Abstract
Purpose
Multifactorial etiological factors contribute to denture stomatitis (DS), a type of oral candidiasis; however, unlike other oral candidiasis, DS can occur in a healthy person wearing a denture. In this study, we therefore attempt to explore the association between candida, denture, and mucosal tissue using 1) exfoliative cytology, 2) the candidal levels present in saliva, on mucosal tissues and on denture surfaces, and 3) the salivary flow rate and xerostomic symptoms.
Materials and Methods
A cross-sectional study enrolled 32 edentulous participants, 17 without DS as controls and 15 with DS (Newton’s classification type II and III). Participants with systemic or other known oral conditions were excluded. Participants completed a xerostomia questionnaire, and salivary flow rates were measured. Samples of unstimulated whole saliva (UWS) and stimulated whole saliva (SWS) were collected. UWS was used for fungal culturing. Periodic acid-Schiff (PAS) stain and quantitative exfoliative cytology were performed on samples from affected and unaffected mucosa from each participant. Levels of Candida species (albicans and non-albicans) were determined in salivary samples (expressed as colony-forming units, CFU), as well as from swab samples obtained from denture fitting surfaces, in addition to affected and unaffected mucosa.
Results
There were no significant differences in salivary flow rates, mucosal wetness, or frequency of reported dry mouth comparing participants with and without DS. Exfoliative cytology of mucosal smears demonstrated significantly higher (P = 0.02) inflammatory cell counts in DS patients, as compared with smears of healthy denture-wearers. C. albicans was significantly more prevalent in saliva (P = 0.03) and on denture surfaces (P = 0.002) of DS participants, whereas mucosal candidal counts and the presence of cytological hyphae did not show significant difference comparing DS to healthy participants.
Conclusions
In this investigation, we presented a unique group of healthy edentulous patients. This population may reflect the general DS population without systemic or other oral diseases. The prominent etiological factor for DS in this population is the presence of candida in denture and saliva. We found that other factors such as saliva flow/xerostomia, fitting of the denture, and the presence of candida in the mucosa, are less important in this population. Therefore, DS treatments in healthy patients should first focus on sanitization of an existing denture and/or fabrication of a new denture.
Keywords: C. albicans, denture, hyposalivation, stomatitis, exfoliative cytology
Multiple etiological factors contribute to denture stomatitis (DS).1-6 These factors include 1) microorganisms such as Candida organisms (in particular Candida albicans) and gram-negative anaerobes; 2) impaired salivary flow and salivary gland function; 3) trauma from ill-fitting dentures; 4) poor denture and oral hygiene; and 5) impaired immune response secondary to systemic conditions.5,7-18 Certain strains of Candida, specifically hyphal-forming C. albicans clonal types, are more commonly found in candidal infections in DS patients. These virulent strains are capable of epithelial binding, disruption of epithelial integrity, and invasion.19-24 Besides Candida, antibiotics have been reported to be effective in some refractory DS cases to provide resolution. This suggests that anaerobic pathogens may potentially play a role in some DS circumstances.13-16 Thus, Candida and gram-negative anaerobes may function together in the pathogenesis of DS.
Impaired salivary flow or altered salivary protein and inorganic composition, often but not always associated xerostomia, have been suggested to lead to a shift in the oral microbiome composition that favors fungal overgrowth. Note that a patient’s report of “dry mouth” does not always reflect the reduction of salivary flow or alteration of salivary composition.25,26 Salivary secretory IgA level is a critical modulator of microbial aggregation, microbial clearance, and surface adherence.25,26 Thus, impaired sIgA has been attributed to microbial overgrowth.27-29 The relation between salivary flow and viscosity has been suggested to also potentially play a role in DS by altering the epithelial resistance to candidal binding and invasion.
DS is often associated with ill-fitting dentures associated with atrophic osseous ridge anatomy.5-7 It is unclear whether the inflammatory state of DS induces ridge resorption resulting in a loose and easily displaced denture, or whether the trauma associated with the denture (e.g., through poor tissue adaption, clenching or inadequate inter-ridge space) can provide a mechanical stress that induces mucosal inflammation and bone resorption via poor tissue perfusion, necrosis, or trauma. Nonetheless, the denture can serve as a habitat for high-density biofilm, which can harbor high levels of bacteria and yeasts, especially in patients with poor oral hygiene, poor denture hygiene, or wearing dentures overnight.7,17,18 Thus, the denture itself is believed to serve as both traumatic inducer and a reservoir for triggering a local microbial infection-mediated inflammatory response.
Current and past literature reveals that patients with compromised immunity due to a systemic condition, such as diabetes, are prone to refractory DS as well as other forms of oral candidiasis.7-12 Overgrowth of Candida in DS is a common finding and can be a precursor to oro-pharyngeal and esophageal candidiasis, which can become a life-threatening disseminating infection among HIV/AIDS patients or in those with other immunocompromised conditions, especially those associated with T-cell functional deficits.22-24
Although several studies have been done to examine these etiologic factors of DS, most focus on only one or a few factors. Moreover, a large body of literature on DS presents results that overlap between DS patients with other forms of oral candidiasis. Note that healthy individuals without dentures will not have any oral candidiasis. On the contrary, healthy denture wearers often present with DS. It is plausible that in the general denture-wearing population, some etiological factors may play a larger role than others. For example, healthy denture wearers would have little problem from systemic and local immunity or from xerostomia or impaired salivary function. The interaction between candida and the denture mediated with normal saliva is perhaps the most prominent DS etiological factor in healthy denture wearers.
To reflect healthy denture wearers with limited influence from systemic conditions and other factors besides DS that contribute to oral candidiasis, we decided to conduct an exploratory cross-sectional investigation. Our goal was to examine the association between clinical signs of DS (measuring the severity of stomatitis with the Newton Classification30 and the denture fit using the Kapur index31) and candidal overgrowth by examining 1) the exfoliative cytology, 2) the candidal levels present in saliva, on mucosal tissues, and on denture surfaces, and 3) salivary flow rate and xerostomic symptoms. We believe that the overall healthy status of the patient population provides a unique cohort for studying the etiology of DS, reflecting the majority of denture-wearing population.
Materials and methods
Study design, participants, and sample size estimates
This was a single-center, case control study design intended to collect biological samples from participants with and without denture stomatitis, specifically with mucosal lesions (Newton’s Classification type II or type III).30 The following biological markers were obtained: 1) gene expression profiles as determined by Affymetrix (Affymetrix Inc., Santa Clara, CA) arrays obtained using tissue biopsy samples, 2) exfoliative cytology was performed on mucosal surfaces to examine for the presence or absence of C. albicans infection as determined by periodic acid-Schiff (PAS) cytology, 3) the presence or absence and relative level of C. albicans on mucosal and denture surfaces was determined using cultivable methods, 4) tissue and denture surfaces were sampled to measure the presence, absence, and levels of 18 dental biofilm organisms by deoxyribonucleic acid-deoxyribonucleic acid (DNA-DNA) checkerboard and cultured for C. albicans, 5) the rate of salivary flow and the levels of selected pro-inflammatory cytokines in saliva were measured and 6) C-reactive protein levels were measured in the serum.
This was an exploratory study, and a targeted sample size of 30 (15 patients per group) was determined to provide 80% power with two-sided alpha = 0.05 significance tests to detect changes in means from continuous variables between diseased and non-diseased patients that are 1.06 times the standard deviation of the variable. The sample size was considered sufficient as effect sizes such as this, or larger, are common for levels of dental biofilm organisms and pro-inflammatory cytokines.7,17,22 To allow for an 8 to 10% possible drop-out rate, 32 patients were enrolled to ensure that 30 (15 participants per group) completed the study. In this report, we limit the analyses to the clinical findings, the cytology, and the cultivable data.
The study protocol was approved by the UNC Institutional Review Board (IRB), No. 07-2014. A total of 32 edentulous patients were enrolled according to the inclusion and exclusion criteria listed in Appendix 1. The control group (n = 17) had no signs or symptoms of DS, and the diseased group (n = 15) presented with type II (n = 8) or III (n = 7) DS (Newton’s classification).30 This level of DS includes the moderate (type II) and severe (type III) forms. Representative clinical appearance of cases and controls appear in Figure 1. All enrolled participants completed the two-visit study. For all parameters of interest, there was no apparent gradient progressing from health to type II to type III; therefore, type II and type III remained grouped as a single DS diseased group, as initially intended.
Figure 1.
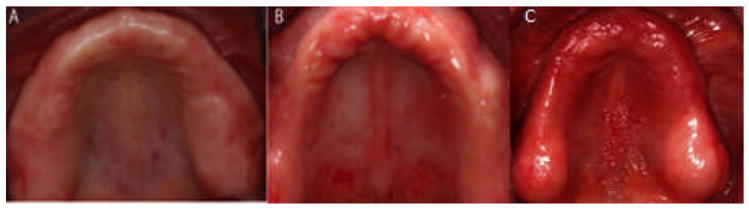
Clinical photographs of (A) a control patient, (B) Newton classification II stomatitis, and (C) Newton classification III stomatitis.
Demographics
The mean age of the 32 participants was 64.8. The healthy group was comprised of 14 women, 3 men, 11 Caucasians, and 6 African Americans. The stomatitis group was comprised of 9 women, 6 men, 9 Caucasians, 3 African Americans, and 3 Asians. There were no significant differences in age, race, or gender in the DS and Healthy groups. Baseline characteristics and Kapur index results are included in Table 1 and show no significant differences between groups.
Table 1.
Demographics and baseline characteristics of the participants
| Control (n = 17) | DS (n = 15) | |
|---|---|---|
| Age (mean ± SD) | 66.2 ± 9.7 | 63.2 ± 8.83 |
| Gender | ||
| Male | 3 (17.6%) | 6 (40%) |
| Female | 14 (82.4%) | 9 (60%) |
| Race | ||
| Caucasian | 11 (64.7%) | 9 (60%) |
| African American | 6 (35.3%) | 3 (20%) |
| Asian | 3 (20%) | |
| Kapur Index of max. Denture (mean ± SD) | 3.56 ± 1.46 | 3.00 ± 1.57 |
Clinical evaluation of dentures (Kapur Index)
A qualified examiner (prosthodontist) assessed the fit of the maxillary and mandibular dentures (if present) using the Kapur Index31 as following:
Retention:
3: Good – maximum resistance to vertical pull and sufficient lateral force.
2: Moderate – moderate resistance to vertical pull and little or no resistance to lateral force.
1: Minimum – slight resistance to vertical pull and little or no resistance to lateral force.
0: No retention – denture displaces itself.
Stability:
2: Sufficient – demonstrates slight or no rocking on its supporting structures under pressure.
1: Some – demonstrates moderate rocking on its supporting structures under pressure.
0: No stability – demonstrates extreme rocking on its supporting structures under pressure.
Because not all of the patients had a mandibular denture, mean values for the total scores of retention and stability for the maxillary denture were calculated.
Clinically poor denture = sum score < 3
Clinically fair denture = sum score 3-4.
Clinically good denture = sum score > 4.
Unpaired T-test was performed to assess statistical difference between means of scores of the maxillary dentures for DS and healthy groups.
Unstimulated saliva collection
Patients were instructed to remove their dentures and refrain from eating, drinking, smoking, brushing their teeth, or chewing gum for 15 minutes prior to salivary collections. All collections were performed between 9:00 and 11:00 AM. Participants were instructed to swallow to clear the mouth of any accumulated saliva, and whole unstimulated saliva was allowed to pool in a sterile polypropylene graduated collection vial for 5 minutes. A fraction of the sample was sent immediately to the microbiology lab for rapid processing to prevent overgrowth of the Candida species. Samples were aliquoted into Eppendorf tubes, centrifuged for 10 minutes at 3000 g, and the supernatant stored at -80°C for proteomic and cytokine analysis.
Stimulated saliva collection
Patients were instructed to place their dentures back before collection began. Stimulated whole saliva was quantified using a modification of the Saxon test,32 where each participant was asked to chew on a folded strip of paraffin for 2 minutes after swallowing to clear the mouth of the accumulated saliva. The collected saliva was then quantified as milliliters of saliva generated per minute (ml/min) and recorded on the CRF. Participants found to have a resting (unstimulated) saliva rate of less than 0.01 ml/min and stimulated salivary rate of less than 0.10 ml/min were characterized as having hyposalivation.33,34
Xerostomia questionnaire
Patients were asked to complete the xerostomia questionnaire (Appendix 2) after the salivary collection. This questionnaire is derived from the validated questionnaire from the Dental ARIC study as described by Beck et al.35 ANOVA (items # 3, 13 and 14) and Spearman correlations (items #5-12) by regression analysis were used to analyze relationships between salivary flow rates and patient-reported symptoms of dry mouth.
Mucosal wetness
Patients were instructed to swallow, and sialopaper was placed on the midline of the anterior third of the dorsum of the tongue for 5 seconds. Sialopaper was then transferred to Periotron (Model 6000) (Ora Flow Inc. Plainview, NY) for reading. After reading, the sialopaper was discarded. Measurement was done twice. T-test was used to determine differences between the means of readings comparing groups.
Exfoliative examination
Smears were taken from the affected palatal mucosa and unaffected palatal mucosa for the stomatitis group, and only one sample from the palatal mucosa for the control group. Samples were taken from the buccal vestibular area and dorsum of the tongue as well. Participants with dry mouth (xerostomia) were instructed to rinse with a small amount of water prior to collection of the sample. Selected areas were wiped firmly, using a wooden tongue blade, until a visible accumulation of oral fluids was present. Accumulated samples were transferred to a clean glass slide until a thin coat was visible when the slide was held against light. Slides were then sprayed with Cytofix/Cytoperm (Becton, Dickinson and Company, Franklin Lakes, NJ) with one or two swipes from a distance of about 1 foot and allowed to dry for 10 minutes.
When adequate samples could not be collected using this procedure, the sampling steps outlined above were repeated in a different area of the palatal mucosa, until an adequate sample was collected. These samples were used for cytology and PAS identification of fungal forms. The following scoring system was used for the PAS-cytology assessments:
0 = Inadequate cell sample, 1 = Benign smear, 2 = Bacteria only, 3 = Benign inflammatory smear, 4 = Bacteria plus inflammatory cells, 5 = Fungal spores, 6 = Fungal organisms.
Culture
BBL CultureSwab (Becton, Dickinson and Company) was used to swab the denture and the mucosal surface, with a total of three swabs for the diseased group (from the denture, mucosal surface of affected area, and unaffected area), and two swabs for the control group (from the denture and the mucosal surface). Samples obtained from swabbing were cultured on Sabouraud Dextrose agar containing quemicetine succinate. Samples were spiral plated to Sabouraud’s dextrose plates to obtain a quantitative value of CFU/ml. Samples were also plated on a total aerobic plate to compute the percentage of total aerobic recoverable CFU on a non-selective medium to allow for identification of both albicans and non-albicans Candida. Candida colonies were counted after 48 hours, and the patients classified according to the number of CFU (colony-forming units) as follows: negative (CFU/ml = 0), carrier (CFU/ml < 400), and positive (CFU/ml > 400). A fraction of the UWS was sampled in Sabouraud Dextrose agar containing quemicetine succinate. Candida colonies were counted after 48 hours, and the patients classified in the same way as for the BBL culture swabs.
Mucosal biopsy
At the end of the appointment, patients were anesthetized, and 4 mm punch biopsies were collected from the palate. In healthy patients, the biopsy was taken from the posterior ridge area. All patients were followed up 1 week post-surgery. In DS participants, two punch biopsies were taken: a diseased sample from the palatal area that was the most severe clinical area, and a second biopsy from a relatively less-affected area of inflammation, sampling the palatal site that was most normal in appearance. All biopsies extended to the periosteum and included a full-thickness mucosal sample from periosteum to keratinized epithelium.
Results
Salivary flow rates
There was no significant difference in the rate of stimulated or unstimulated salivary flow comparing healthy individuals to DS patients (Table 2).
Table 2.
Flow rate of unstimulated and stimulated whole saliva in control and DS participants
| Participants | Salivary flow (ml/min) UWS (mean ± SD) |
SWS (mean ± SD) |
|---|---|---|
| Control (n = 17) | 0.5 ± 0.23 | 1.43 ± 0.57 |
| DS type II (n = 8) | 0.5 ± 0.15 | 1.14 ± 0.43 |
| DS type III (n = 7) | 0.55 ± 0.23 | 1.34 ± 0.57 |
| P value (ANOVA) | 0.84 | 0.46 |
| DS combined (n = 15) | 0.53 ± 0.19 | 1.23 ± 0.5 |
| P value (unpaired T-test) | 0.71 | 0.31 |
- UWS: unstimulated whole saliva collected for 5 minutes.
- SWS: stimulated whole saliva collected by chewing folded strip of paraffin for 2 minutes.
- Hyposalivation: unstimulated saliva rate of less than 0.01 ml/min, and stimulated saliva rate of less than 0.10 ml/min.
Xerostomia symptoms
There was no significant difference in the frequency of reported dry mouth comparing DS to control patients; however, among all patients there were significant associations between symptomology and flow and wetness measures. A significant correlation was found between unstimulated salivary flow rate and perceived “Rate the dryness of your tongue” (r2 = 0.36, p = 0.0405). There was also a significant association between stimulated salivary flow rate and “Rate the level of your thirst” (r2 = 0.51, p = 0.0028). All other correlations were not significant and were in the range of (-0.23 to 0.10). For questions #3, 13, and 14 there were no significant associations between the reported symptoms and the flow rate of both unstimulated and stimulated saliva.
Cytology
According to the cytology results of the palatal mucosal swabs (Fig 2, Table 3), it was noticed that type II and type III DS are associated with higher scores reflecting inflammatory cells, bacteria, and fungal morphotypes. Inflammatory cells in the palatal mucosa showed a significant difference (P = 0.02) between DS and control participants. It is noteworthy that fungal forms were often found in control participants. There was no statistically significant difference in the cytology of the vestibular and tongue swabs comparing DS type II or type III to health; however, the vestibular area and the tongue as well as the palate had a trend for more prevalent fungal forms in DS patients.
Figure 2.
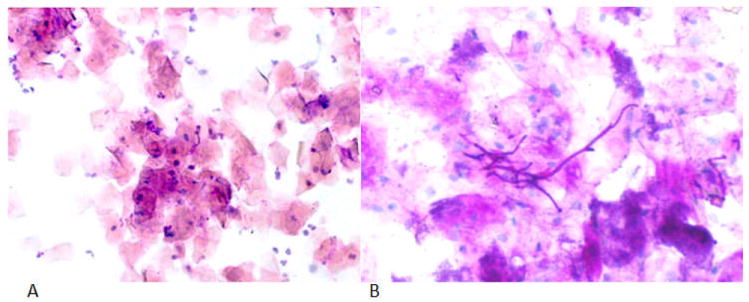
PAS exfoliative cytology representative slides. (A) a healthy control benign smear; oral cytologic smear of palatal mucosa showing typical squamous cells and scattered chronic inflammatory cells. (10 X), (B) DS fungal hyphae; oral cytologic smear from palatal mucosa showing candidal hyphae. (40X).
Table 3.
PAS exfoliative cytology results for palatal and vestibular area and tongue
| Palatal area | Vestibular area and tongue | |||||
|---|---|---|---|---|---|---|
| Healthy | Diseased | p-value | Healthy | Diseased | p-value | |
| Adequate (Yes) | 12 (44.4%) | 15 (55.6%) | 17 (53.1%) | 15 (46.9%) | ||
| No | 5 (100.0%) | 0 (0.0%) | 0.02 | .. | .. | .. |
| Bacteria (Yes) | 6 (40.0%) | 9 (60.0%) | 12 (46.2%) | 14 (53.9%) | ||
| No | 11 (64.7%) | 6 (35.3%) | 0.16 | 5 (83.3%) | 1 (16.7%) | 0.10 |
| Inflammatory cells (Yes) | 0 (0.0%) | 4 (100.0%) | 3 (37.5%) | 5 (62.5%) | ||
| No | 17 (60.7%) | 11 (39.3%) | 0.02* | 14 (58.3%) | 10 (41.7%) | 0.31 |
| Fungal spores (Yes) | 5 (35.7%) | 9 (64.3%) | 12 (46.2%) | 14 (53.9%) | ||
| No | 12 (66.7%) | 6 (33.3%) | 0.08 | 5 (83.3%) | 1 (16.7%) | 0.10 |
| Fungal hyphae (Yes) | 4 (40.0%) | 6 (60.0%) | 7 (43.8%) | 9 (56.3%) | ||
| No | 13 (59.1%) | 9 (40.9%) | 0.32 | 10 (62.5%) | 6 (37.5%) | 0.29 |
| Atypical cells (Yes) | 0 (0.0%) | 1 (100.0%) | .. | .. | ||
| No | 17 (54.8%) | 14 (45.2%) | 0.28 | 17 (53.1%) | 15 (46.9%) | .. |
| Score | ||||||
| Inadequate cell sample | 6 (100.0%) | 0 (0.0%) | .. | .. | ||
| Benign smear | 4 (57.1%) | 3 (42.9%) | 4 (100.0%) | 0 (0.0%) | ||
| Bacteria only | 2 (50.0%) | 2 (50.0%) | 1 (50.0%) | 1 (50.0%) | ||
| Benign inflammatory cells | 0 (0.0%) | 1 (100.0%) | .. | .. | ||
| Bacteria plus inflammatory cells | .. | .. | .. | .. | ||
| Fungal spores | 1 (25.0%) | 3 (75.0%) | 5 (50.0%) | 5 (50.0%) | ||
| Fungal organisms | 4 (40.0%) | 6 (60.0%) | 0.13 | 7 (46.6%) | 8 (53.3%) | 0.28 |
| Score dichotomized (fungal) | 5 (35.7%) | 9 (64.3%) | 12 (48.0%) | 13 (52.0%) | ||
| No fungal | 12 (66.7%) | 6 (33.3%) | 0.08 | 5 (83.3%) | 1 (16.7%) | 0.12 |
Statistically significant (P < 0.05)
Cultivable C. albicans levels in saliva and on denture and mucosal surfaces
In the saliva, C. albicans was detected in 80% (12/15) of the DS participants, but only 41.2% (7/17) of the control participants (p = 0.03, Chi Square). There was no greater detection rate of C. albicans in severe (type III) DS as compared to mild (type II) DS. In the dentures, C. albicans was present in 73.2% of dentures sampled from DS participants and only in 11.8% of dentures sampled from healthy individuals (p = 0.002), Chi Square. In the mucosal swabs there was no significant difference in C. albicans counts between diseased and healthy mucosa (p = 0.2). There was a high degree of concordance between the presence of C. albicans in the saliva vs. that detectible on the denture (r2 = 0.31, p = 0.0007). C. albicans prevalence by location comparing saliva vs. healthy mucosa was not significant (p = 0.059), nor was it significant when compared to diseased mucosa (p = 0.79). Among participants with detectible C. albicans, the level (CFU) of C. albicans within the saliva tended to be higher among DS participants, compared to healthy controls, but the difference was not statistically significant (Fig 3). When C. albicans was present, the mean salivary level of log CFU was 5.03 in DS and 4.4 in health. This difference (0.63) indicates that the mean level was 4.3-fold higher in the saliva of participants with DS when detected (Fig 3). Non-albicans Candida was also significantly detected, especially in DS patients. Table 4 shows the prevalence of both C. albicans and non-albicans in all patients.
Figure 3.
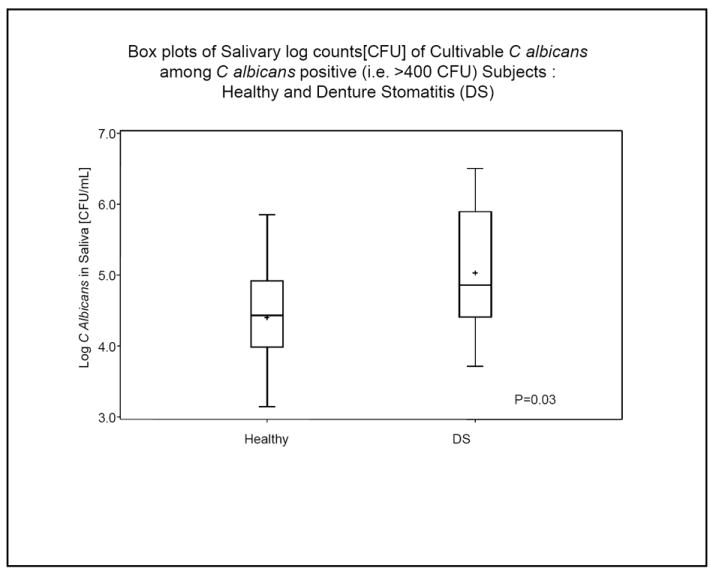
Mean log C. albicans counts in saliva among DS and control participants. The boxplot indicates the log CFU C albicans / mL of saliva. The plot shows median, upper, and lower 25th percentile range (box boundaries) and range as outer markings. The plus sign shows the mean log values.
Table 4.
Prevalence of detectable C. Albicans and non-albicans in saliva, denture, and mucosal surfaces for control and DS participants. The table indicates the number of patients positive for Candida [albicans and non-albicans] and the percent positive within the disease category. The p-values are chi-square statistics between the control and the DS groups.
| Saliva (N = 32) | Denture (N = 32) | Unaffected mucosa (N = 32) | Affected mucosa (N = 15) | |||||
|---|---|---|---|---|---|---|---|---|
| C. albicans | Non-albicans | C. albicans | Non-albicans | C. albicans | Non-albicans | C. albicans | Non-albicans | |
| Control (N = 17) | 7 (41.2%) | 7 (41.2%) | 2 (11.8%) | 8 (47.1%) | 1 (5.9%) | 7 (41.18%) | ||
| DS type II (N = 8) | 7 (87.5%) | 0 (0.00) | 6 (75.0%) | 0 (0.00%) | 2 (25.0%) | 2 (25.0%) | 2 (25.0%) | 3 (37.5%) |
| DS type III (N = 7) | 5 (71.4%) | 0 (0.00) | 5 (71.4%) | 2 (28.6%) | 2 (28.6%) | 4 (57.1%) | 4 (57.1%) | 3 (42.9%) |
| Total (N,%) | 19 (59.4%) | 7 (21.9%) | 13 (40.6%) | 10 (31.3%) | 5 (15.6%) | 13 (40.6%) | 6 (40.0%) | 6 (40.0%) |
| P values | 0.03* | 0.02* | 0.002* | 0.06* | 0.27 | 0.45 | 0.20 | 0.83 |
Statistically significant (P < 0.05)
Discussion
Role of saliva
In healthy denture wearers without a systemic condition, saliva is known to be an important etiological factor in the development of DS. In this study, there was no difference in the frequency of reported dry mouth, or in the rate of stimulated or unstimulated salivary flow comparing control individuals to DS patients. Note also that most of the participant-reported symptoms of dry mouth did not correlate with saliva flow. Only three questions (out of 14) in the xerostomia questionnaires had any statistical significance. Torres et al9 found that 67.9% of the individuals with xerostomia were colonized by Candida spp.; however, the difference between these patients and those without xerostomia was not statistically significant. Närhi et al8 found significantly higher counts of yeasts in individuals with salivary flow rates below normal. According to Pereira-Cenci et al36 patients with low or impaired salivary flow and/or composition presented higher Candida species counts when compared with saliva from patients with normal salivary flow. Hibino et al37reported that both stimulated and unstimulated salivary flow rates of the non-carriers were higher than the carriers, although the difference was not statistically significant.
In their study to determine risk factors associated with oral candidiasis onset and chronic maintenance, Campisi et al38 found both denture wearing and xerostomia to be local risk factors according to their analysis. The sample size in our study is too small to refute or resolve any of these conflicting findings. Our results, however, suggest that at least in healthy denture wearers, DS can develop with normal saliva flow. One key issue is that the typical assessments of salivary function from a physiological perspective (e.g., salivary flow rate, mucosal wetness, xerostomia symptoms) may not be associated with the antifungal capacity of the saliva. Clearly, future studies intending to examine the role of salivary immunoglobulins (e.g., sIgA) and specific anti-fungal components, such as lactoferrin, histatin-5, lysozyme, and histidine-rich polypeptides in recoverable yeast counts, in addition to physiological assessments, would be informative.
Denture fitting
In this study, the fit of the dentures does not appear to be a strong contributing factor for DS in healthy denture wearers. There was no statistical difference in Kapur index between DS and control subjects (Table 1); however, it is important to point out that the average Kapur index for the control patients was in the middle of the fair range, whereas the average index for the DS patients was at the cutoff between the fair to poor range. The small sample size limits our interpretation of the contribution of denture fit to DS in healthy patients, and it is possible that this factor may be statistically significant in a larger sample size.
Role of Candida
In this study, C. albicans was approximately twice as likely to be present in the saliva of DS patients (80%) as compared to control patients (35.3%); however, there was no clear trend for the CFU levels of salivary C. albicans to show a gradient of higher numbers associated with the transition from type II to type III DS. When C. albicans was detected in healthy or DS participants, the level within the saliva was found to be 4.3-fold higher among DS participants.
A strong relationship between DS and the presence of Candida in saliva has been reported previously.39,40 On the other hand, patients with Candida in saliva may not develop DS.41 This is in agreement with our findings. Since the frequency of detection of C. albicans is lower on the denture (11.8%) than in the saliva (41.2%) of healthy patients, it would appear that saliva and/or mucosa represents the reservoir of infectious agent in these healthy individuals and that the denture is less frequently colonized with C. albicans. According to Pires et al,42 the clinical resolution of DS was not related to the levels of Candida in saliva and, furthermore, a decrease of Candida counts in saliva usually is not followed by clinical improvement of DS.
In our study, there was a significant difference in the prevalence of detectible C. albicans counts from denture surface between DS and control participants (p = 0.03). C. albicans was 20.6-fold more likely to be cultured from the denture in DS patients than in healthy ones (i.e., 11/15 vs. 2/17, Table 4). If we examine the levels of C. albicans cultured from dentures there was a non-significant trend for higher denture counts among those with DS. Grouping the denture counts for healthy patients into three categories as no growth, [1+, 2+], and [3+, 4+] resulted in a distribution of 41.2%, 47.1%, and 11.7%, respectively. Similarly for denture stomatitis samples the distribution was 26.7%, 40%, and 33.3% (p = 0.18), suggesting the trend toward higher denture counts of C. albicans in DS participants. The notion that greater numbers of Candida spp. were recovered from smears prepared from the fitting surfaces of the dentures than from those on the palatal mucosa has been known since the 1970s.43,44 It has been shown that C. albicans colonies were recovered more frequently from the tissue fitting surface of the acrylic resin denture than from the corresponding palatal mucosa in DS patients.40 However, according to Radford et al,45 it is difficult to attribute the etiology of the condition entirely to the presence of C. albicans in denture plaque, as they found in their review that methods of sample collection accounted for individual variation, since palatal imprints yielded 55% of participants with yeast present, denture plaque sampling 80%, and saliva sampling 95%. Interestingly, in our study we found a high degree of concordance between the presence of C. albicans in the saliva vs. that detectible on the denture (r2 = 0.31, p = 0.0007). It was also noticed that non-albicans species are more prevalent in control patients, especially upon comparing prevalence in the unaffected mucosa in these individuals; however, whether non-albicans presence in patient’s saliva or mucosa is associated with any protective effect cannot be concluded from this small cohort.
Exfoliative cytology findings in DS
Reports have been variable on whether the inflammation associated with denture stomatitis is secondary to denture trauma or if it is simply a result of candidal infection. According to Barbeau et al,1 the presence of yeasts on the denture in denture-related stomatitis is probably linked to extensive inflammation. Considering the hypothesis that inflammation could be present before Candida colonization, this could explain the variable results in the treatment of denture-related stomatitis with antifungal treatment alone.1
According to Edgerton and Levine,46 stomatitis pathogenesis can occur through two separate mechanisms. They state, “Alterations in the composition of pellicle formed in stomatitis conditions, such as degradation of pellicle components, may directly promote colonization of C. albicans on “stomatitis” pellicle. Alternatively, C. albicans may be a secondary colonizer or may require bacterial cell products to stimulate adhesion, as has been shown with in vitro studies of Streptococcus mutans and C. albicans.47 In this case, altered pellicle deposition in the disease process may initially enhance adhesion of other bacteria, which would subsequently promote adhesion of Candida.”46
Ritchie et al48 found that bacteria, leukocytes, and yeast hyphae could be detected in all patients even when cultures were negative. Examination of PAS-stained smears prepared from denture scrapings showed higher number of yeast cells in DS patients by Budtz-Jorgensen et al13 as well; however, in our study, the presence of hyphae, as determined from the PAS smear from tissue was not pathognomic for disease.
Conclusions
The overall healthy status of the patient population in this study provides a unique cohort for the study of three important DS etiological factors including candida, denture, and saliva. DS in healthy patients appears to have a unique pathogenesis different from other oral candidiasis. We selected participants to limit the influence of systemic conditions and others (e.g., medications) that may compromise immunity and saliva factors. In this study, we confirm that DS is perhaps a result of the denture acting as a reservoir of candidal organism. Normal saliva in healthy patients appears to be the medium for candidal organisms’ movement between the denture and tissue, but plays a limited role in DS development. Denture fitting and xerostomic factors are perhaps not primary factors, at least in this same sample size, healthy, denture-wearing population. The results also suggest that unlike other oral candidiasis, there is no association between the fungal presence in the tissue and the clinical symptoms of denture stomatitis. Therefore, treatments for DS should first focus on sanitization of an existing denture or fabrication of a new denture, rather than antifungal treatment in healthy denture wearers.
Acknowledgments
The authors thank C. R. Mack for proofreading this manuscript.
This work was supported by NIH research grants UL-1-RR025746 and R21HL092338. GSK Consumer Healthcare, and the American College of Prosthodontists Educational Fund.
The funder has no role in manuscript preparation or submission of the manuscript; however, Authors ZGL and LG are, respectively, former and current employees of GSK, one of the funders.
Appendix 1
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
References
- 1.Barbeau J, Seguin J, Goulet JP, et al. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:51–59. doi: 10.1067/moe.2003.44. [DOI] [PubMed] [Google Scholar]
- 2.Budtz-Jorgensen E. Etiology, pathogenesis, therapy, and prophylaxis of oral yeast infections. Acta Odontol Scand. 1990;48:61–69. doi: 10.3109/00016359009012735. [DOI] [PubMed] [Google Scholar]
- 3.Cumming CG, Wight C, Blackwell CL, et al. Denture stomatitis in the elderly. Oral Microbiol Immunol. 1990;5:82–85. doi: 10.1111/j.1399-302x.1990.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Glass RT, Belobraydic KA. Dilemma of denture contamination. J Okla Dent Assoc. 1990;81:30–33. [PubMed] [Google Scholar]
- 5.Webb BC, Thomas CJ, Willcox MD, et al. Candida-associated denture stomatitis. Aetiology and management: a review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 6.Webb BC, Thomas CJ, Willcox MD, et al. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J. 1998;43:160–166. doi: 10.1111/j.1834-7819.1998.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramage G, Tomsett K, Wickes BL, et al. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Narhi TO, Ainamo A, Meurman JH. Salivary yeasts, saliva, and oral mucosa in the elderly. J Dent Res. 1993;72:1009–1014. doi: 10.1177/00220345930720060301. [DOI] [PubMed] [Google Scholar]
- 9.Torres SR, Peixoto CB, Caldas DM, et al. Clinical aspects of Candida species carriage in saliva of xerotomic subjects. Med Mycol. 2003;41:411–415. [PubMed] [Google Scholar]
- 10.Jeganathan S, Lin CC. Denture stomatitis--a review of the aetiology, diagnosis and management. Aust Dent J. 1992;37:107–114. doi: 10.1111/j.1834-7819.1992.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 11.Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 12.Cross LJ, Williams DW, Sweeney CP, et al. Evaluation of the recurrence of denture stomatitis and Candida colonization in a small group of patients who received itraconazole. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:351–358. doi: 10.1016/j.tripleo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Budtz-Jorgensen E, Theilade E, Theilade J. Quantitative relationship between yeast and bacteria in denture-induced stomatitis. Scand J Dent Res. 1983;91:134–142. doi: 10.1111/j.1600-0722.1983.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 14.Koopmans AS, Kippuw N, de Graaff J. Bacterial involvement in denture-induced stomatitis. J Dent Res. 1988;67:1246–1250. doi: 10.1177/00220345880670091901. [DOI] [PubMed] [Google Scholar]
- 15.Pesci-Bardon C, Fosse T, Madinier I, et al. In vitro new dialysis protocol to assay the antiseptic properties of a quaternary ammonium compound polymerized with denture acrylic resin. Lett Appl Microbiol. 2004;39:226–231. doi: 10.1111/j.1472-765X.2004.01569.x. [DOI] [PubMed] [Google Scholar]
- 16.Redding S, Bhatt B, Rawls HR, et al. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:669–672. doi: 10.1016/j.tripleo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Sachdeo A, Haffajee AD, Socransky SS. Biofilms in the edentulous oral cavity. J Prosthodont. 2008;17:348–356. doi: 10.1111/j.1532-849X.2008.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Coco BJ, Bagg J, Cross LJ, et al. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol Immunol. 2008;23:377–383. doi: 10.1111/j.1399-302X.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 19.Salerno C, Pascale M, Contaldo M, et al. Candida-associated denture stomatitis. Med Oral Patol Oral Cir Bucal. 2011;16:e139–143. doi: 10.4317/medoral.16.e139. [DOI] [PubMed] [Google Scholar]
- 20.Dahlen G, Blomquist S, Carlen A. A retrospective study on the microbiology in patients with oral complaints and oral mucosal lesions. Oral Dis. 2009;15:265–272. doi: 10.1111/j.1601-0825.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- 21.Bilhan H, Sulun T, Erkose G, et al. The role of Candida albicans hyphae and Lactobacillus in denture-related stomatitis. Clin Oral Investig. 2009;13:363–368. doi: 10.1007/s00784-008-0240-6. [DOI] [PubMed] [Google Scholar]
- 22.Leigh JE, Steele C, Wormley F, et al. Salivary cytokine profiles in the immunocompetent individual with Candida-associated denture stomatitis. Oral Microbiol Immunol. 2002;17:311–314. doi: 10.1034/j.1399-302x.2002.170508.x. [DOI] [PubMed] [Google Scholar]
- 23.Nikawa H, Jin C, Makihira S, et al. Susceptibility of Candida albicans isolates from the oral cavities of HIV-positive patients to histatin-5. Prosthet Dent. 2002;88:263–267. doi: 10.1067/mpr.2002.127907. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Archilla A, Urquia M, Cutando A, et al. Denture stomatitis: quantification of interleukin-2 production by mononuclear blood cells cultured with Candida albicans. J Prosthet Dent. 1996;75:426–431. doi: 10.1016/s0022-3913(96)90036-0. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi Y, Ansai T, Awano S, et al. Salivary levels of hyaluronic acid in female patients with dry mouth compared with age-matched controls: a pilot study. Biomed Res. 2009;30:63–68. doi: 10.2220/biomedres.30.63. [DOI] [PubMed] [Google Scholar]
- 26.Wolff M, Kleinberg I. Oral mucosal wetness in hypo- and normosalivators. Arch Oral Biol. 1998;43:455–462. doi: 10.1016/s0003-9969(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima C, Matsuse H, Saeki S, et al. Salivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroid. J Asthma. 2005;42:601–604. doi: 10.1080/02770900500216259. [DOI] [PubMed] [Google Scholar]
- 28.Hagewald S, Bernimoulin JP, Kottgen E, et al. Salivary IgA subclasses and bacteria-reactive IgA in patients with aggressive periodontitis. J Periodontal Res. 2002;37:333–339. doi: 10.1034/j.1600-0765.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Tanida T, Okamoto T, Okamoto A, et al. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J Oral Pathol Med. 2003;32:586–594. doi: 10.1034/j.1600-0714.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 30.Newton AV. Denture sore mouth. A possible etiology. Br Dent J. 1962;112:357–360. [Google Scholar]
- 31.Kapur KK. A clinical evaluation of denture adhesives. J Prosthet Dent. 1967;18:550–558. doi: 10.1016/0022-3913(67)90221-1. [DOI] [PubMed] [Google Scholar]
- 32.Kohler PF, Winter ME. A quantitative test for xerostomia. The Saxon test, an oral equivalent of the Schirmer test. Arthritis Rheum. 1985;28:1128–1132. doi: 10.1002/art.1780281008. [DOI] [PubMed] [Google Scholar]
- 33.Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79:1652–1658. doi: 10.1177/00220345000790090301. [DOI] [PubMed] [Google Scholar]
- 34.Bergdahl M. Salivary flow and oral complaints in adult dental patients. Community Dent Oral Epidemiol. 2000;28:59–66. doi: 10.1034/j.1600-0528.2000.280108.x. [DOI] [PubMed] [Google Scholar]
- 35.Beck JD, Elter JR, Heiss G, et al. Relationship of periodontal disease to carotid artery intimamedia wall thickness: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21:1816–1822. doi: 10.1161/hq1101.097803. [DOI] [PubMed] [Google Scholar]
- 36.Pereira-Cenci T, Del Bel Cury AA, Crielaard W, et al. Development of Candida-associated denture stomatitis: new insights. J Appl Oral Sci. 2008;16:86–94. doi: 10.1590/S1678-77572008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibino K, Samaranayake LP, Hagg U, et al. The role of salivary factors in persistent oral carriage of Candida in humans. Arch Oral Biol. 2009;54:678–683. doi: 10.1016/j.archoralbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Campisi G, Panzarella V, Matranga D, et al. Risk factors of oral candidosis: a twofold approach of study by fuzzy logic and traditional statistic. Arch Oral Biol. 2008;53:388–397. doi: 10.1016/j.archoralbio.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Berdicevsky I, Ben-Aryeh H, Szargel R, et al. Oral candida of asymptomatic denture wearers. Int J Oral Surg. 1980;9:113–115. doi: 10.1016/s0300-9785(80)80047-0. [DOI] [PubMed] [Google Scholar]
- 40.Webb BC, Thomas CJ, Willcox MD, et al. Candida-associated denture stomatitis. Aetiology and management: a review. Part 3. Treatment of oral candidosis. Aust Dent J. 1998;43:244–249. doi: 10.1111/j.1834-7819.1998.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J. 1998;185:380–384. doi: 10.1038/sj.bdj.4809821. [DOI] [PubMed] [Google Scholar]
- 42.Pires FR, Santos EB, Bonan PR, et al. Denture stomatitis and salivary Candida in Brazilian edentulous patients. J Oral Rehabil. 2002;29:1115–1119. doi: 10.1046/j.1365-2842.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- 43.Davenport JC. The oral distribution of candida in denture stomatitis. Br Dent J. 1970;129:151–156. doi: 10.1038/sj.bdj.4802540. [DOI] [PubMed] [Google Scholar]
- 44.Lamfon H, Al-Karaawi Z, McCullough M, et al. Composition of in vitro denture plaque biofilms and susceptibility to antifungals. FEMS Microbiol Lett. 2005;242:345–351. doi: 10.1016/j.femsle.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 46.Edgerton M, Levine MJ. Characterization of acquired denture pellicle from healthy and stomatitis patients. J Prosthet Dent. 1992;68:683–691. doi: 10.1016/0022-3913(92)90387-p. [DOI] [PubMed] [Google Scholar]
- 47.Branting C, Sund ML, Linder LE. The influence of Streptococcus mutans on adhesion of Candida albicans to acrylic surfaces in vitro. Arch Oral Biol. 1989;34:347–353. doi: 10.1016/0003-9969(89)90108-8. [DOI] [PubMed] [Google Scholar]
- 48.Ritchie GM, Fletcher AM, Main DM, et al. The etiology, exfoliative cytology, and treatment of denture stomatitis. J Prosthet Dent. 1969;22:185–200. doi: 10.1016/0022-3913(69)90246-7. [DOI] [PubMed] [Google Scholar]


