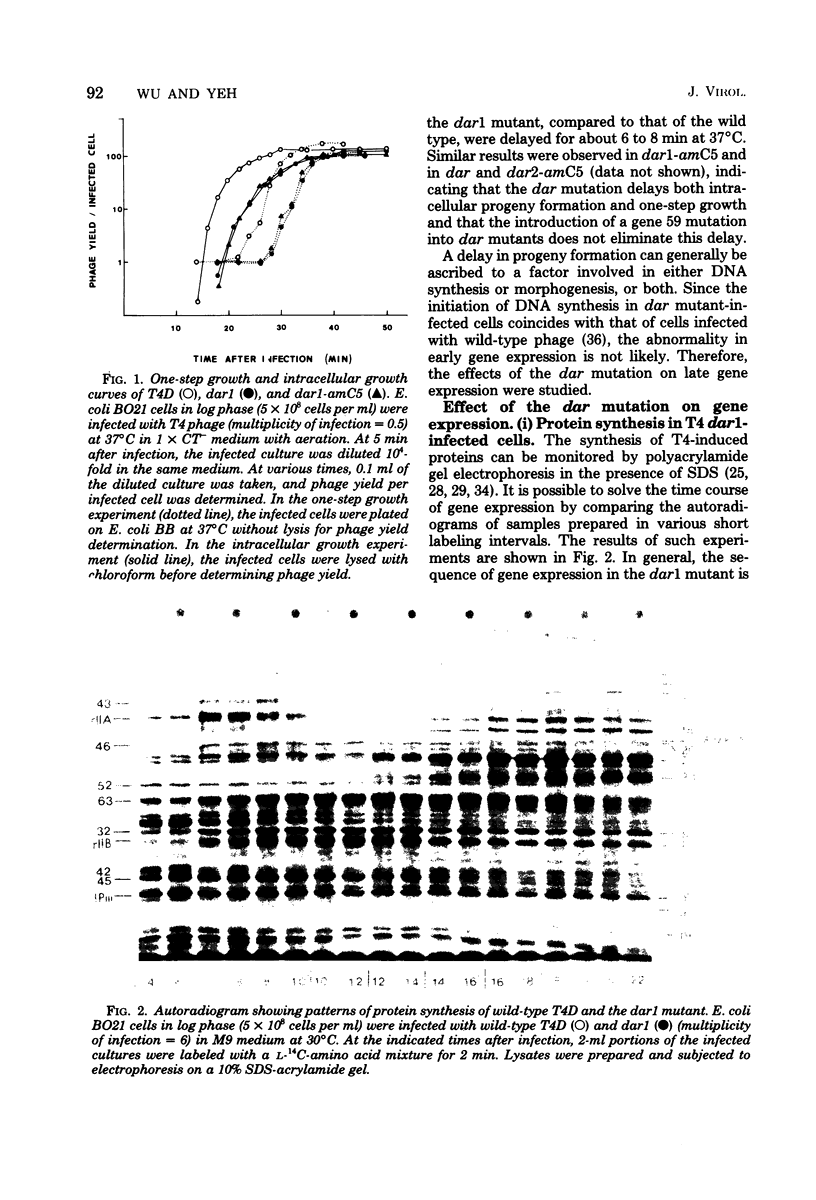
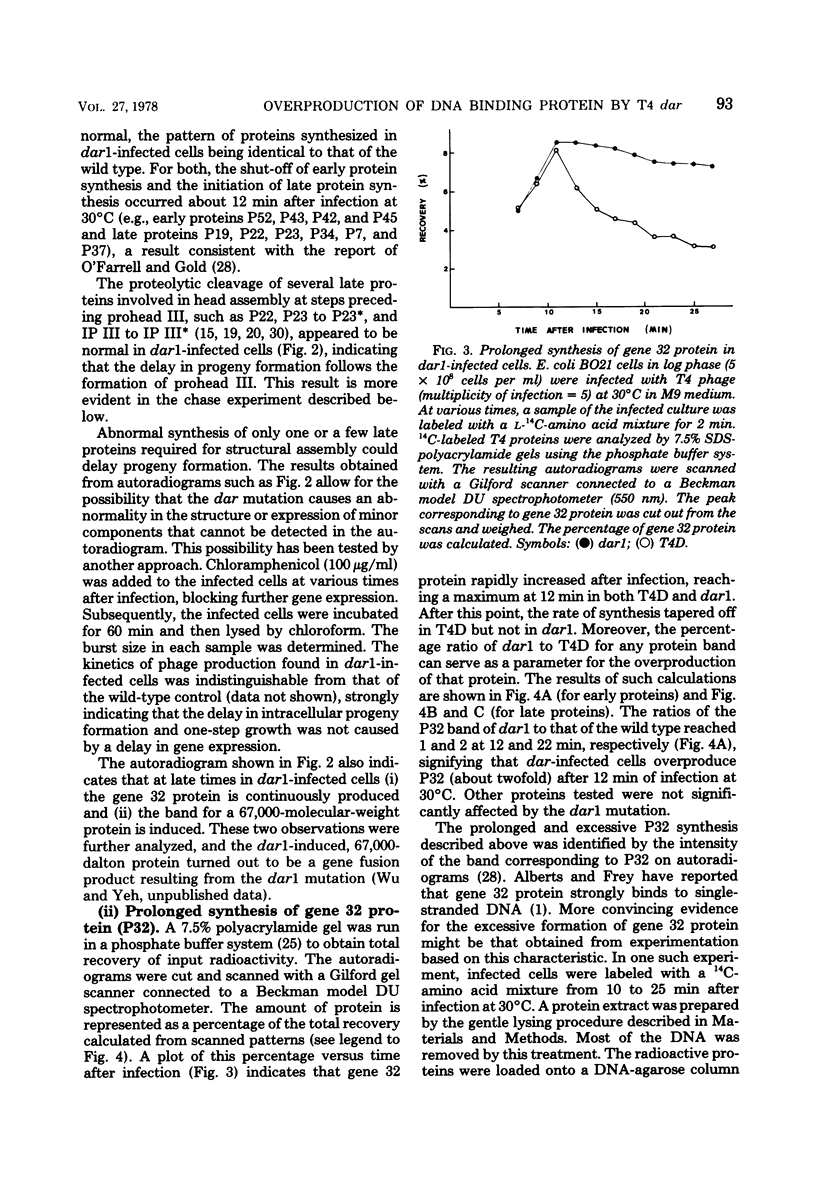
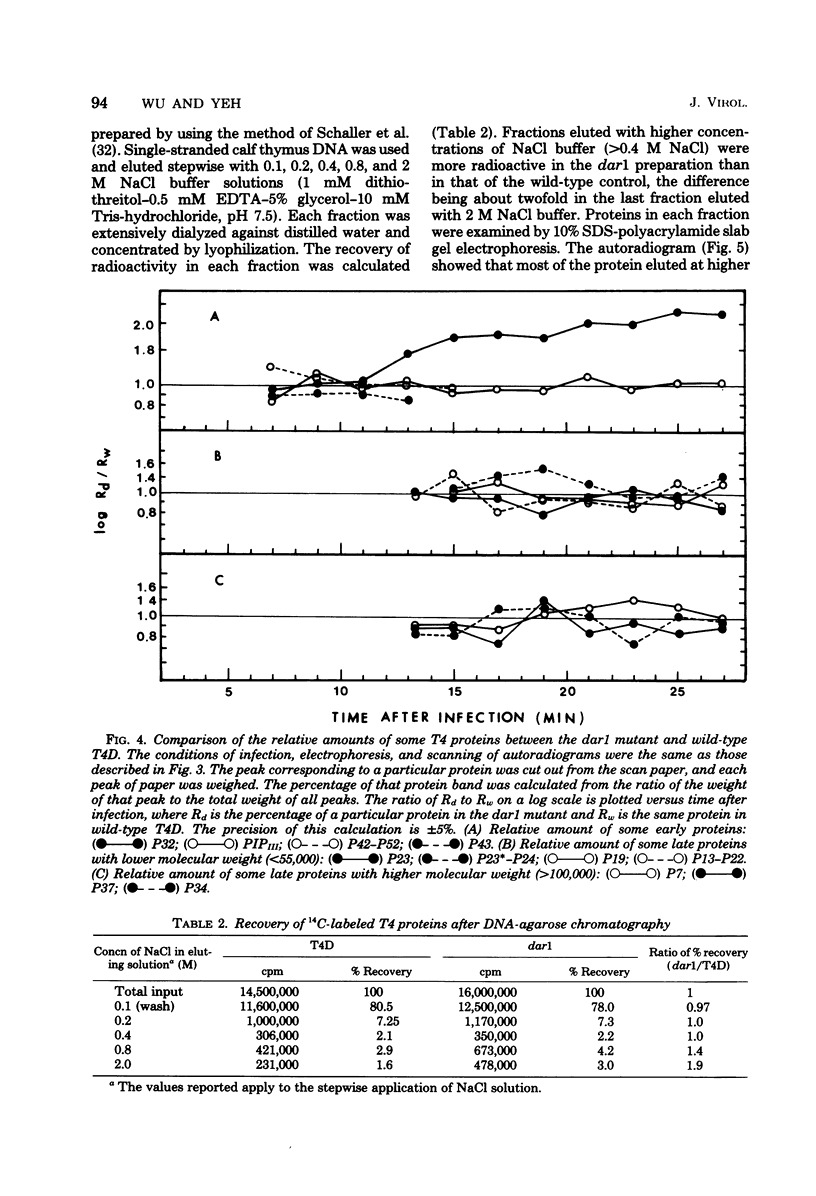
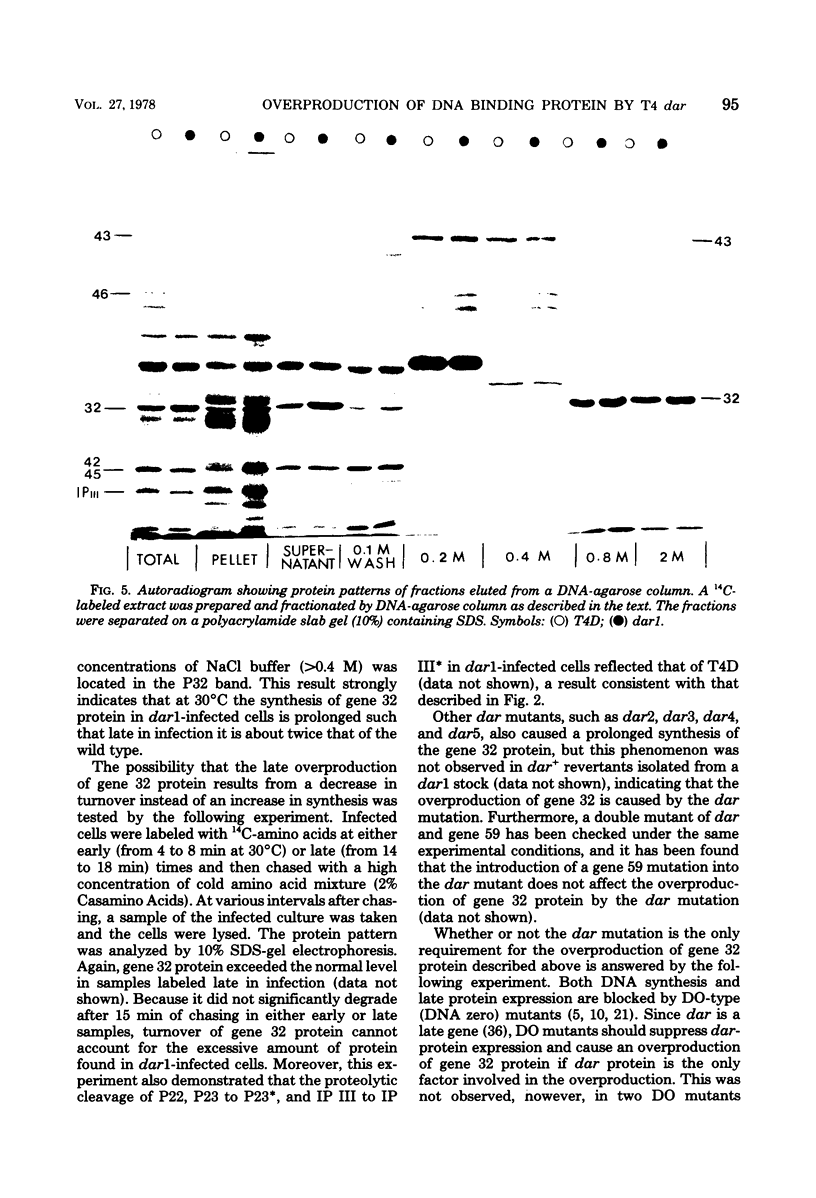
Abstract
We have previously shown that the arrested DNA synthesis of mutant defective in T4 phage gene 59 can be reversed by a mutation in dar. In this paper, we have examined the effect of the dar mutation on the kinetics of gene 32 protein (DNA binding protein) synthesis, DNA packaging, progeny formation, and several other porcesses. Several lines of evidence are presented showing that the regulation of synthesis of gene 32 protein is abnormal in dar 1-infected cells. In these cells, gene 32 protein, an early protein, is also expressed late in the infectious cycle. Our data also indicate that the packaging og DNA into T4 phage heads is delayed in dar mutant-infected cells, and this in turn results in a 6- to 8-min delay in intracellular progeny formation, although the synthesis of late proteins appears to be normal, as shown by gel electrophoresis. We have also studied the phenotypes of the double mutant dar-amC5 (gene 59). The increased sensitivity to hydroxyurea caused by a mutation in the dar gene can be alleviated by a second mutation in gene 59, but an increased sensitivity to UV irradiation caused by a mutation in gene 59 cannot be alleviated by a second mutation in the dar gene. Therefore, the double mutant still exhibits abnormalities in the repair of UV lesions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Beguin C. F. Degradation of T4 DNA synthesized in the absence of phage ligase. Virology. 1973 Apr;52(2):488–501. doi: 10.1016/0042-6822(73)90344-9. [DOI] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Structural aberrations in T-even bacteriophage. IV. Parameters of induction and formation of lollipops. J Virol. 1974 Jun;13(6):1368–1377. doi: 10.1128/jvi.13.6.1368-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Carlson K., Kozinski A. W. Nonreplicated DNA and DNA fragments in T4 r- bacteriophage particles: phenotypic mixing of a phage protein. J Virol. 1974 Jun;13(6):1274–1290. doi: 10.1128/jvi.13.6.1274-1290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K., Lorkiewicz Z. K., Kozinski A. W. Host-mediated repair of discontinuities in DNA from T4 bacteriophage. J Virol. 1973 Aug;12(2):310–319. doi: 10.1128/jvi.12.2.310-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Gold L., O'Farrell P. Z., Russel M. Regulation of gene 32 expression during bacteriophage T4 infection of Escherichia coli. J Biol Chem. 1976 Nov 25;251(22):7251–7262. [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Hamlett N. V., Berger H. Mutations altering genetic recombination and repair of DNA in bacteriophage T4. Virology. 1975 Feb;63(2):539–567. doi: 10.1016/0042-6822(75)90326-8. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D., Bowles M. G. Mutation to overproduction of bacteriophage T4 gene products. J Virol. 1974 Feb;13(2):428–438. doi: 10.1128/jvi.13.2.428-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. IV. Comparison of gene 16-, 17-, and 49-defective head structures. J Virol. 1972 Sep;10(3):545–554. doi: 10.1128/jvi.10.3.545-554.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. V. The role of DNA synthesis in maturation of an intermediate in head assembly. Virology. 1973 Feb;51(2):432–442. doi: 10.1016/0042-6822(73)90442-x. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Kozinski A. W., Litwin S. Molecular Recombination in T4 Bacteriophage Deoxyribonucleic Acid: III. Formation of Long Single Strands During Recombination. J Virol. 1970 Mar;5(3):368–380. doi: 10.1128/jvi.5.3.368-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Distances separating genetic markers in T4 DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Onorato L., Showe M. K. Gene 21 protein-dependent proteolysis in vitro of purified gene 22 product of bacteriophage T4. J Mol Biol. 1975 Mar 5;92(3):395–412. doi: 10.1016/0022-2836(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
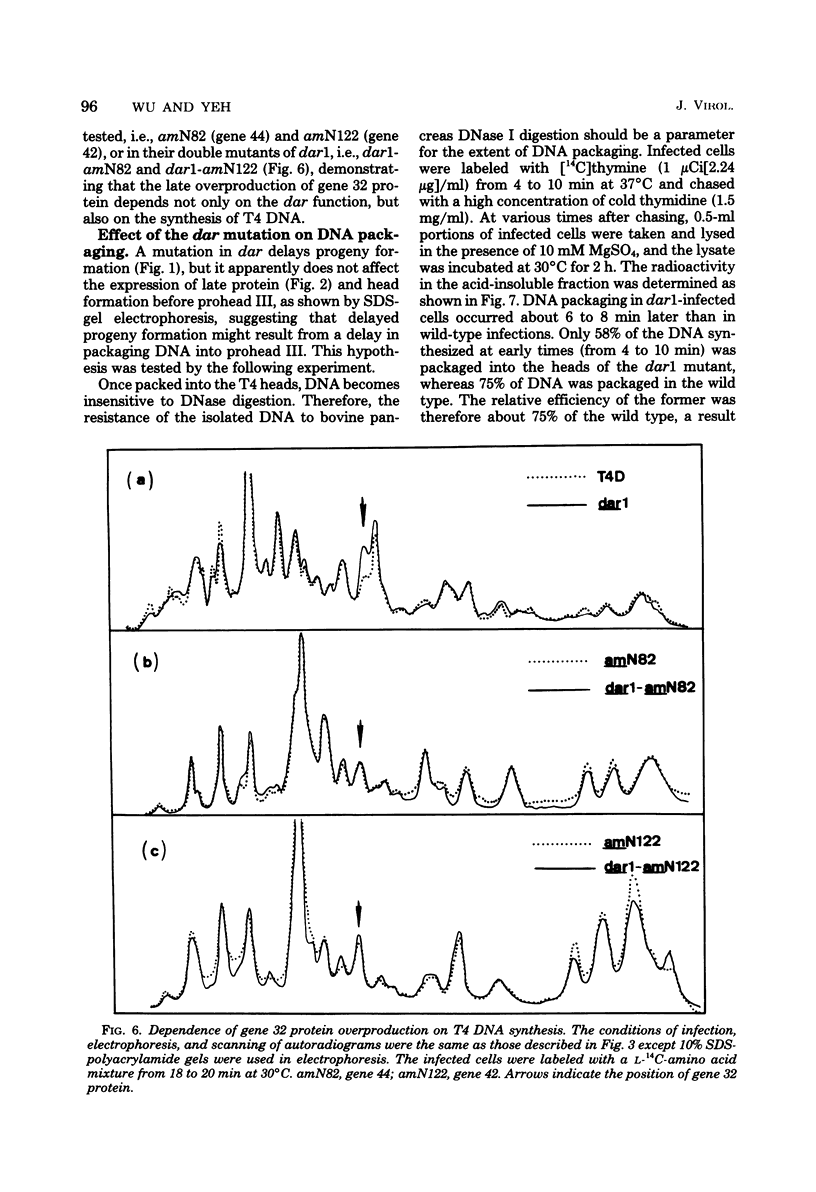
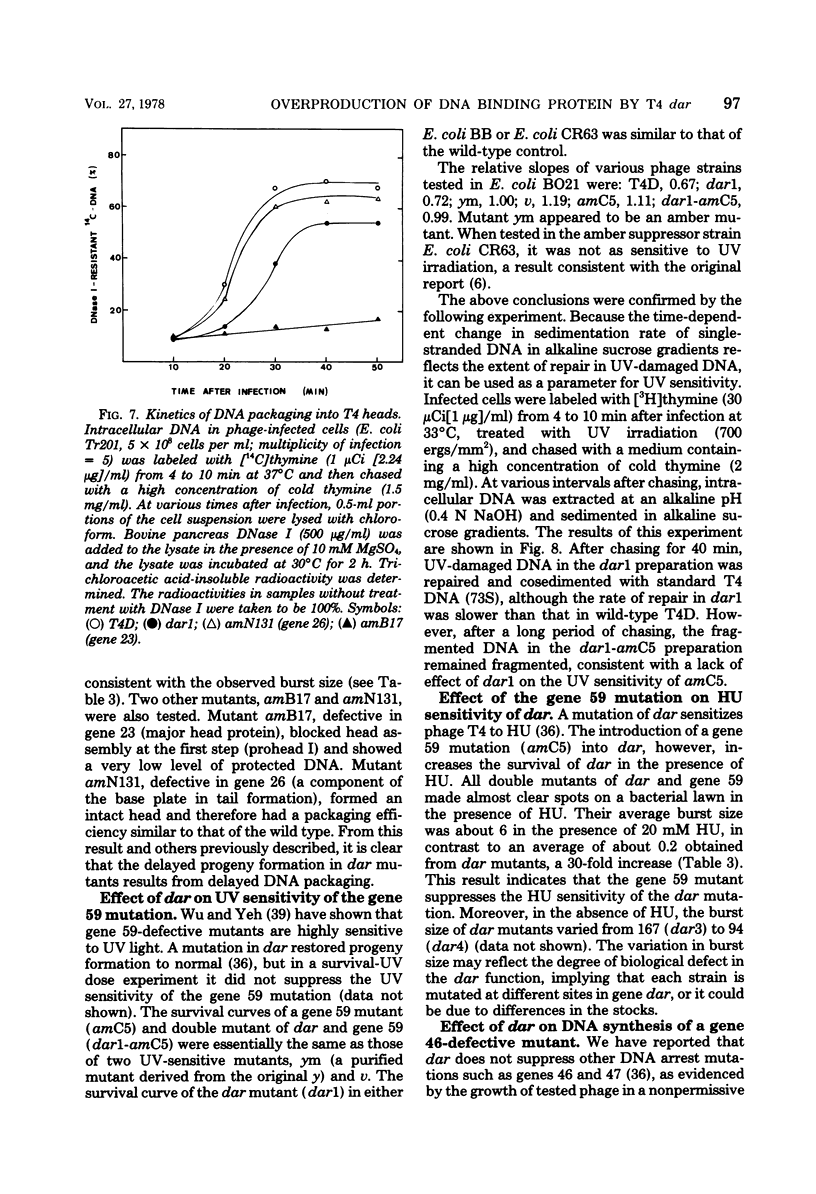
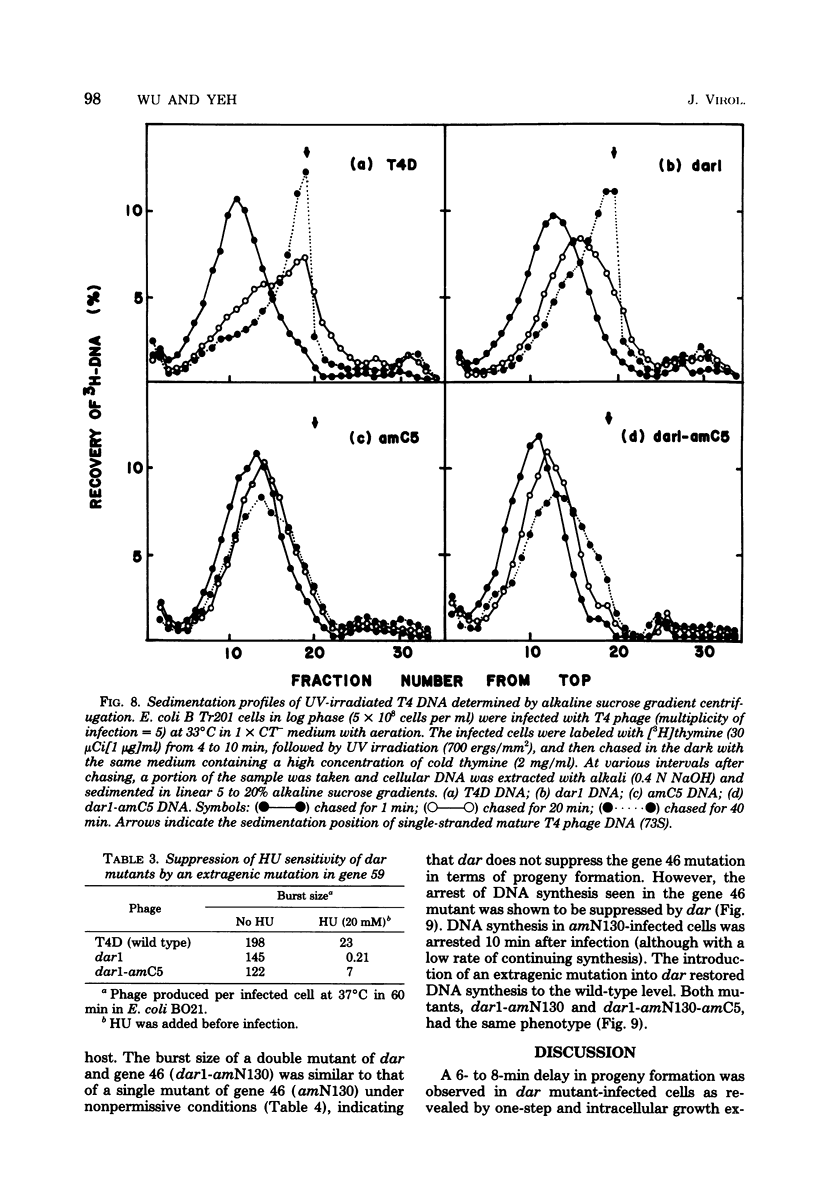
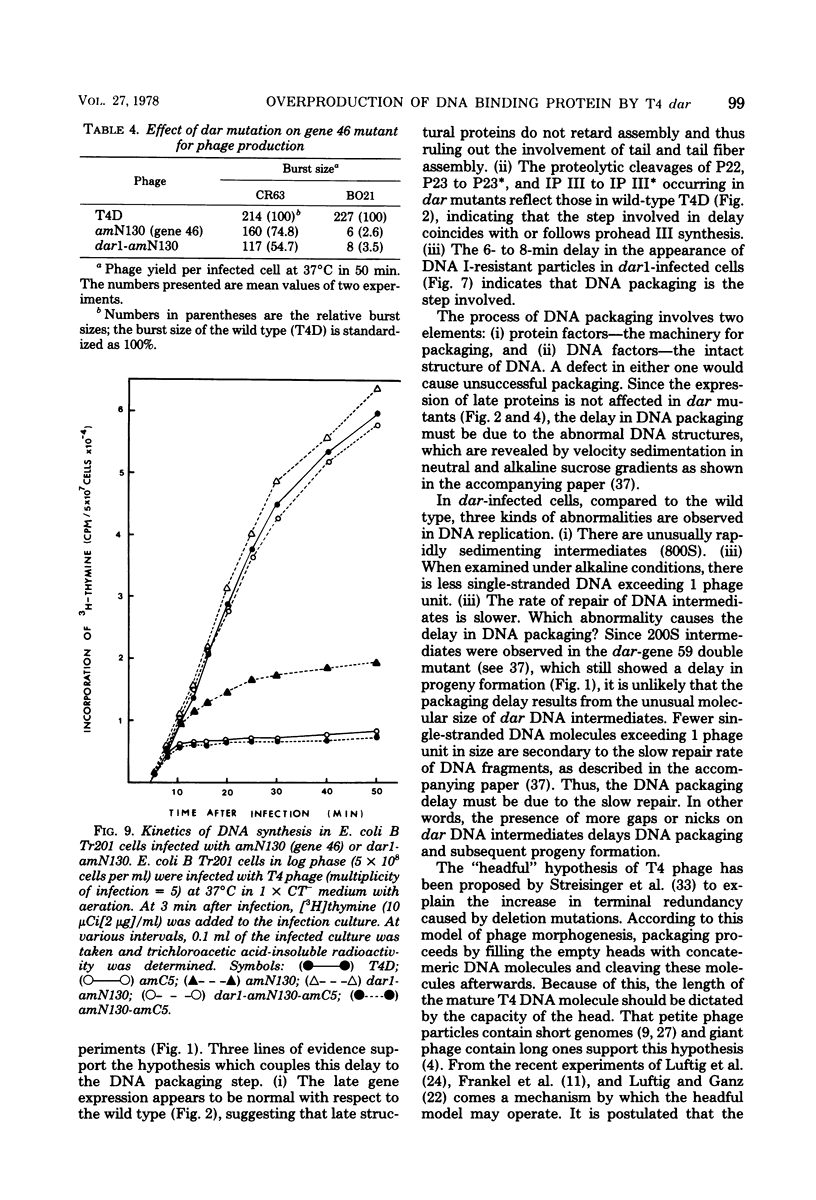
- Wu J. R., Yeh Y. C. New Late Gene, dar, Involved in DNA Replication of Bacteriophage T4 I. Isolation, Characterization, and Genetic Location. J Virol. 1975 May;15(5):1096–1106. doi: 10.1128/jvi.15.5.1096-1106.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. New late gene, dar, involved in the replication of bacteriophage T4 DNA. III. DNA replicative intermediates of T4 dar and a gene 59 mutant suppressed by dar. J Virol. 1978 Jul;27(1):103–117. doi: 10.1128/jvi.27.1.103-117.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. Requirement of a functional gene 32 product of bacteriophage T4 in UV, repair. J Virol. 1973 Oct;12(4):758–765. doi: 10.1128/jvi.12.4.758-765.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Wu J. L., Yeh Y. C. Role of gene 59 of bacteriophage T4 in repair of UV-irradiated and alkylated DNA in vivo. J Virol. 1975 Jul;16(1):5–16. doi: 10.1128/jvi.16.1.5-16.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Yeh Y. C. DNA arrested mutants of gene 59 of bacteriophage T4. II. Replicative intermediates. Virology. 1974 May;59(1):108–122. doi: 10.1016/0042-6822(74)90209-8. [DOI] [PubMed] [Google Scholar]




