α-OHCh, 7β-OHCh, 5α, 6α- and 5β, 6β-epoxycholesterol, and 7-ketocholesterol (7-KCh) as major products.(12) The conversion of 7-KCh into 7β-OHCh in vivo was previously reported.(13) The oxidation of 7-OHCh by either 7α-hydroxycholesterol dehydrogenase(14) or by non-enzymatic autoxidation yields 7-KCh. 7β-OHCh may be regarded as a marker of free radical-mediated oxidation. Oxysterols are present in vivo in different forms, namely the esterified, sulfated, and conjugated forms, as well as free oxysterols.(15)
Measurement of oxidation products
With increasing evidence that indicates the involvement of lipid peroxidation in various disorders and diseases, biomarkers of lipid peroxidation have gained increasing attention. The detection and identification of lipid peroxidation products are easier and more reliable than the detection of reactive oxygen species, reactive nitrogen species, and other active oxidants by using various probes and techniques such as fluorescence probes, chemiluminescence probes, and the ESR spin trapping technique. Coordination ion-spray mass spectrometry and electrospray ionization, or matrix-assisted laser desorption and ionization time-of-flight mass spectrometry have been found to be powerful tools for detecting and identifying complex mixtures of lipid peroxidation products such as IsoPs and NPs.(16) The oxidation products of linoleates are now measured by gas chromatography (mass spectrometry [GC-MS]) or high performance liquid chromatography (tandem mass spectrometry) with high accuracy and sensitivity. However, needless to say, there are limitations in the widespread use of each apparatus. The development of simpler and more convenient detection and quantification systems such as enzyme-linked immunosorbent assay is thus desirable.
Linoleates and cholesterol are abundant lipids in vivo, and their free radical-mediated oxidation yields HPODE and 7-OOHCh as major and primary products with high selectivity. We recently proposed the measurement of total hydroxyoctadecanoic acid (tHODE) and total 7-hydroxycholesterol (t7-OHCh) as biomarkers of oxidative stress in vivo.(17–28) In this method, biological samples such as plasma, erythrocytes, urine, and tissues are first reduced by sodium borohydride or triphenylphosphine followed by saponification with potassium hydroxide. The hydroperoxides and ketones (in the case of sodium borohydride) as well as hydroxides of both free and ester forms of linoleic acid and cholesterol are measured as tHODE and t7-OHCh, respectively.
The efficacy for scavenging peroxyl radicals by the antioxidant in vivo can be estimated from the ratio of cis, trans-HODE to trans, trans-HODE, which is expressed by the following equation:
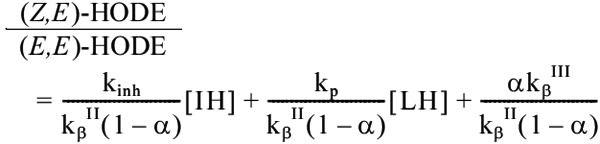 |
Here, we assume that free radical-mediated oxidation takes place and that the steady state applies, and kinh and kp are the second order rate constants in the reaction between the radical scavenging antioxidant (IH) and peroxyl radicals and the chain propagation reaction, respectively. In addition, kβII, kβIII, and α are the constants that are shown in the Fig. 2. Thus, these biomarkers may be useful for evaluating the beneficial effects of antioxidant foods, spices, beverages, supplements, and drugs.
Evaluating Oxidative Stress in vivo
A number of studies have been performed to measure the level of lipid oxidation products in humans. Above all, thiobarbituric reactive substances, malonaldehyde (MDA), HODE, isoprostanes, and oxysterols have been measured most frequently. Ethane and pentane in exhaled gas are also used as biomarkers of lipid oxidation in vivo. Each method has its own advantages and disadvantages. However, usually these levels are not measured simultaneously from a single subject, making it difficult to compare the levels of different lipid oxidation products as biomarkers. Only a few recent studies measured several parameters simultaneously for the same subjects.(22,29)
Animal models
There are now many reports that show that the levels of oxidation products of lipids and modified proteins and DNA are elevated under oxidative stress conditions. It was previously found that the intraperitoneal administration of 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH) to mice induces oxidative stress in vivo.(30) AAPH generates free radicals by spontaneous thermal decomposition without biotransformation. It is interesting that mice do not drink water with AAPH but 2,2'-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH), although the reason for this is unknown at present. The levels of tHODE and t8-iso-PGFα in plasma and erythrocytes of mice were clearly increased by drinking water with AIPH for 7 weeks.(31)
A choline-deficient diet induces liver damage. Significant increases in tHODE and t8-iso-PGF2α in the liver and plasma were observed when mice were fed a choline-deficient diet.(21) This increase in tHODE and t8-iso-PGFα was decreased to a normal level when α-tocopherol or BO-653 was mixed with the diet. The decrease in the HODE stereo isomer ratio due to a choline-deficient diet was also reversed by these antioxidants. However, the increase in plasma glutamic-pyruvic transaminase and fatty acids in the liver that was induced by a choline-deficient diet was not recovered by the antioxidants. Similar protective effects of antioxidants against liver damage that was induced by carbon tetrachloride, which is a well-established liver toxin, were observed.(18)
Ts65Dn mouse is used as a model of Down syndrome. It is reported that the levels of t8-iso-PGFα and tHODE in cortex and hippocampus, and plasma, respectively, of Ts65Dn mice at the age of 12 weeks were significantly higher than control mice with the observation of cognitive deficits.(32) Interestingly, the increase in levels of oxidative markers and cognitive deficits were ameliorated by α-tocopherol which was administered to pregnant mice from the day conception throughout the pregnancy, and to pups over their entire lifetime. As arachidonates, parent compounds of t8-iso-PGF2α, are more abundant in brain than plasma, t8-iso-PGF2α can be more sensitive biomarkers than tHODE in brain.
These results clearly show that the oxidative stress that is induced by free radicals, oxidative toxic compounds, and decreases in antioxidants enhance lipid oxidation, and that free radical scavenging antioxidants ameliorate lipid oxidation. Furthermore, the antioxidant capacity in vivo may be assessed from these experiments. Obviously, it is important to examine whether the level of lipid oxidation that is measured by the biomarkers is associated with the disease state and if the antioxidants prevent or ameliorate the diseases in appropriate animal experiments and well programmed human studies.
Human diseases
It has been reported in numerous studies that the extent of lipid oxidation is elevated in diseased patients compared to normal subjects.(33,34) The levels of tHODE, t8-iso-PGF2α, and t7-OHCh in the plasma and erythrocytes of 44 healthy human subjects were measured to assess the level of lipid oxidation in humans.(22) The plasma and erythrocytes were treated with sodium borohydride and potassium hydroxide to convert both free and ester forms of linoleic acid, arachidonic acid, and cholesterol to their free forms. The average concentrations of tHODE, t8-iso-PGF2α, and t7-OHCh in the plasma were 203, 0.727, and 243 nmol/L, and in the erythrocytes, their concentrations were 1,917, 12.8, and 5,226 nmol/packed L, respectively. The ratios of tHODE and t7-OHCh to the parent substrates were 194 and 3,519 µmol tHODE/mol linoleates, and 40.9 and 686 µmol t7-OHCh/mol cholesterol in the plasma and erythrocytes, respectively. Thus, the level of lipid oxidation products in the erythrocytes was higher than that in the plasma.
In another experiment, the levels of tHODE, t8-iso-PGF2α, and t7-OHCh in human LDL were measured. LDL that was isolated from normal human plasma was sub-fractionated into 3 fractions, LDL-1, LDL-2, and LDL-3, according to the surface electronegativity of the LDL particles with anion-exchange HPLC.(35) Each fraction consisted of 75.1%, 19.3%, and 5.6% total LDL. The concentrations of tHODE, t7-OHCh, and t8-iso-PGFα in each LDL sub-fraction were assessed after extraction followed by reduction and saponification. It was found that the levels of tHODE, t8-iso-PGF2α, and t7-OHCh were well correlated with the negative charge of the LDL particles. These results clearly indicate that the extent of oxidation increases in the order of LDL-1<LDL-2<<LDL-3.
The oxidative modification hypothesis that was proposed by Steinberg and his colleagues (1989) indicates that the oxidative modification of LDL is an important initial event for the development of atherosclerosis. A large number of in vitro and animal studies support this hypothesis. Lipid oxidation products have been measured in human atherosclerotic lesions. It was found in many studies that human atherosclerotic lesions contained increased amounts of lipid oxidation products when compared with non-atherosclerotic vessel walls.(36,37) For example, it was reported that the contents in an human atherosclerotic lesion were 4.7 mmol HODE/mol linoleic acid and 13.6 µmol isoprostane/mol arachidonic acid (after hydrolysis), which were much higher than the levels in normal umbilical veins.(38)
The relationship between oxidative stress and cataracts was studied.(39) Forty healthy men and postmenopausal women aged 50 to 70 years (F25, M15) underwent eye examinations. Blood samples were collected to analyze the major well-known antioxidants tHODE and t8-iso-PGF2α by using GC-MS. Twenty-seven (F17, M10) of the 40 subjects were diagnosed with early cataracts at the onset of the study, which were regarded as age appropriate lens opacities. There were no significant differences in plasma major antioxidants and lipid peroxidation determined by MDA as well as t8-iso-PGF2α between the groups with and without early cataracts. However, the levels of isomers of 9- and 13-(Z,E)-HODE were significantly higher in subjects with early cataracts compared with non-cataract subjects (p<0.05).
In another study, the levels of lipid oxidation products were measured in the plasma and livers of hepatitis C- and B-infected patients and compared with those of control subjects.(24) It was found that tHODE, tHETE, and t7-OHCh were the major products in both the plasma and liver, and that the level of t8-iso-PGF2α was much smaller. The levels of lipid oxidation products in the plasma and livers of the patients were more elevated than those of the healthy subjects.
It is important to determine the method of analysis according to the targeted samples and diseases. We previously analyzed urine and cerebrospinal fluid (CSF) of human and found there is little difference in the levels between total and free forms of 8-iso-PGF2α and HODE. We have measured the levels of free 8-iso-PGF2α and HODE in CSF between patients with and without symptomatic vasospasm (SVS) in order to elucidate the involvement of oxidative stress in vasospasm generation after subarachnoid hemorrhage (SAH).(40) It was found that the levels of free 8-iso-PGF2α and HODE and plasma platelet-activating factor-acetyl hydrase (PAF-AH) activity showed higher in patients without SVS than with SVS. Thus, we speculated that the plasma PAF-AH can hydrolyze oxidized phospholipids and attenuate the spreading of lipid peroxidation.
The role of oxidative stress in neurodegenerative diseases has received much attention recently, whereby elevated levels of lipid oxidation products have been observed in patients with neurological diseases.(41–43) We also found that the levels of tHODE and oxidatively modified peroxiredoxin-2 and -6 in Alzheimer’s disease were significantly higher than those in healthy controls, and that they increased with increasing clinical dementia ratings.(27)
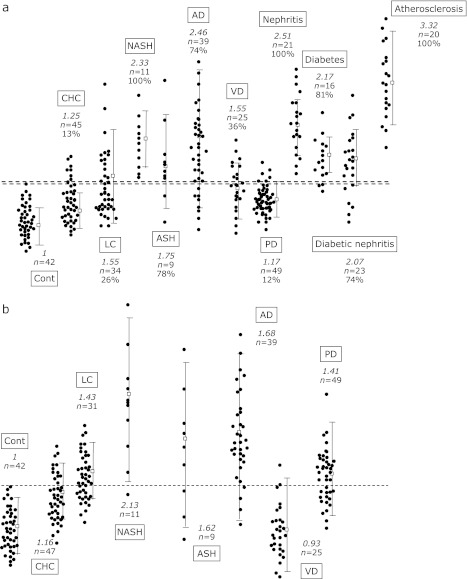
Fig. 4 shows the levels of tHODE in the plasma (Fig. 4a) and erythrocytes (Fig. 4b) of healthy volunteers and patients with several diseases, including the above results. These data clearly shows the increase in plasma and erythrocyte HODE levels due to the diseases. However, one might argue that these lipid peroxidation products are by no means specific to liver damage. Admittedly, this lack of specificity is an inherent drawback that is associated with oxidative stress biomarkers of lipids. Some diseases show large overlaps e.g., liver diseases and Parkinson’s disease. Nevertheless, the combination of several biomarkers should be effective in improving their accuracy and they may be used as surrogate biomarkers to monitor disease progression and evaluate therapies.
Fig. 4.
Relative levels of hydroperoxyoctadecadienoic acid in the plasma (a) and erythrocytes (b) of patients compared to healthy volunteers (Cont). The italic number in the figure show the average level compared to Cont and the number studied is shown as ”n=” individually. Percentages shown in the figure are the number above the highest level of Cont. CHC, chronic hepatitis C; LC, liver cirrhosis; NASH, non-alcoholic steatohepatitis; ASH, alcoholic steatohepatitis; AD, Alzheimer’s disease; VD, vascular disease; and PD, Parkinson’s disease.
It should be noted that, as is often pointed out, the accurate determination of lipid oxidation products in human samples is difficult and great care should be taken when trying to ascertain the levels of these compounds. The potential generation or loss of lipid oxidation products during sampling, storage, isolation, and analysis may take place. The use of an appropriate internal standard is recommended. Furthermore, the diet contains various lipid oxidation products and the measurement of their levels in biological fluids and tissues may be confounded by the diet unless the subjects are fasting. Therefore, blood should be withdrawn after sufficient fasting has taken place.
Assessing Antioxidant Capacity in vivo
As described above, the assessment of free radical-scavenging antioxidant capacity in vivo is an important subject and has been studied extensively.(44–46) Antioxidant capacity in vivo is determined by several factors such as bioavailability, metabolism, localization, distribution, fate of antioxidant-derived radicals, and interaction with other antioxidants as well as reactivity toward free radicals. It is important to elucidate each factor by employing basic studies for the sound interpretation of experimental results. However, it is quite difficult to estimate the antioxidant capacity in vivo based on these individual factors. In practice, it is essential to examine the effects of the antioxidant in appropriate animal model studies and human intervention studies. Biomarkers are necessary for these studies, and HODE is a potential biomarker for assessing antioxidant capacity to inhibit lipid oxidation and attenuate oxidative stress in vivo.
The beneficial effects of antioxidants in vivo have been assessed in various animal experiments under normal conditions and under oxidative stress. This is important because it is difficult to estimate the antioxidant capacity using the results of in vitro studies. It is essential to examine the effects of antioxidants in animal model experiments and to elucidate the capacity and underlying mechanisms of antioxidant actions in vivo. Appropriate biomarkers are indispensable and HODE may serve this purpose. More recent studies showed that the administration of azo compounds to mice increased their levels of tHODE and t8-iso-PGF2α while decreasing the (cis, trans-HODE/trans, trans-HODE) ratio in their livers.(19)
It was confirmed that removing vitamin E from the diet also increased the levels of tHODE and decreased the cis, trans-HODE/trans, trans-HODE ratio, whereas the administration of natural and synthetic antioxidants such as vitamin E isoforms, chlorogenic acid, caffeic acid, coffee, and 2,3-dihyro-5-hydroxy-4,6-di-tert-butyl 2,2-dipentylbenzoylfuran (BO-653) reduced the tHODE level and at the same time increased the cis, trans-HODE/trans, trans-HODE ratio.(22,25,26) In another study, it was found that coenzyme Q9 decreased the tHODE and t8-iso-PGF2α levels in the plasma, erythrocytes, livers, and brains of mice that were fed a vitamin E-free diet in a dose-dependent manner.(21) Coenzyme Q9 increased the stereo isomer ratio that was mentioned above.
Conclusions
Lipid peroxidation yields complex products, including hydroperoxides, cleavage products such as aldehydes, and polymeric materials. These products exert cytotoxic and genotoxic effects.(47–56) Their applications as biomarkers to diagnose disease progression, evaluate therapies, and in health examinations have been the focus of intensive study; some markers have been proposed, as mentioned above. However, it should be noted that there is no sole marker and that inclusive assessments of oxidation products are needed. Additionally, the physiological significance of biological effects of lipid peroxidation products in vivo has to be established in future studies.
Acknowledgments
Our works described in this article were partially supported by Grant-in-Aid for Young Scientists (A) 22680051 and Scientific Research (B) 22300242 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Prof. Niki and our colleagues for their invaluable contribution.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gershman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and X-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;119:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JWC. Free radicals in biology and medicine, fourth ed. Oxford UK: Clarendon; 2007. [Google Scholar]
- 4.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Re Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi N. Role of oxidative stress in adaptive responses in special reference to atherosclerosis. J Clin Biochem Nutr. 2008;43:131–138. doi: 10.3164/jcbn.2008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zmijewski JW, Landar A, Watanabe N, Dickinson DA, Noguchi N, Darley-Usmar VM. Cell signaling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33:1385–1389. doi: 10.1042/BST20051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 10.Roberts LJ, 2nd, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol. 2005;15:143–148. doi: 10.1111/j.1750-3639.2005.tb00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cracowski JL. Isoprostanes: an emerging role in vascular physiology and disease? Chem Phys Lipids. 2004;128:75–83. doi: 10.1016/j.chemphyslip.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Diczfalusy U. Analysis of cholesterol oxidation products in biological samples. J AOAC Int. 2004;87:467–473. [PubMed] [Google Scholar]
- 13.Erickson SK, Cooper AD, Matsui SM, Gould RG. 7-Ketocholesterol. Its effects on hepatic cholesterogenesis and its hepatic metabolism in vivo and in vitro. J Biol Chem. 1977;252:5186–5193. [PubMed] [Google Scholar]
- 14.Song W, Pierce WM, Jr., Saeki Y, Redinger RN, Prough RA. Endogenous 7-oxocholesterol is an enzymatic product: characterization of 7α-hydroxycholesterol dehydrogenase activity of hamster liver microsomes. Arch Biochem Biophys. 1996;328:272–282. doi: 10.1006/abbi.1996.0173. [DOI] [PubMed] [Google Scholar]
- 15.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 16.Schiller J, Süss R, Arnhold J, et al. Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Prog Lipid Res. 2004;43:449–488. doi: 10.1016/j.plipres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Niki E. Detection of lipid peroxidation in vivo: total hydroxyoctadecadienoic acid and 7-hydroxycholesterol as oxidative stress marker. Free Radic Res. 2004;38:787–794. doi: 10.1080/10715760410001700460. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y, Itoh N, Hayakawa M, et al. Lipid peroxidation induced by carbon tetrachloride and its inhibition by antioxidant as evaluated by an oxidative stress marker, HODE. Toxicol Appl Pharmacol. 2005;208:87–97. doi: 10.1016/j.taap.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Hayakawa M, Niki E. Total hydroxyoctadecadienoic acid as a marker for lipid peroxidation in vivo. BioFactors. 2005;24:7–15. doi: 10.1002/biof.5520240102. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y, Itoh N, Hayakawa M, et al. Lipid peroxidation in mice fed a choline-deficient diet as evaluated by total hydroxyoctadecadienoic acid. Nutrition. 2006;22:303–311. doi: 10.1016/j.nut.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Hayakawa M, Habuchi Y, Niki E. Evaluation of the dietary effects of coenzyme Q in vivo by the oxidative stress marker, hydroxyoctadecadienoic acid and its stereoisomer ratio. Biochim Biophys Acta. 2006;1760:1558–1568. doi: 10.1016/j.bbagen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y, Saito Y, Hayakawa M, et al. Levels of lipid peroxidation in human plasma and erythrocytes: comparison between fatty acids and cholesterol. Lipids. 2007;42:439–449. doi: 10.1007/s11745-007-3037-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E. Evaluation of lipophilic antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid and isoprostane. Lipids. 2007;42:463–472. doi: 10.1007/s11745-007-3043-7. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y, Kodai S, Takemura S, Minamiyama Y, Niki E. Simultaneous measurement of F2-isoprostane, hydroxyoctadecadienoic acid, hydroxyeicosatetraenoic acid, and hydroxycholesterols from physiological samples. Anal Biochem. 2008;379:105–115. doi: 10.1016/j.ab.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida Y, Hayakawa M, Cynshi O, Jishage K, Niki E. Acceleration of lipid peroxidation in α-tocopherol transfer protein-knock out mice following the consumption of drinking water containing a radical initiator. J Oleo Sci. 2008;57:577–583. doi: 10.5650/jos.57.577. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Hayakawa M, Niki E. Evaluation of the antioxidant effects of coffee and its components using the biomarkers hydroxyoctadecadienoic acid and isoprostane. J Oleo Sci. 2008;57:691–697. doi: 10.5650/jos.57.691. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida Y, Yoshikawa A, Kinumi T, et al. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol Aging. 2009;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Yin H, Ogawa Y, Yoshida Y, Niki E, Porter NA. Ex vivo oxidation in tissue and plasma assays of hydroxyoctadecanoates: Z,E/E,E stereoisomer ratios. Chem Res Toxicol. 2010;23:986–995. doi: 10.1021/tx1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CY, Huang SH, Jenner AM, Halliwell B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radic Biol Med. 2008;44:1314–1322. doi: 10.1016/j.freeradbiomed.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Terao K, Niki E. Damage to biological tissues induced by radical initiator 2,2'-azobis(2-amidinopropane) dihydrochloride and its inhibition by chain-breaking antioxidants. J Free Radic Biol Med. 1986;2:193–201. doi: 10.1016/s0748-5514(86)80070-8. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y, Hayakawa M, Cynshi O, Jishage K, Niki E. Acceleration of lipid peroxidation in α-tocopherol transfer protein-knockout mice following the consumption of drinking water containing a radical initiator. J Oleo Sci. 2008;57:577–583. doi: 10.5650/jos.57.577. [DOI] [PubMed] [Google Scholar]
- 32.Shichiri M, Yoshida Y, Ishida N, et al. α-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Rad Biol Med. 2011;50:1801–1811. doi: 10.1016/j.freeradbiomed.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Bachi A, Zuccato E, Baraldi M, Fanelli R, Chiabrando C. Measurements of urinary 8-Epi-prostaglandin F2α, a novel index of lipid peroxidation in vivo, by immunoaffinity extraction/gas chromatography-mass spectrometry. Basal levels in smokers and nonsmokers. Free Radic Biol Med. 1996;20:619–624. doi: 10.1016/0891-5849(95)02087-x. [DOI] [PubMed] [Google Scholar]
- 34.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 35.Kitano S, Yoshida Y, Kawano K, Hibi N, Niki E. Oxidative status of human low density lipoprotein isomated by anion-exchange high-performance liquid chromatography—Assessment by total hydroxyoctadecadienoic acid, 7-hydroxycholesterol, and 8-iso-prostaglandin F2α. Anal Chim Acta. 2007;585:86–93. doi: 10.1016/j.aca.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids. 1998;91:1–11. doi: 10.1016/s0009-3084(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 37.Platicò D, Iuliano L, Mauriello A, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gniwotta C, Morrow JD, Roberts LJ, 2nd, Kühn H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Atheroscler Thromb Vasc Biol. 1997;17:3236–3241. doi: 10.1161/01.atv.17.11.3236. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Duker JS, Yoshida Y, et al. Oxidative stress and antioxidant status in older adults with early cataract. Eye (Lond) 2009;23:1464–1468. doi: 10.1038/eye.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirashima Y, Doshi M, Hayashi N, et al. Plasma platelet-activating factor-acetyl hydrolase activity and the levels of free forms of biomarker of lipid peroxidation in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage. Neurosrgery. 2012;70:602–609. doi: 10.1227/NEU.0b013e3182333c69. [DOI] [PubMed] [Google Scholar]
- 41.Montine T, Neely MD, Quinn JF, et al. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 42.Roberts LJ, 2nd, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol. 2005;15:143–148. doi: 10.1111/j.1750-3639.2005.tb00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su B, Wang X, Nunomura A, et al. Oxidative stress signaling in Alzheimer’s disease. Curr Alzheimer Res. 2008;6:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004;430:97–103. doi: 10.1016/j.abb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 46.Niki E, Omata Y, Fukuhara A, Saito Y, Yoshida Y. Assessment of radical scavenging capacity and lipid peroxidation inhibiting capacity of antioxidant. J Agric Food Chem. 2008;56:8255–8260. doi: 10.1021/jf800605x. [DOI] [PubMed] [Google Scholar]
- 47.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 48.Kawamoto Y, Nakamura Y, Naito Y, et al. Cyclopentenone prostaglandins as potential inducers of phase II detoxification enzymes. J Biol Chem. 2000;275:11291–11299. doi: 10.1074/jbc.275.15.11291. [DOI] [PubMed] [Google Scholar]
- 49.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-Δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 50.Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004:157–176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- 51.Musiek ES, Milne GL, McLaughlin B, Morrow JD. Cyclopentenone eicosanoids as mediators of neurodegeneration: a pathogenic mechanism of oxidative stress-mediated and cyclooxygenase-mediated neurotoxicity. Brain Pathol. 2005;15:149–158. doi: 10.1111/j.1750-3639.2005.tb00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura T, Inoue T, Shibata N, et al. Inhibitation of cholesterol biosynthesis inhibition by 25-hydoxycholesterol is independent of OSBP. Genes Cells. 2005;10:793–801. doi: 10.1111/j.1365-2443.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 54.Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signaling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 55.Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: protein new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 56.Brown HA, Marnett LJ. Introduction to lipid biochemistry, metabolism, and signaling. Chem Rev. 2011;111:5817–5820. doi: 10.1021/cr200363s. [DOI] [PubMed] [Google Scholar]