Abstract
Purpose
To longitudinally investigate racial differences in serum adiponectin and leptin in European-American [EA] and African-American [AA] women in the overweight and weight-reduced states.
Methods
Sixty-two EA and 58 AA premenopausal women were weight-reduced from BMI 27–30 kg/m2 to BMI ≤24. Fasting serum adiponectin and leptin were determined; body composition and intra-abdominal adipose tissue [IAAT] were measured with dual-energy X-ray absorptiometry and computed tomography, respectively.
Results
In repeated-measures MANOVA there was a significant race-effect for IAAT and total fat mass; compared to AA women, EA women had higher IAAT and total fat mass [p<.0001 and p=0.027, respectively]. In the mixed-model for adiponectin that adjusted for IAAT, limb fat and total fat, race was significantly associated with adiponectin [p=0.046]. AA women had significantly lower adjusted adiponectin compared to EA women at baseline [7.67 [6.85, 8.60] versus 9.32 [8.34, 10.4] µg/ml, p<0.05] and following weight loss [9.75 [8.70, 10.9] versus 11.8 [10.6, 13.2] µg/ml, p<0.05]. In a mixed-model for leptin that adjusted for insulin, estradiol and fat mass, race was significantly associated with leptin [p<.0001]. African-American women had significantly higher adjusted leptin compared to EA women at baseline [24.7 [22.3, 27.4] versus 19.9 [18.1, 21.8] ng/dl, p<0.05] and following weight loss [11.7 [10.2, 13.3] versus 8.48 [7.50, 9.57] ng/dl, p<0.05].
Conclusions
Despite having a more favorable body fat distribution, AA women had lower adjusted adiponectin and higher leptin. Differences in body composition and fat distribution do not appear to be significant factors in explaining lower adiponectin and higher leptin in AA women.
INTRODUCTION
Adipose tissue is an active endocrine organ producing several hormones associated with disease risk. Specifically, the adipocyte-derived hormones adiponectin and leptin play pivotal roles in energy homeostasis and are important links between adiposity and risk for hyperinsulinemia, type 2 diabetes and metabolic syndrome. Adiponectin, produced exclusively by white adipose tissue, is the most abundant adipokine in the blood and concentrations of adiponectin are inversely associated adiposity [1]. Higher serum concentrations of adiponectin are considered to be protective and are positively associated with insulin sensitivity and glucose utilization [2, 3]. Conversely, lower adiponectin levels are seen in individuals with type 2 diabetes [4] and metabolic syndrome [5]. In contrast to adiponectin, leptin concentrations are positively correlated with body fat and associated with obesity-related insulin resistance [6, 7]. Hyperleptinemia, which occurs with obesity, has been reported to be independently associated with lower insulin-sensitivity [8].
As detailed in the recent Endocrine Society Scientific Statement on health disparities in endocrine disorders, African-American (AA) women have increased risk for metabolic disorders compared to European-American (EA) women independent of body weight [9]. Specifically, AA women tend to have lower insulin-sensitivity [10], higher acute response to glucose [11] and increased risk for type 2 diabetes [12] compared to European-Americans [EA]. Given, the role that adiponectin and leptin play in glucose homeostasis it is plausible that racial differences in these adipokines may exist. Indeed, previous studies have investigated racial/ethnic differences in adiponectin and leptin. Recent investigations have reported that AA children have lower serum adiponectin concentrations compared to EA children [13, 14]. Another study observed racial differences between postmenopausal AA and EA women for serum adiponectin [15]. A recent large epidemiological study provided additional evidence of racial differences in adiponectin and leptin in premenopausal and postmenopausal AA and EA women. In the SWAN study, Khan et al observed that AA women have significantly lower adiponectin and higher leptin relative to EA women independent of total fat mass and waist circumference [16].
These previous investigations have observed racial differences in adiponectin and leptin; however, most have been limited by their cross-sectional design. Further, studies in female populations have used BMI or total fat mass determined by bioelectrical impedance or waist circumference as measures of adiposity and may not have adequately adjust for body fat distribution [15,16]. Both limb fat and intra-abdominal adipose tissue (IAAT) have been shown to influence adiponectin and leptin concentrations [13, 14,17]. Because racial differences have been reported in body fat distribution it may be important to adjust for these covariates [18, 19, 20].
The purpose of this study was to longitudinally explore racial differences in circulating concentrations of adiponectin and leptin in healthy premenopausal AA and EA women after adjusting for body composition and body fat distribution in order to elucidate whether these potential ethnic differences could be latent underlying factors contributing to increased risk for metabolic disorders in AA women.
METHODS
Participants
Participants were premenopausal women enrolled in a weight loss study at the University of Alabama at Birmingham (UAB). Eligibility criteria included sedentary lifestyle (defined as exercising <1 time per week for the past year); having normal menstrual cycles; not taking oral contraceptives; negative for a history of eating disorder; normoglycemic (determined by 2-hour blood glucose following oral glucose load); non-smoker; not taking medications known to alter metabolism; and identified themselves as either EA or AA. Written informed consent was obtained from all participants prior to testing. All procedures and protocols pertaining to the study were approved by the UAB Institutional Review Board.
Study Intervention
This study was a diet and weight loss intervention to reduce BMI from 27–30 to BMI≤24. Participants were randomized to one of three weight loss groups: 1.) caloric restriction (CR), 2.) CR+ aerobic exercise (AE), or 3.) CR+ resistance training (RT), based on matching for age, race and body composition.
Protocol
At baseline, following 4-weeks of energy balance to ensure weight stability, body composition measurements and fasting blood were collected during the follicular phase of the menstrual cycle (within 10 days of menses). All blood collections were obtained in the morning following an overnight stay in the General Clinical Research Center (GCRC).
Participants were then provided meals that consisted of 800 kcal/day diet with 20–22% of energy as fat, 18–22 % as protein, and 58–62% from carbohydrate during the weight loss period. Additionally, participants randomized to the CR+AE and CR+RT groups performed supervised physical activity at a UAB exercise clinic. The aerobic exercise program consisted of fast walking or light jogging or stationary cycling for 40 minutes at 80% maximum heart-rate 3 days per week. Resistance training consisted of upper and lower body weight training at 80% maximum lifting 3 days per week.
All participants were weighed regularly until they reached BMI≤24 kg/m2. Subsequent to meeting the weight loss goal, participants again underwent 4 weeks of energy balance and weight stability. Body composition measurements and fasting serum were again collected during the follicular phase of the menstrual cycle following an overnight stay at the GCRC.
Body composition measurements
Body mass index (BMI) was calculated using weight in kilograms divided by height in meters squared. Dual-energy X-ray absorptiometry (DXA; GE-Lunar Prodigy software version 1.33) was used to assess total and regional body composition. Computed tomography scanning with HiLight/Advantage Scanner was used to determine IAAT. A 5 mm scan at the L4-L5 region was taken while subjects were in the supine position with arms above their head. Cross sectional area of adipose tissue was analyzed using density contour as we have previously described [21].
Hormones
Adiponectin, insulin and leptin were measured in duplicate using double-antibody radio-immunoassays (RIA) from Linco/Millipore [Billerica, MA]. The intra- and interassay coefficient of variation (CV) for adiponectin, insulin and leptin were 5.36% and 6.26%, 3.49% and 5.57%, and 5.0% and 5.6%, respectively. Estradiol was measured using an AIA-600 II immunoassay analyzer (TOSOH Bioscience, Toyoma, Japan). The mean intra- and interassay CV was 4.2%, 12.9%.
Statistical analyses
The mean and standard error are shown for body composition measures. Serum hormones and adipokines were non-normally distributed and were log-transformed. The geometric mean and 95% confidence interval (CI) are shown for these variables. Repeated-measures multivariate analysis of variance (MANOVA) was used to assess the change from baseline to follow-up for body composition measurements and serum analytes. Initial repeated-measures MANOVA analyses showed that there was no effect of weight loss method (CR alone or combined with aerobic or resistance training) on total fat mass, IAAT and adiponectin and leptin; therefore we combined the weight loss groups for subsequent MANOVA analyses and explored racial differences in adiponectin and leptin. Repeated-measure mixed-models were used to test the independent effect of race on adiponectin and leptin after adjusting for covariates. In the mixed-models for adiponectin the independent variables included IAAT, limb fat, total fat mass and race. In the mixed-models for leptin the independent variables included total fat mass, insulin, estradiol, and race. We included estradiol and insulin in the models for leptin because these hormones stimulate adipocyte production of leptin [22,23,24] and were shown to be significantly correlated with leptin in preliminary univariate analysis (data not shown). All models were adjusted for age and race was entered into the models as a dichotomous variable (coded as EA=0 and AA=1). Residuals from models were tested and confirmed to be normally distributed. JMP version 8.0 (SAS Institute, Inc., Cary NC) was used for statistical analyses, all p-values were two-sided and a p-value ≤0.05 was considered statistically significant.
RESULTS
Fifty-eight AA and 62 EA premenopausal women mean age 34.6±0.56 years underwent weight reduction for 22.3±0.09 weeks. As shown in Table 1 at baseline AA and EA women had similar BMI but EA women had significantly greater body weight, fat mass and IAAT [p<0.05 for all].
Table 1.
Anthropometric measurements from baseline and weight-reduced time periods.
| AA n=58 | EA n=62 | P for time |
P for race |
P for time |
|
|---|---|---|---|---|---|
| Body Composition1 | |||||
| Weight, kg | |||||
| Baseline | 76.7±0.73a | 78.2±1.04b | <.0001 | 0.455 | 0.06 |
| Weight-reduced | 65.0±0.65 | 65.7±0.94 | |||
| BMI | |||||
| Baseline | 28.3±0.14 | 28.2±0.17 | <.0001 | 0.366 | 0.313 |
| Weight-reduced | 24.0±0.12 | 23.7±0.16 | |||
| Fat mass, kg | |||||
| Baseline | 31.1±0.47a | 33.0±0.70b | <.0001 | 0.027 | 0.266 |
| Weight-reduced | 20.1±0.43a | 21.6±0.61b | |||
| Limb fat, kg | |||||
| Baseline | 16.0±0.30 | 15.9±0.41 | <.0001 | 0.944 | 0.219 |
| Weight-reduced | 10.6±0.26 | 10.8±0.32 | |||
| Lean mass, kg | |||||
| Baseline | 45.7±0.51 | 45.2±0.50 | <.0001 | 0.364 | 0.235 |
| Weight-reduced | 45.0±0.50 | 44.1±0.53 | |||
| IAAT, cm2 | |||||
| Baseline | 64.1±3.32a | 93.3±3.45b | <.0001 | <.0001 | 0.001 |
| Weight-reduced | 39.6±2.50a | 57.4±2.72b | |||
| Serum Hormones2 | |||||
| Insulin, µIU/ml | |||||
| Baseline | 12.1 (11.0, 13.1) | 10.9 (10.1, 11.7) | <.0001 | 0.323 | 0.066 |
| Weight-reduced | 8.61 (7.72, 9.50) | 8.54 (7.76, 9.31) | |||
| Adiponectin, µg/ml | |||||
| Baseline | 9.24 (8.15, 10.3) | 9.24 (8.40, 10.1) | <.0001 | 0.784 | 0.579 |
| Weight-reduced | 11.7, 10.3, 13.1) | 12.1 (10.8, 13.4) | |||
| Leptin, ng/dl | |||||
| Baseline | 25.1 (23.0, 27.5) | 22.7 (20.1, 25.3) | <.0001 | 0.093 | 0.732 |
| Weight-reduced | 12.7 (11.0, 14.4) | 10.5 (8.96, 12.0) | |||
| Estradiol, pg/ml | |||||
| Baseline | 69.9 (57.1, 85.6) | 58.9 (50.5, 68.7) | 0.907 | 0.036 | 0.615 |
| Weight-reduced | 73.6 (59.0, 91.8) | 57.0 (48.1, 67.6) |
Mean±SEE,
Geometric mean (95% CI).
a and b denote significant differences across rows (p<.05). BMI, body mass index; IAAT, intra-abdominal adipose tissue.
The results from repeated measures MANOVA for body composition and serum hormones are shown in Table 1. Following weight loss there was a significant time effect for all measures of body composition (p<.0001) indicating that the weight loss intervention significantly decreased body weight, BMI, fat mass, limb fat, lean mass and IAAT. There was a significant time effect for serum hormones with the exception of estradiol indicating that insulin and leptin significantly decreased (p<.0001 for both) whereas adiponectin significantly increased (p<.0001). Significant main effects of race were observed for fat mass (p=0.027) and IAAT (p<.0001) indicating that EA women had greater amounts of fat mass and IAAT. A significant main effect of race was observed for serum estradiol with AA women having higher concentrations than EA women (p=0.036). The interaction term time×race was significant for IAAT indicating EA women lost greater amounts of IAAT over the course of the weight loss.
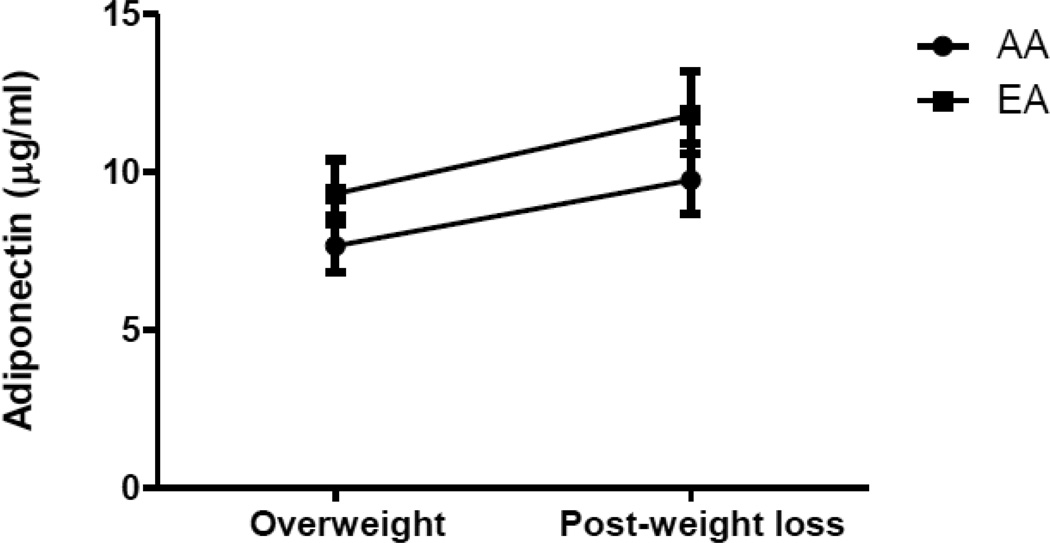
The repeated-measures mixed-models for the dependent variable adiponectin are shown in Table 2. In Model 1, limb fat, fat mass, and IAAT were included as independent variables. A significant effect for limb fat was observed (p=0.043). In Model 2, after race was added to the model, significant effects were observed for limb fat (p=0.014), IAAT (p=0.045) and race (p=0.042). We tested the interaction term time×race in the model however it was not significant, indicating that adiponectin increased over time similarly in AA and EA women. Adjusted least squared means for adiponectin are illustrated in Figure 1. African-American women had significantly lower adjusted adiponectin compared to EA women at baseline [7.67 (6.85, 8.60) versus 9.32 (8.34, 10.4] µg/ml, p<0.05) and following weight loss [9.75 (8.70, 10.9) versus 11.8 (10.6, 13.2) µg/ml, p<0.05].
Table 2.
Repeated measures mixed-models for dependent variable adiponectin
| Model 1 | Term | Estimate | SEE | P |
| Intercept | 2.3553 | 0.6355 | 0.0003 | |
| Limb fat | 0.8093 | 0.3974 | 0.043 | |
| Total fat mass | −0.4891 | 0.4430 | 0.271 | |
| IAAT | −0.1118 | 0.0777 | 0.152 | |
| Model 2 | Term | Estimate | SEE | P |
| Intercept | 2.6775 | 0.6456 | <.0001 | |
| Limb fat | 0.9992 | 0.4008 | 0.014 | |
| Fat mass | −0.6901 | 0.4469 | 0.124 | |
| IAAT | −0.1628 | 0.0806 | 0.045 | |
| Race | 0.0773 | 0.0376 | 0.042 |
Models adjusted for age.
Figure 1.
Geometric mean [95% CI] for circulating concentrations of adiponectin in AA and EA women in the overweight [BMI 27–30 kg/m2] and weight-reduced states [BMI ≤24]. African-American women had significantly lower adiponectin in both the overweight and weight-reduced states after adjusting for covariates [p<0.05 for both].
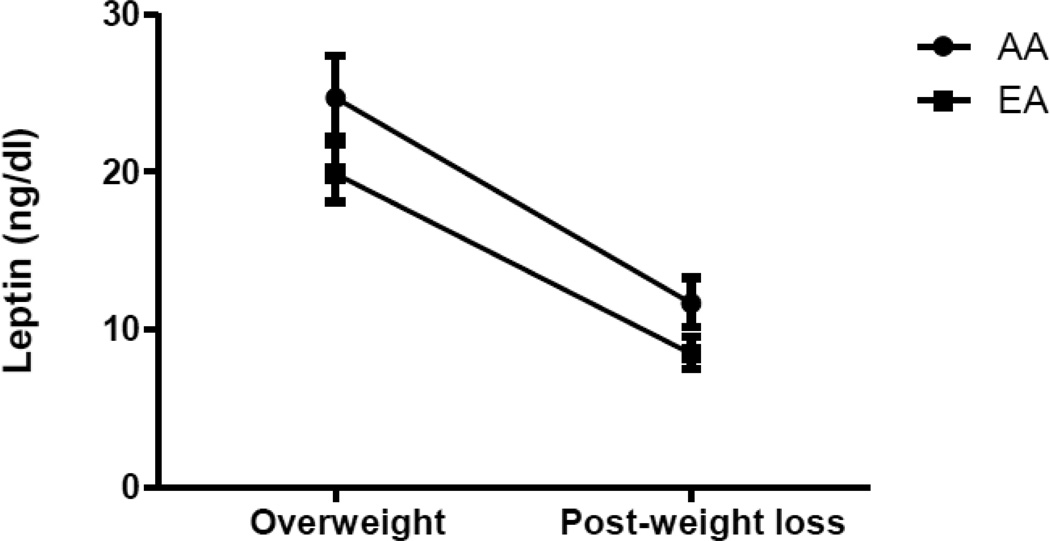
The repeated-measures mixed-models for the dependent variable leptin are shown in Table 3. In Model 1, there were significant effects for fat mass (p<.0001), insulin (p=0.002) and estradiol (p<0.001). In Model 2, race was added to the model and the effects of fat mass, insulin and estradiol remained significant (p<.0001, p=0.003, p<0.001, respectively) and there was a significant race effect observed (p=0.001). We tested the interaction term time×race in the model however it was not significant, indicating that leptin decreased over time similarly in AA and EA women. Adjusted least squared means for leptin are illustrated in Figure 2. African-American women had significantly higher adjusted leptin compared to EA women at baseline [24.7 (22.3, 27.4) versus 19.9 (18.1, 21.8) ng/dl, p<0.05] and following weight loss [11.7 (10.2, 13.3) versus 8.48 (7.50, 9.57) ng/dl, p<0.05].
Table 3.
Repeated measures mixed-models for dependent variable leptin.
| Model 1 | Term | Estimate | Std Error | P |
| Intercept | −3.9581 | 0.7186 | <.0001 | |
| Fat mass | 1.6965 | 0.1932 | <.0001 | |
| Insulin | 0.2319 | 0.0750 | 0.002 | |
| Estradiol | 0.1463 | 0.0389 | <0.001 | |
| Model 2 | Term | Estimate | Std Error | P |
| Intercept | −4.0882 | 0.6894 | <.0001 | |
| Fat mass | 1.7732 | 0.1860 | <.0001 | |
| Insulin | 0.2204 | 0.0729 | 0.003 | |
| Estradiol | 0.1337 | 0.0384 | <0.001 | |
| Race | −0.1308 | 0.0356 | 0.001 |
Models adjusted for age and time (weight loss)
Figure 2.
Geometric mean [95% CI] for circulating concentrations of leptin in AA and EA women in the overweight [BMI 27–30 kg/m2] and weight-reduced states [BMI ≤24]. African-American women had significantly higher leptin in both the overweight and weight-reduced states after adjusting for covariates [p<0.05 for both].
DISCUSSION
In this study we determined that race was significantly associated with adiponectin and leptin concentrations in premenopausal AA and EA women. In both the overweight and normal weight states, EA women had higher adiponectin and lower leptin concentrations compared to AA women after adjusting for body composition and other circulating hormones shown to influence adipokines. Our results indicate that AA women have a more unfavorable adipokine profile despite having a more favorable body composition relative to EA women of similar age and BMI. To our knowledge, this is among the first studies to longitudinally explore differences in adiponectin and leptin in AA and EA premenopausal women.
Although the racial differences in adiponectin and leptin observed in the present study were modest these observations may be clinically important given that both adiponectin and leptin are strongly linked with glucose metabolism, insulin resistance and type 2 diabetes. Adiponectin has been shown to be insulin-sensitizing in several models [25] while hyperleptinemia as a result of obesity impairs insulin signaling [26]. Notably, AAs have a greater risk of developing impaired glucose metabolism [11], insulin-resistance [10] and type 2 diabetes [12] independent of obesity. The factors that contribute to the increased risk for these metabolic disorders in AAs are largely unclear. In the present study we observed that AA women matched with EA women for age and BMI had lower adiponectin and higher leptin before weight loss. These differences continued to be observed after both the AA and EA women were weight-reduced to a normal weight state. Importantly, these differences were independent of fat mass and fat distribution suggesting that there are inherit racial differences in serum concentrations of adiponectin and leptin. Because adiponectin and leptin are important biological factors involved in glucose homeostasis and insulin signaling the observed differences may be clinically relevant in the pathobiology of these metabolic disorders in AA women.
Factors that may contribute to race differences in leptin include estradiol and insulin. In our study AA women had significantly higher estradiol compared to EA women. This observation is in agreement with previous reports [27]. AA women in our study tended to have higher insulin concentrations although this did not reach statistical significance. Other studies have shown that AA women have greater acute insulin response to glucose [AIRg] than EA women and tend to be more insulin-resistant [11]. Both insulin and estradiol drive adipocyte leptin production and could potentially explain the differences seen in leptin between AA and EA women [22,23,24]. In our analyses, both insulin and estradiol made significant independent contributions to the variance in leptin. However, greater leptin in AA was independent of both insulin and estradiol. Further research is needed to identify the factors responsible for greater leptin in AA.
Circulating concentrations of adiponectin are partly influenced by body fat distribution. Central adiposity, particularly IAAT, is inversely associated with adiponectin [25] while there appears to be a positive association with limb fat [13]. In our subject population, AA and EA women had similar amounts of limb fat but AA women had significantly less IAAT compared to EA women. Further, we observed that IAAT was independently associated with adiponectin as was limb fat. However, we observed that AA women had significantly lower adiponectin independent of body fat distribution. Thus, we conclude that differences in body composition and fat distribution do not appear to be significant factors in explaining lower adiponectin in AA.
Our data is consistent with a growing body of literature supporting differences in circulating adipokines in AA and EA women [13,14,15,16, 20]. Although the biological mechanisms explaining these differences are unclear we speculate that racial differences in fat cell metabolism within specific fat-depots may play a role. A previous study by Tittelbach et al showed that gluteal fat cell size is similar between AA and EA women however differences in gluteal fat cell metabolism appear to exist [19]. In AA women, gluteal fat cell size was significantly correlated with higher insulin whereas this relationship was not present in EA women [19]. This study also reported significant positive associations between fat cell size and higher triglycerides and lower HDL in AA but not EA women. Although this previous investigation did not measure adiponectin or leptin, these data support that racial differences in fat-depot specific fat cell size can influence several metabolic parameters and could explain the differences in adipokines we observed in the current study. Further, studies have determined that 30–70% of the variation in adiponectin is attributable to genetic factors [28] and therefore it is possible that race differences in adiponectin have a genetic basis. However, we did not determine the influence of genetic variation on serum concentrations of adipokines and this is a limitation of the present study. Future studies are needed to determine whether differences in fat cell metabolism and/or variations in the adiponectin gene are associated with reduced adiponectin in AA.
The overall strengths of this investigation include the longitudinal study design, controlled weight loss protocol, robust repeated-measure statistical analyses and inclusion of age, race and BMI-matched AA and EA women in both the overweight and weight- reduced states that allowed for the assessment of potential racial differences in serum adiponectin and leptin. We acknowledge that the racial differences observed in this population of healthy premenopausal AA and EA women were modest and that the clinical relevance of these findings needs to be confirmed in larger cohorts.
In conclusion, we found that despite having a more favorable body fat distribution, AA women had significantly lower serum adiponectin and higher leptin concentrations relative to EA women. These differences were independent of body composition, body fat distribution, insulin, and estradiol. Further research is needed both to identify the factors responsible for lower adiponectin and higher leptin in AA, and to determine if these differences contribute to ethnic disparities in the prevalence of metabolic disorders related to glucose metabolism.
Acknowledgments
Grants and fellowships: RO1DK49779, NCI Cancer Prevention & Control Training Program [R25 CA047888], UAB Nutrition and Obesity Research Center [P30 DK56336], UAB Diabetes Research and Training Center [P60 DK079626] and UL 1RR025777 for core lab support.
Footnotes
The authors have no disclosures or conflicts of interest. The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Author contributions: Maria Azrad [MA] conceived the idea to test the hypothesis in the manuscript and constructed the text and tables of the manuscript. Barbara A. Gower and Gary R. Hunter provided MA with access to the database and biological samples used in this manuscript and assisted in editing the manuscript. Tim R. Nagy oversaw the construction of the manuscript and helped in editing the final draft.
REFERENCES
- 1.Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br. J. Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 5.Yu D, Yu Z, Sun Q, Sun L, Li H, et al. Effects of Body Fat on the Associations of High-Molecular-Weight Adiponectin, Leptin and Soluble Leptin Receptor with Metabolic Syndrome in Chinese. PLoS ONE. 2011 doi: 10.1371/journal.pone.0016818. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siklova-Vitkova M, Klimcakova E, Polak J, et al. Adipose Tissue Secretion and Expression of Adipocyte-Produced and Stromavascular Fraction-Produced Adipokines Vary during Multiple Phases of Weight-Reducing Dietary Intervention in Obese Women. J Clin Endocrinol Metab. 2012;97:E1176–E1181. doi: 10.1210/jc.2011-2380. [DOI] [PubMed] [Google Scholar]
- 8.Huang KC, Lin RC, Kormas N, et al. Plasma leptin is associated with insulin resistance independent of age body mass index, fat mass, lipids, and pubertal development in nondiabetic adolescents. Int J Obes Relat Metab Disord. 2004;28:470–475. doi: 10.1038/sj.ijo.0802531. [DOI] [PubMed] [Google Scholar]
- 9.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: Biological, clinical, and nonclinical factors – An Endocrine Society Scientific Statement. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyatt TC, Phadke RP, Hunter GR, et al. Insulin sensitivity in African-American and white women: association with inflammation. Obesity. 2009;17:276–282. doi: 10.1038/oby.2008.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brancati FL, Kao WH, Folfom AR, et al. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 13.Bush NC, Darnell BE, Oster RA, et al. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Bacha F, Gungor N, et al. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Nicklas BJ, Toth MJ, Goldberg AP, et al. Racial differences in plasma leptin concentrations in obese postmenopausal women. J Clin Endocrinol Metab. 1997;82:315–317. doi: 10.1210/jcem.82.1.3659. [DOI] [PubMed] [Google Scholar]
- 16.Khan UI, Wang D, Sowers MR, Mancuso P, Everson-Rose SA, Scherer PE, Wildman RP. Race–ethnic differences in adipokine levels: the Study of Women's Health Across the Race-ethnic differences in adipokine levels: the Study of Women's Health Across the Nation [SWAN] Metabolism. 2012;61:1261–1269. doi: 10.1016/j.metabol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 18.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–771. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 19.Tittelbach TJ, Berman DM, Nicklas BJ, Ryan AS, Goldberg AP. Racial differences in adipocyte size and relationship to the metabolic syndrome in obese women. Obes Res. 2004;12:990–998. doi: 10.1038/oby.2004.121. [DOI] [PubMed] [Google Scholar]
- 20.Sumner AE, Micklesfield LK, Ricks M, et al. Waist circumference, BMI, and visceral adipose tissue in white women and women of African descent. Obesity. 2011;19:671–674. doi: 10.1038/oby.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter GR, Brock DW, Byrne NM, et al. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity. 2010;18:690–695. doi: 10.1038/oby.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu H, Shimomura Y, Nakanishi Y, et al. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154:285–292. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 23.Alonso A, Fernandez R, Moreno M, et al. Leptin and its receptor are controlled by 17β-estradiol in peripheral tissues of ovariectomized rats. Exp Biol Med. 2007;232:542–549. [PubMed] [Google Scholar]
- 24.Fried SK, Ricci MR, Russell CD, et al. Regulation of leptin production in humans. J Nutr. 2000;130:3127S–3131S. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 25.Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocr. 2010;37:11–21. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 26.Pérez C, Fernández-Galaz C, Fernández-Agulló T, Arribas C, Andrés A, Ros M, et al. Leptin impairs insulin signaling in rat adipocytes. Diabetes. 2004;53:347–353. doi: 10.2337/diabetes.53.2.347. [DOI] [PubMed] [Google Scholar]
- 27.Woods MN, Barnett JB, Spiegelman D, et al. Hormone levels during dietary changes in premenopausal African-American women. J Natl Cancer Inst. 1996;88:1369–1374. doi: 10.1093/jnci/88.19.1369. [DOI] [PubMed] [Google Scholar]
- 28.Enns JE, Taylor CG, Zahradka P. Variations in adipokine genes AdipoQ, Lep, LepR are associated with risk for obesity-related metabolic disease: The modulatory role of gene-nutrient interactions. J Obes. 2011 doi: 10.1155/2011/168659. [DOI] [PMC free article] [PubMed] [Google Scholar]