Summary
Background
The optimum time to start antiretroviral therapy for children diagnosed with HIV infection after 1 year of age is unknown. We assessed whether antiretroviral therapy could be deferred until CD4 percentages declined to less than 15% without affecting AIDS-free survival.
Methods
In our multicentre, randomised, open-label trial at nine research sites in Thailand and Cambodia, we enrolled children aged 1–12 years who were infected with HIV and had CD4 percentages of 15–24%. Participants were randomly assigned (1:1) by a minimisation scheme to start antiretroviral therapy at study entry (early treatment group) or antiretroviral therapy to start when CD4 percentages declined to less than 15% (deferred treatment group). The primary endpoint was AIDS-free survival (based on US Centers for Disease Control and Prevention category C events) at week 144, assessed with the Kaplan-Meier analysis and the log-rank approach. This study is registered with ClinicalTrials.gov, number NCT00234091.
Findings
Between March 28, 2006, and Sept 10, 2008, we enrolled 300 Thai and Cambodian children infected with HIV, with a median age of 6·4 years (IQR 3·9–8·4). 150 children were randomly allocated early antiretroviral therapy (one participant was excluded from analyses after withdrawing before week 0) and 150 children were randomly allocated deferred antiretroviral therapy. Median baseline CD4 percentage was 19% (16–22%). 69 children (46%) in the deferred treatment group started antiretroviral therapy during the study. AIDS-free survival at week 144 in the deferred treatment group was 98·7% (95% CI 94·7–99·7; 148 of 150 patients) compared with 97·9% (93·7–99·3; 146 of 149 patients) in the early treatment group (p=0·6).
Interpretation
AIDS-free survival in both treatment groups was high. This low event rate meant that our study was underpowered to detect differences between treatment start times and thus additional follow-up of study participants or future studies are needed to answer this clinical question.
Funding
US National Institutes of Health, Division of AIDS; National Institute of Allergy and Infectious Diseases; National Institute of Child Health and Human Development; and National Institute of Mental Health.
Introduction
2·5 million children live with HIV infection worldwide, and 370 000 children are newly infected every year.1 More than 90% of children infected with HIV live in Africa and Asia,1 where diagnosis usually occurs after the first year of life because of restricted access to HIV PCR testing.2 Meta-analysis has shown the effectiveness of antiretroviral therapy for children infected with HIV in low-resource settings.3 The distribution of disease progression of HIV infection is biphasic in children who are infected perinatally; without antiretroviral therapy, AIDS-related mortality is 20–30% in the first year of life, and about 5% per year thereafter.4,5 In the Children with HIV Early Antiretroviral Therapy (CHER) trial, which enrolled HIV-positive infants aged 6–12 weeks, early infant mortality was 4% in an early antiretroviral therapy group compared with 16% for infants starting anti-retroviral therapy after their CD4 percentages had declined to less than 25%.6 However, the optimum time to start antiretroviral therapy in children infected with HIV who have survived past their first year without treatment is unknown.7,8 Because initiation of antiretroviral therapy in children is a lifelong commitment, the timing of treatment initiation needs to balance the risks of morbidity and mortality related to HIV9 with the problems associated with long-term anti-retroviral therapy, including toxicity, poor adherence, development of drug resistance, and the few alternative drug formulations suitable for children. Moreover, whether early initiation of antiretroviral therapy can prevent or reverse neurodevelopmental deficits is unknown.
In 2004, when our study was being planned, the US Department of Health and Human Services guidelines recommended treatment of all children with CD4 percentages of less than 25%. However, because of restricted access to antiretroviral drugs in low-resource settings, this aim was not achievable. Instead, children were treated when they became symptomatic (CDC category C illness) or when CD4 percentages declined to less than 15%. We designed the Pediatric Randomised Early versus Deferred Initiation in Cambodia and Thailand (PREDICT) trial to compare AIDS-free survival in children with moderate immunosuppression starting antiretroviral therapy when CD4 percentages were 15–24% (early treatment group) with equivalent children starting antiretroviral therapy when CD4 percentages declined to less than 15% (deferred treatment group). We postulated that antiretroviral therapy could be deferred until CD4 declined to less than 15% without affecting AIDS-free survival.
Methods
Study design and participants
In our multicentre, randomised, open-label trial, we enrolled children aged 1–12 years at nine tertiary referral hospitals or research sites in the Comprehensive International Program for Research in AIDS (CIPRA) Thailand and Cambodia Network. Eligible children had HIV infection (defined as positive HIV DNA PCR or positive RNA PCR twice in children aged 12–18 months or positive HIV antibody test in children aged >18 months), had a CD4 percentage of 15–24%, had no history of AIDS illness, and had not received anti-retroviral therapy except for exposure as part of prevention of mother-to-child transmission. Participants were excluded if they had active AIDS-defining illnesses or previous use of immunosuppressive drugs (eg, for systemic cancer therapy), immunomodulators within 30 days of study entry, or abnormal laboratory results, including absolute neutrophil count of less than 750 cells per μL, haemoglobin of less than 7·5 g/dL, platelet counts of less than than 50 000 per μL, and alanine aminotransferase more than four times the upper limit of normal. All participants who had not had previous antiretroviral therapy presenting to the clinical research sites were screened for entry to the study. The study was approved by institutional review boards at all sites. Caregivers provided written informed consent.
Randomisation and masking
After validation of eligibility criteria, participants were randomly allocated (1:1) to early treatment (for patients with a CD4 percentage of 15–24%) or deferred treatment (started after a decline in CD4 percentage to <15% or development of US Centers for Disease Control and Prevention [CDC] category C events10) by an independent biostatistician at the trial coordinating centre (HIV Netherlands Australia Thailand Research Collaboration [HIV NAT], Bangkok, Thailand) with a program written in SAS 9.1. Randomisation was minimised by research site and history of nevirapine exposure for prevention of mother-to-child transmission of HIV. Treatment-group assignment was immediately communicated to the site investigator via fax. Patients and treating physicians were not masked to treatment-group assignment.
Procedures
In December, 2008, immunological criteria to start antiretroviral therapy for children aged 1–3 years were modified to CD4 percentages of less than 20%, because of changes in the WHO11 and national treatment guidelines. After December, 2008, we modified treatment criteria to start antiretroviral therapy if CD4 percentages declined to less than 20%. The criteria were applied to all children who were younger than 3 years on Dec 16, 2008. If CD4 percentages declined to less than 15%, we started co-trimoxazole prophylaxis and continued for at least 6 months until two subsequent results were more than 15%. A neurodevelopmental substudy of PREDICT was started in August, 2007. We used one of the endpoints of that study, the Beery visual motor integration (VMI) assessment, as a secondary endpoint in this study. The Beery VMI assessment provides an overall neuro-development index and assessments are done for almost all Thai and Cambodian children aged 2 years or older. Children in the early treatment group were seen at clinical research sites at 2, 4, 8, and 12 weeks and every 12 weeks thereafter; children in the deferred treatment group were seen at clinical research sites at 8 weeks and 12 weeks and every 12 weeks thereafter. We did clinical assessments, including for toxicity or HIV-related events, at every visit. We did a complete blood count and measured CD4 percentages, cell counts, and serum electrolytes and alanine transaminase concentrations every 12 weeks. We assessed plasma HIV RNA and did Beery VMI tests every 24 weeks. We used adherence questionnaires and pill counts to assess adherence to antiretroviral therapy. We used the US National Institutes of Health, Division of AIDS (DAIDS) toxicity grading table to grade toxicity and abnormalities not related to drugs, such as thrombocytopenia.12
First-line antiretroviral therapy was zidovudine, lamivudine, and nevirapine, and protease inhibitor (lopinavir–ritonavir or nelfinavir) was substituted for children with previous exposure to nevirapine (nelfinavir was not used after September, 2007).13 Abacavir was substituted for zidovudine in participants with grade 3 or 4 haematological toxicity. Efavirenz or lopinavir-ritonavir or nelfinavir was substituted for nevirapine in children with hypersensitivity to nevirapine, dependent on the severity. Children needing treatment for tuberculosis received zidovudine, lamivudine, and abacavir. We defined failure of antiretroviral therapy as HIV RNA of more than 1000 copies per mL after 6 months or more of treatment, and selection of second-line antiretroviral therapy was based on genotypic resistance testing.
The primary outcome was CDC category C event-free survival at 144 weeks. The main secondary outcomes were CDC clinical category B events and the Beery VMI standard score. To interpret the Beery VMI test score, we enrolled and collected data from 155 children exposed to but uninfected with HIV and 164 HIV-negative children as controls. Other secondary outcomes included rates of hospital admission, changes in CD4 percentage and growth, toxicity related to antiretroviral therapy, and cumulative proportions of children with virological failure. CDC category B and C endpoints and hospital admissions were reviewed by independent committees who were masked to randomisation, CD4 values, and antiretroviral therapy status, and made decisions based on prespecified definitions and criteria.
Statistical analysis
When the study was designed in 2004, AIDS-free survival at week 144 was assumed to be 85% in the early treatment group, on the basis of data from a paediatric Thai cohort who had received two-drug antiretroviral therapy.14 Sample size calculations made with Stata showed that randomisation of 127 patients to each group was needed to detect a reduction in AIDS-free survival from 85% to 70% in the deferred treatment group at week 144 with 80% power and a two-sided significance level of 5% in a superiority design. We increased this number to 150 per group to allow for up to 20% loss to follow-up.
We included all participants who were randomly allocated treatment and remained in the study past week 0 in the main analyses of all outcomes. Poisson regression models used total person follow-up time in the study as the exposure variable. We also did a per-protocol analysis for the primary endpoint, which censored participants at the time of protocol deviation. Effect differences between treatment groups were estimated with the early treatment group as the reference. For secondary outcome analyses, we counted participants lost to follow-up as failures in binary analyses, and used the last-observation-carried-forward approach for continuous endpoints. We assessed AIDS-free survival with the Kaplan-Meier product limit method, and used the log-rank test to compare equality of the survivor function between treatment groups. We used Cox proportional hazards regression to calculate the hazard ratio (HR) for AIDS-free survival between treatment groups. We compared rates of CDC category B events and hospital admissions with Poisson regression. Rate calculations for AIDS-free survival used a denominator of total person-years of follow-up until AIDS or death, and participants who remained free of disease were censored at their last study visit. Other rates and incidence rate ratios derived from Poisson regression models used total person follow-up time in the study as the exposure variable. We used the Mantel-Haenszel test to assess homogeneity of rate outcomes by site.
We compared continuous study endpoints including CD4 percentage and growth by calculating the mean difference between randomised groups in parameter change from week 0 to 144, and derived p values from the corresponding 95% CI and t tests. We compared CD4 percentage changes in each 12 week interval between children in the early treatment group and those in the deferred treatment group after antiretroviral therapy initiation with a random-effects regression model. The Beery VMI standard score assessment commenced after the main study began in August, 2007, and therefore comparisons between groups were made at final study visit. We did a sensitivity analysis by comparing the change in Beery standard score from first to last test between treatment groups overall, and with those who had a baseline visit. We assessed normality by use of residual plots; Schoenfeld residuals were used to assess the proportional hazards assumption in time-to-event models. Significance was regarded as a two-sided level of 5% (p<0·05), without adjustment for multiple comparisons. Because of a sex imbalance in the treatment groups, and because of age and sex differences in CD4 count and HIV RNA in children,15 we did further analyses of disease progression, virological suppression, CD4 recovery, and Beery VMI data after adjustment for age (≤3 years vs >4 years) and sex.
Data were entered electronically at the HIV NAT, Thai Red Cross AIDS research centre through an internet interface with electronic data management-eDM version 4.0. Data analysis was done with SAS version 9.2 and Stata version 11.
This study is registered with ClinicalTrials.gov, number NCT00234091.
Role of the funding source
Representatives from the US National Institutes of Health were part of the study team and were involved in the design of the study, data interpretation, and writing of this report. Pharmaceutical companies providing study drugs had no role in the study design, analysis, or preparation of the report. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
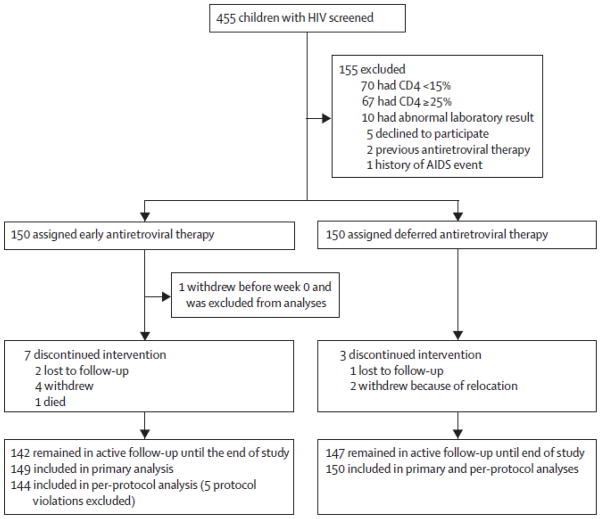
Between March 28, 2006 and Sept 10, 2008, we screened 455 children and enrolled 180 Thai and 120 Cambodian children (figure 1). Five children in the early treatment group had protocol deviations (three did not start antiretroviral therapy immediately) and were excluded from per-protocol analyses. Baseline characteristics were much the same between the two treatment groups (table 1), but the deferred treatment group had a higher proportion of girls than did the early treatment group. The median baseline CD4 in 186 children aged 5 years or older was 548 cells per μL (IQR 399–729). 18 (6%) of 300 children had been exposed to nevirapine for prevention of mother-to-child HIV transmission.
Figure 1.
Trial profile
Table 1.
Baseline characteristics
| Early antiretroviral therapy (n=149) | Deferred antiretroviral therapy (n=150) | Overall (n=299) | |
|---|---|---|---|
| Age, years | 6·4 (3·6–8·0) | 6·5 (4·2–8·7) | 6·4 (3·9–8·4) |
| Age group | |||
| 1–3 years | 45(30%) | 33(22%) | 78(26%) |
| 4–6 years | 43(29%) | 50(33%) | 93(31%) |
| 7–9 years | 44(30%) | 49(33%) | 93(31%) |
| 10–12 years | 17(11%) | 18(12%) | 35(12%) |
| Sex, female | 77(52%) | 96(64%) | 173(58%) |
| Ethnicity | |||
| Thai | 90(60%) | 89(59%) | 179(60%) |
| Cambodia | 59(40%) | 61(41%) | 120(40%) |
| HIV clinical staging | |||
| CDC category N | 3 (2%) | 2(1%) | 5(2%) |
| CDC category A | 91(61%) | 94(63%) | 185(62%) |
| CDC category B | 55(37%) | 54(36%) | 109(36%) |
| CD4 percentage | 19% (16–22) | 20% (17–23) | 20% (17–23) |
| CD4 cell count, cells per μL | 620 (425–851) | 619(466–847) | 619(437–850) |
| HIV-RNA, log10 copies per mL | 4·9 (4·4–5·0) | 4·7 (4·3–5·0) | 4·8 (4·3–5·0) |
| Weight-for-age Z score | −1·3 (−2·0 to −0·8) | −1·3 (−2·0 to −0·8) | −1·3 (−2·0 to −0·8) |
| Height-for-age Z score | −1·6 (−2·5 to −0·8) | −1·7 (−2·6 to −0·9) | −1·7(−2·5 to −0·8) |
| Haemoglobin, g/dL | 11·0 (10·3–11·7) | 11·5 (10·6–12) | 11·2 (−10·5–11·9) |
Data are median (IQR) or n (%). CDC=US Centers for Disease Control and Prevention.
By May 23, 2011, 289 children (96%) had completed 144 weeks of follow-up; one child died, seven children withdrew from the study, and three children were lost to follow-up (figure 1). By May 23, 2011, 69 children (46%) in the deferred treatment group had started antiretroviral therapy: three of whom started because of clinical criteria (one with Pneumocystis jirovecii pneumonia and two severe thrombocytopenia) and 66 started because of immunological criteria (63 with CD4 percentages <15% and three with CD4 percentages <20%). Mean CD4 percentage at time of initiation of antiretroviral therapy in the deferred treatment group was 13·8% (SD 2·8); mean CD4 cell count was 591 cells per μL (SD 508) in 17 children younger than 5 years old and 309 cells per μL (SD 141) in 52 children aged 5 years or older.
201 children received zidovudine, lamivudine, and nevirapine (140 children in early treatment group vs 61 children in deferred treatment group), ten children received zidovudine, lamivudine, lopinavir, and ritonavir (seven vs three), and seven received other regimens (two vs five). In the early treatment group, children received antiretroviral therapy for 99% of the total study period, compared with 25% in the deferred treatment group. Good adherence, defined as average adherence by pill count of more than 95% while receiving study drug, was reported in 88% of children in the early treatment group and 90% children in the deferred treatment group who started antiretroviral therapy.
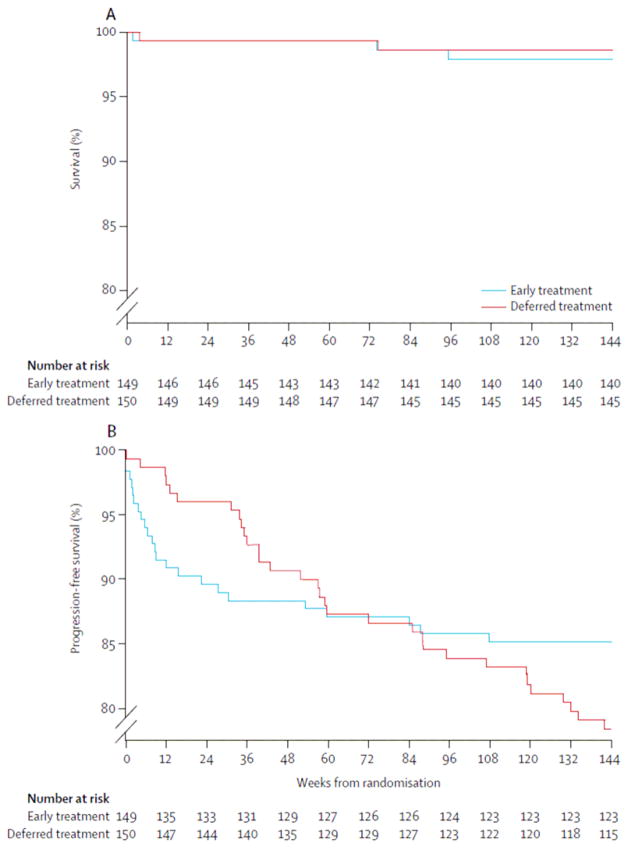
Only five children progressed to CDC category C disease (table 2). In the primary analysis, AIDS-free survival rates at week 144 were 98·7% (95% CI 94·7–99·7) in the deferred treatment group and 97·9% (93·7–99·3) in the early treatment group (p=0·6; figure 2). In per-protocol analysis, AIDS-free survival rates at week 144 were 98·7% (94·7–99·7) in the deferred treatment group and 98·6% (94·5–99·6) in the early treatment group (p=0·96). In the main analysis, incidence of CDC category C events per 1000 person-years of follow-up was 4·9 in the deferred treatment group and 7·6 in the early treatment group (unadjusted HR 0·7, 95% CI 0·1–3·9, p=0·6; and adjusted HR 0·8, 0·1–4·6, p=0·8). In the per-protocol analysis, incidence of CDC category C events was unchanged in the deferred treatment group but incidence in the early treatment group declined to 5·2 per 1000 person-years of follow-up (unadjusted HR 0·95, 0·13–6·7, p=0·96; adjusted HR 1·17, 0·16–8·5, p=0·9).
Table 2.
CDC category C events and deaths
| Age at onset, years | Diagnosis | Study week | Weeks of antiretroviral therapy | Duration, days | CD4 percentage* | Plasma HIV RNA, log10 copies per mL* | |
|---|---|---|---|---|---|---|---|
| Early treatment | |||||||
| Girl | 1·6 | Pneumocystis jirovecii pneumonia | 1 | 1 | 11 | 17 | 5·29 |
| Boy | 5·0 | Pneumonia and sepsis, death | 74 | 74 | 9 | 7 | 1·70 |
| Boy | 10·6 | Disseminated penicilliosis | 95 | −3 | 42 | 4 | 5·78 |
| Deferred treatment | |||||||
| Girl | 2·1 | Oesophageal candidiasis | 3 | −4 | 20 | 22 | 6·00 |
| Girl | 10·0 | Extrapulmonary tuberculosis | 74 | 22 | 190 | 27 | 1·70 |
CDC=US Centers for Disease Control and Prevention.
Closest CD4 percentage and viral load preceding the progression event.
Figure 2.
Kaplan-Meier estimates of the probability of AIDS-free survival (A) and time to the first Centers for Disease Control and Prevention category B or category C event or death (B)
Table 3 shows incidence and type of CDC category B events. Overall, incidence of such events was 110 events per 1000 person-years of follow-up (95% CI 80–147) in the deferred treatment group and 88 events per 1000 person-years of follow-up (61–122) in the early treatment group (incidence rate ratio 1·25, 95% CI 0·8–1·9, p=0·3). Incidence of thrombocytopenia was higher in the deferred treatment group (24·4 events vs 2·5 events per 1000 person-years of follow-up; incidence rate ratio 9·7, 95% CI 1·2–75·9, p=0·03), as was incidence of herpes zoster (31·8 vs 5·0; 6·3, 1·4–28·0, p=0·02). Incidence of bacterial pneumonia did not differ between groups (22·0 vs 40·2; 0·55, 0·24–1·24, p=0·15). Figure 2 shows time to development of first CDC clinical category B or C event. For children who had CDC category B and C events, the median time on antiretroviral therapy before development of first CDC B or C event was 9 weeks (IQR 4–30) in the early treatment group and median time from enrolment to development of first CDC B or C event in the deferred treatment group was 57 weeks (34–101; p=0·0005, by Wilcoxon rank sum test). Rate of hospital admissions did not differ between groups (incidence rate ratio 0·7, 95% CI 0·5–1·1, p=0·1; table 3). We noted no evidence of differences in CDC category B and C events or hospitalisation rate between treatment groups by site.
Table 3.
HIV clinical disease progression and rate of hospital admissions
| Early treatment group (n=149) | Deferred treatment group (n=150) | |
|---|---|---|
| CDC category C events | ||
| Incidence per 1000 patient-years | 7·6 (2·5–23·6) | 4·9 (1·2–19·7) |
| Number of children | 3 | 2 (1, 1) |
| Pneumocystis jirovecii pneumonia | 1 | 0 |
| Disseminated penicilliosis | 1 | 0 |
| Severe sepsis | 1 | 0 |
| Oesophageal candidiasis | 0 | 1 (1, 0) |
| Extrapulmonary tuberculosis | 0 | 1 (0, 1) |
| CDC category B events | ||
| Incidence per 1000 patient-years | 88 (61–122) | 110 (80–147) |
| Number of children | 20 | 32 |
| Number of events | 35 | 45 (34, 11) |
| Bacterial pneumonia | 16 | 9 (7, 2) |
| Lymphoid interstitial pneumonia | 9 | 0 |
| Thrombocytopenia | 1 | 10 (10, 0) |
| Herpes zoster | 2 | 13 (9, 4) |
| Herpes simplex | 1 | 6 (4, 2) |
| Complicated chickenpox | 1 | 1 (1, 0) |
| Pulmonary tuberculosis | 1 | 1 (1, 0) |
| Oral candidiasis | 2 | 3 (1, 2) |
| Diarrhoea | 1 | 2 (1, 1) |
| Molluscum contagiosum | 1 | 0 |
| Hospital admissions | ||
| Incidence per 1000 patient-years | 123 (91–163) | 88 (62–122) |
| Number of admissions | 49 | 36 |
| HIV related* | 14 | 11 |
| Antiretroviral adverse drug reactions | 7 | 2 |
| Not HIV related | 28 | 23 |
Data are incidence (95% CI), n, or n (events before antiretroviral therapy, events after antiretroviral therapy). CDC=US Centers for Disease Control and Prevention.
One child in the early treatment group had six admissions for pneumonia, and subsequently died.
Because the neurodevelopment substudy was phased in after the main study had started, we only did baseline Beery VMI assessments in 39 children in the early treatment group (mean 81·5, SD 17·2) and 32 children in the deferred treatment group (87·1, 14·4). At week 144, the standardised score on the Beery VMI in 132 children in the early treatment group was 85·2 (SD 13·8), compared with 86·6 (14·0) for 140 children in the deferred treatment group (absolute difference 1·4, 95% CI −1·89 to 4·77, p=0·4). Distribution of age, sex, and treatment group did not differ for children completing the baseline assessment from those who did not complete the assessment. For 71 children who had a baseline assessment, the mean difference in Beery standard score change from baseline from week 0 to 144 between the early and the deferred arms was −4·37 (−12·00 to 3·26; p=0·26).
The mean Beery VMI standard scores in children exposed to but uninfected with HIV was 94 (SD 13) and in children uninfected with HIV was 98 (16). Beery VMI scores in children infected with HIV in both early and deferred treatment groups were significantly lower than were those for children in the control group (p<0·0001).
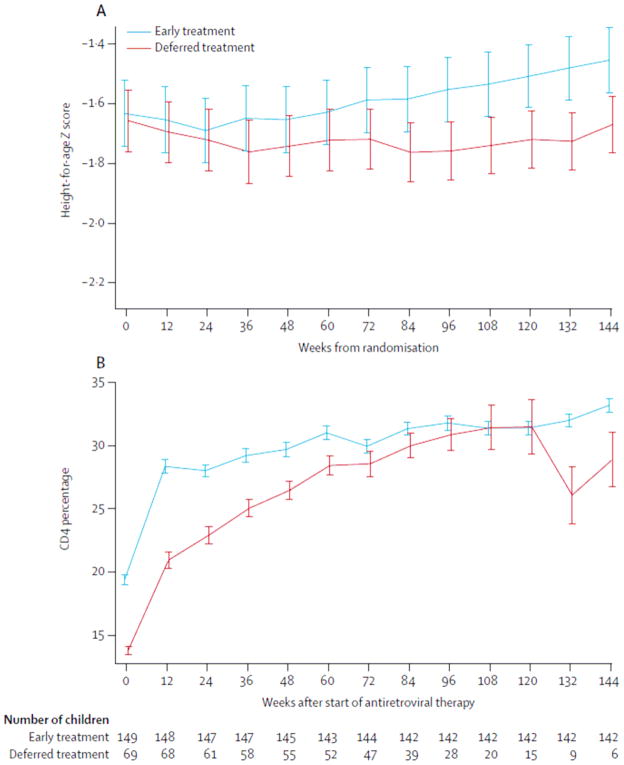
Table 1 shows median weight-for-age and height-for-age Z scores at baseline. The mean body weight gain per year was 2·2 kg (SD 1·1) in the early treatment group and 2·1 kg (1·2) in the deferred treatment group (absolute difference −0·1, 95% CI −0·3 to 0·2, p=0·69). The mean height gain per year was 5·4 cm (SD 1·4) in the early treatment group and 4·9 cm (1·2) in the deferred treatment group (absolute difference −0·5, −0·8 to −0·2, p=0·001). The mean height-for-age Z score at week 144 was −1·45 (SD 1·30) in the early and −1·67 (1·14) in the deferred treatment group (mean difference −0·22, −0·38 to −0·08, p=0·003; figure 3).
Figure 3.
Differences in mean height-for-age Z score between treatment groups at each study visit (A), and mean CD4 percentage after start of antiretroviral therapy in early treatment group versus children in the deferred treatment group who started antiretroviral therapy (B) Error bars show one standard error.
The mean CD4 percentage at week 144 in the early treatment group was 33·2% (SD 6·4) compared with 24·8% (7·4) in the deferred treatment group (p<0·0001). In the early treatment group, the mean baseline CD4 was 19·4%, with an increase of 13·8% by week 144 of treatment (figure 3). In the deferred treatment group, the mean baseline CD4 was 13·8%, with an increase of 15·2% by week 144 for children who started antiretroviral therapy (figure 3). In a random-effects model, the difference in CD4 percentage change after starting of antiretroviral therapy in the entire trial follow-up in the deferred versus the early arms was −0·46 (−1·87 to 0·94, p=0·52). With the Kaplan-Meier method, the probability of achievement of a CD4 percentage of 30% or more by week 96 was 83% (95% CI 77–89) in the early and 74% (62–84) in the deferred treatment group (p=0·0006).
For children with at least 48 weeks of antiretroviral therapy, 121 (81%) of 149 children in the early treatment group and 47 (85%) of 55 children in the deferred treatment group had HIV RNA of fewer than 50 copies per mL and remained on first-line antiretroviral therapy (absolute difference −4%, 95% CI −15 to 7, p=0·5). Cumulatively, 13 (9%) children in the early treatment group and four (6%) of 69 in the deferred treatment group who started antiretroviral therapy switched to second-line antiretroviral therapy regimens (p=0·59). 32 grade 3 or 4 antiretroviral therapy-related events occurred, including nine zidovudine-related neutropenias (seven in the early treatment group vs two in the deferred treatment group), nine zidovudine-related anaemias (four vs five), nine nevirapine-related drug hypersensitivities (nine vs zero), and five nevirapine-related grade 3 or 4 hepatotoxicities (five vs zero). 25 (17%) of 149 children in the early treatment group and seven (10%) of 69 children in the deferred treatment group who started antiretroviral therapy developed grade 3 and 4 events related to antiretroviral therapy (p=0·19).
In analyses adjusted for sex and baseline age, treatment groups did not differ for rates of CDC category B and C events, hospital admissions, CD4 percentage recovery on antiretroviral therapy, undetectable plasma HIV RNA after 48 weeks on antiretroviral therapy, and Beery VMI.
Discussion
Almost all HIV-infected children older than 1 year of age with moderate immunosuppression and no severe HIV symptoms at baseline were alive and had high AIDS-free survival after 3 years of follow-up, irrespective of whether antiretroviral therapy was started early or deferred until CD4 dropped to less than 15%. In addition, Beery VMI test scores in children in the early treatment group were not significantly better than were those for children in the deferred treatment group, and test scores in both groups were significantly worse than for controls uninfected with HIV. The PREDICT study is the only randomised trial to assess when to start antiretroviral therapy in children older than 1 year (panel).
We noted an extremely low rate of CDC category C clinical events in our study. This low rate could be explained by the bimodal disease progression in paediatric HIV with a rapid disease progression during the first 2 years of life and a slower disease progression thereafter. The median age at study entry was 6·4 years, and thus most enrolled children had survived the first 2 years of life without antiretroviral therapy and were in the slow progressor subgroup.4 Additionally, CD4 was monitored every 12 weeks and antiretroviral therapy was promptly started when the CD4 percentage was less than 15%, possibly contributing to the low rate of CDC category C events and death. This supposition is in line with data from Asian children showing AIDS-free survival rates of 91·7–94·8% at 5 years in children who started antiretroviral therapy when CD4 percentages were less than 15%.16,17 Our findings differ from those of the CHER trial, which showed early antiretroviral therapy reduced mortality by 76% compared with deferral of treatment until CD4 percentages dropped to less than 25% in children younger than 3 months.6 This difference could be explained by the reduced HIV disease progression in older children who survive infancy without development of advanced immunodeficiency, compared with most infants infected with HIV. A meta-analysis of untreated HIV-positive children in the USA and Europe showed a lower short-term risk of clinical progression in older children: for example, 3-month-old infants with CD4 percentage of 25% had a 12 month risk of AIDS progression of 34% compared with 3% in children aged 6 years.18
Rates of herpes zoster infection, a CDC B event, were significantly higher in the deferred treatment group than in the early treatment group. This difference is akin to the higher incidence of herpes zoster reported before highly active antiretroviral therapy (HAART) became available.19 By contrast with results from a randomised study in adults,20 we did not note an increase in cases of pulmonary tuberculosis with deferring antiretroviral therapy. We reported a low rate of tuberculosis in this study, and children received isoniazid prophylaxis if a member of their household had tuberculosis.
The Beery VMI is designed to assess the extent to which individuals can integrate their visual and motor abilities (hand-eye coordination) and can be used across cultures and languages.21–23 We noted no differences between study groups in Beery VMI standard scores at the last study week, and no differences in the change from baseline score between groups in children who had had a baseline assessment. However, the Beery test score was significantly lower than that reported in our control children. This distinction is much the same as in a report from Ugandan children aged 6–12 years with high CD4 counts (CD4 >15%) who had not previously had antiretroviral therapy, suggesting poorer cognitive function compared with controls uninfected with HIV.24 Our study also showed that starting of antiretroviral therapy in children aged older than 1 year with CD4 percentages of more than 15% (whether early or deferred) did not affect neuro-developmental function. This finding is similar to a report in Thai HIV-positive children with advanced disease,25 in whom the intelligence score was not improved after 3 years of antiretroviral therapy. This lack of response might be related to early HIV insult to the brain, and the optimum window of opportunity to protect brain damage could be in early infancy. The CHER study reported improved Griffiths Mental Development Scores at 10–15 months in infants who received antiretroviral therapy at less than 12 weeks of age compared with deferred treatment until CD4 percentages declined to less than 25%.26 Important socioeconomic issues related to living with HIV, such as parents’ health, poverty, and appropriate developmental stimulation, could have affected the Beery VMI scores but were not captured in our study.
Children in the early treatment group had similar weight gain but better height gain than did those in the deferred treatment group, which is consistent with data from Ugandan children whose baseline age and immunological status before antiretroviral therapy affected height but not weight gain.27 Long-term monitoring is needed to assess if the deferred strategy will affect the final growth of children when they enter puberty. Immune recovery related to antiretroviral therapy in children in the deferred treatment group after starting of antiretroviral therapy was unexpectedly good. Despite the lower median baseline CD4 percentages when starting antiretroviral therapy, CD4 recovery rates did not differ by treatment group (figure 3). However, this factor meant that time to reach CD4 percentages of 30% or more took longer in the deferred treatment group than the early treatment group, as shown by the significant log-rank p value of 0·0006. Nevertheless, the cumulative proportion of children who achieved CD4 percentages of 30% or more at week 96 was 74% (95% CI 62–84) in the deferred treatment group compared with 83% (77–89) in the early treatment group. This finding differs from reports from HIV-positive children with advanced disease.28 More than 80% of children on antiretroviral therapy in our study had virological suppression at 3 years, which is superior to the 70% reported from a meta-analysis of children in low-resource settings.3 This discrepancy could be explained by higher baseline CD4 in our study than a mean CD4 of only 8% in the real-life scenarios in low-resource settings.29
Strengths of the study include the randomised design and high participant retention rate for 3 years, with systematic assessment of morbidity and mortality related to HIV and comprehensive secondary outcomes including neurodevelopment, growth, immunological response, and virological response. Our study, however, has several limitations. First, only 26% of our study population were aged 1–3 years, and only 6% were aged 1–2 years. Thus, the generalisability of our result to young children (≤3 years) is restricted. Second, despite our best efforts to obtain relevant data to inform sample size calculation during the design phase, disease progression or death rates were very low, and consequently our trial was underpowered to detect differences in the primary study endpoints. Nevertheless, the low rate of progression (See online for appendix) in our trial provides strong evidence that close monitoring of CD4 every 3 months and commencement of antiretroviral therapy when CD4 percentages decline to less than 15% is a potentially safe strategy for older HIV-positive children who survived the first year of life without antiretroviral therapy, especially in settings where drug availability is restricted or when children and their caregivers are not ready to embark on lifelong antiretroviral therapy. Because CD4 cell counts change rapidly in the first 5 years of life, our study was designed to use CD4 percentage endpoints, which are relatively constant across age groups. In this study, CD4 of 15% equates to a CD4 cell count of about 300 cells per μL in children older than 5 years, which is quite similar to present recommendations for initiation of antiretroviral therapy for adults and adolescents infected with HIV.30 Nevertheless, our findings might not be applicable to settings in which routine CD4 monitoring is not available and the burden of disease and malnutrition is high. Third, the incidence of tuberculosis was low in our study, and deferral of antiretroviral therapy in children infected with HIV living in areas with high tuberculosis disease burden might have increased risk for adverse clinical outcomes. Fourth, although treatment groups did not differ with respect to Beery VMI scores at week 144, only 24% had a baseline neurodevelopmental assessment. However, the absence of difference in the change from baseline scores between treatment groups in children with a baseline measurement, and knowledge that baseline measurements did not differ by study treatment group in those children who had a baseline assessment partly mitigates this weakness. Finally, the long-term effects of deferred treatment, such as final effect on growth, is unknown and warrants further investigation.
In settings where access to antiretroviral therapy is restricted and children or caregivers might not be ready to start antiretroviral therapy, treatment in older HIV-positive children might plausibly be delayed without compromising 3 year AIDS-free survival, provided that close CD4 monitoring can be done and antiretroviral therapy started promptly when CD4 declines to less than 15%. However, both treatment strategies in this older paediatric population resulted in poorer neuro-developmental function compared with children without HIV. Thus, early HIV diagnosis in infants born to mothers infected with HIV and prompt initiation of antiretroviral therapy in infants who are infected with HIV is crucial, and policy makers in low-resource settings should be strongly encouraged to strengthen relevant treatment programmes.
Supplementary Material
Systematic review.
We searched the PubMed database without language or date restriction with the search terms “randomised” AND “antiretroviral therapy” AND “HIV” AND “children” AND “start”. We identified only one randomised trial comparing CD4 thresholds to start of antiretroviral therapy in infants and children (the Children with HIV Early Antiretroviral Therapy trial [CHER]).3
Interpretation.
Our study is the first randomised trial assessing CD4 percentage as a threshold to start antiretroviral therapy in children who survived the first year of life without treatment. We did not note a difference in AIDS-free survival and neurodevelopmental outcomes by Beery visual motor integration scores between HIV-positive children older than 1 year who started early or deferred antiretroviral therapy. However, the study was underpowered to detect a difference, and therefore additional follow-up of study participants or future studies are needed to answer this clinical question. The CHER study,3 which was done in HIV-positive infants aged younger than 12 weeks, showed that early antiretroviral therapy reduced mortality by 76% compared with deferral of antiretroviral therapy until CD4 declined to less than 25%.6 This contrast could be explained by differences in the study populations. Children who survived their first year of life without treatment represent a group of children with slow HIV disease progression. The data from our study suggesting that impaired neurodevelopment function does not improve after initiation of antiretroviral therapy in older children infected with HIV, emphasises the need for early HIV diagnosis and prompt treatment in infancy.
Acknowledgments
The trial was supported by a grant from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health through the Comprehensive International Program of Research on AIDS Network (U19 AI53741), and was co-funded by the Eunice Shriver Kennedy National Institute of Child Health and Human Development and the National Institute of Mental Health, the National Research Council of Thailand, and National Health Security Office, Thailand. Antiretroviral drugs were provided by ViiV Healthcare, GlaxoSmithKline, Boehringer Ingelheim, Merck, Abbott, and Roche. The views in this report do not necessarily reflect the views of the National Institutes of Health or US Department of Health and Human Services. We thank the families and children who participated in the trial, and June Piraporn Ohata for her help with preparation of the report for submission.
Footnotes
Contributors
TP, SV, JA, WTS, RP, LF, MGL, and KR designed the PREDICT trial. TP, VS, PK, RH, UV, SJK, CN, JW, WL, and NN-G-H were project leaders at each of the clinical research sites collecting study data. TP, KC, TC, TS, and SU coordinated the trial. SJK did the data safety monitoring board analysis, which was overseen by MGL. SJK wrote the statistical analysis plan, which was reviewed by all authors. SJK did the final analysis. All authors contributed to the interpretation of the findings. TP, JA, and SJK wrote the first draft of the paper. All authors commented extensively, critically revised the report, and approved the final version. See appendix for a full list of collaborators and research sites.
Conflicts of interest
JA has received consulting fees or speaker’s honoraria from ViiV Healthcare, Abbott, and Gilead. KR has received consulting fees or speaker’s honoraria from ViiV Healthcare and Abbott, and support through grants HR1161A from the Thai National Research University Project of the Commission for Higher Education and the Ratchadaphiseksomphot Endowment Fund, Thailand; the Professional Researcher Strengthen Grant from the National Science and Technology Development Agency, BIOTEC; and Senior researcher scholar from the Thai Research Fund. All other authors declare that they have no conflicts of interest.
References
- 1.UNAIDS. [accessed June 22, 2012];UNAIDS report on the global AIDS epidemic. 2010 http://www.unaids.org/globalreport/Global_report.htm.
- 2.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–27. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanche S, Newell ML, Mayaux MJ, et al. Morbidity and mortality in European children vertically infected by HIV-1. The French Pediatric HIV Infection Study Group and European Collaborative Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:442–50. doi: 10.1097/00042560-199704150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chearskul S, Chotpitayasunondh T, Simonds RJ, et al. and the Bangkok Collaborative Perinatal HIV Transmission Study Group. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics. 2002;110:e25. doi: 10.1542/peds.110.2.e25. [DOI] [PubMed] [Google Scholar]
- 6.Violari A, Cotton MF, Gibb DM, et al. and the CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Recommendations for a public health approach. Geneva: World Health Organization; 2006. Antiretroviral therapy for HIV infection in infants and children: towards universal access. [PubMed] [Google Scholar]
- 8.WHO. Recommendations for a public health approach. Geneva: World Health Organization; 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access. [PubMed] [Google Scholar]
- 9.de Martino M, Tovo PA, Balducci M, et al. and the Italian Register for HIV Infection in Children and the Italian National AIDS Registry. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA. 2000;284:190–97. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep. 1994;43:1–10. [Google Scholar]
- 11.WHO. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Geneva: World Health Organization; 2006. [Google Scholar]
- 12.NIH, DAIDS. Table for grading the severity of adult and pediatric adverse events. National Institute of Allergy and Infectious Diseases, Division of AIDS; 2004. [Google Scholar]
- 13.US Department of Health and Human Services Pediatric Panel. [accessed Aug 29, 2011];Notice on nelfinavir FDA-Pfizer letter. 2007 Sep 11; http://aidsinfonih.gov/contentfiles/PedNFVnotice1.pdf.
- 14.Chokephaibulkit K, Vanprapar N, Sutthent R, et al. Initiation of antiretroviral treatment with dual nucleoside reverse transcriptase inhibitors in human immunodeficiency virus-infected infants with less advanced disease in a resource-limited setting: a multi-center study in Thailand 1998–2000. J Med Assoc Thai. 2005;88 (suppl 8):S1–8. [PubMed] [Google Scholar]
- 15.Ruel TD, Zanoni BC, Ssewanyana I, et al. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis. 2011;53:592–99. doi: 10.1093/cid/cir484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumbiganon P, Kariminia A, Aurpibul L, et al. Survival of HIV-infected children: a cohort study from the Asia-Pacific region. J Acquir Immune Defic Syndr. 2011;56:365–71. doi: 10.1097/QAI.0b013e318207a55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis. 2010;51:1449–57. doi: 10.1086/657401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.HIV Paediatric Prognostic Markers Collaborative Study Group. Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–11. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 19.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor KM. Relationship between visual motor integration skill and academic performance in kindergarten through third grade. Optom Vis Sci. 1999;76:159–63. doi: 10.1097/00006324-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Sortor JM, Kulp MT. Are the results of the Beery-Buktenica Developmental Test of Visual-Motor Integration and its subtests related to achievement test scores? Optom Vis Sci. 2003;80:758–63. doi: 10.1097/00006324-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Dunn M, Loxton H, Naidoo A. Correlations of scores on the developmental test of visual-motor integration and copying test in a South African multi-ethnic preschool sample. Percept Mot Skills. 2006;103:951–58. doi: 10.2466/pms.103.3.951-958. [DOI] [PubMed] [Google Scholar]
- 24.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54:1001–09. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puthanakit T, Aurpibul L, Louthrenoo O, et al. Poor cognitive functioning of school-aged children in Thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24:141–46. doi: 10.1089/apc.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–90. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–92. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 28.Puthanakit T, Kerr S, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. Pediatr Infect Dis J. 2009;28:488–92. doi: 10.1097/inf.0b013e318194eea6. [DOI] [PubMed] [Google Scholar]
- 29.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. and the Academic Alliance for AIDS Care and Prevention in Africa. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach 2010 revision. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.