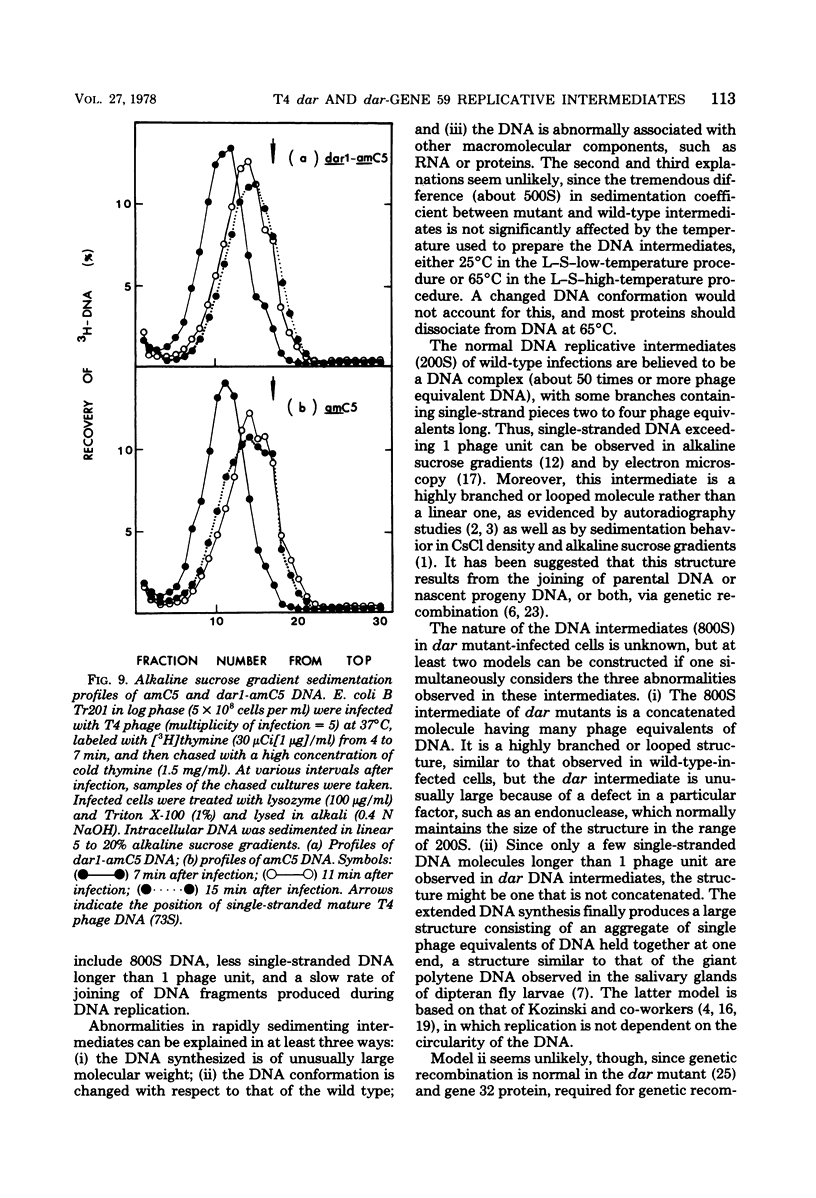
Abstract
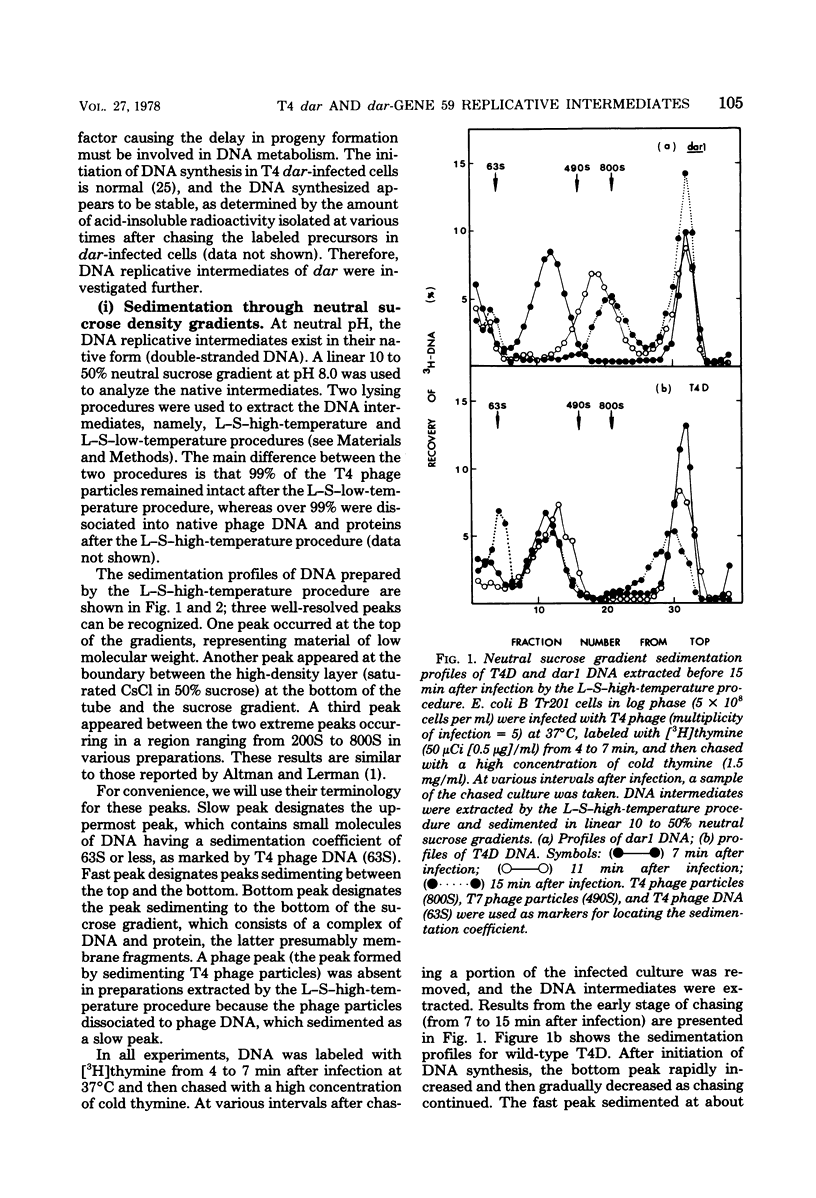
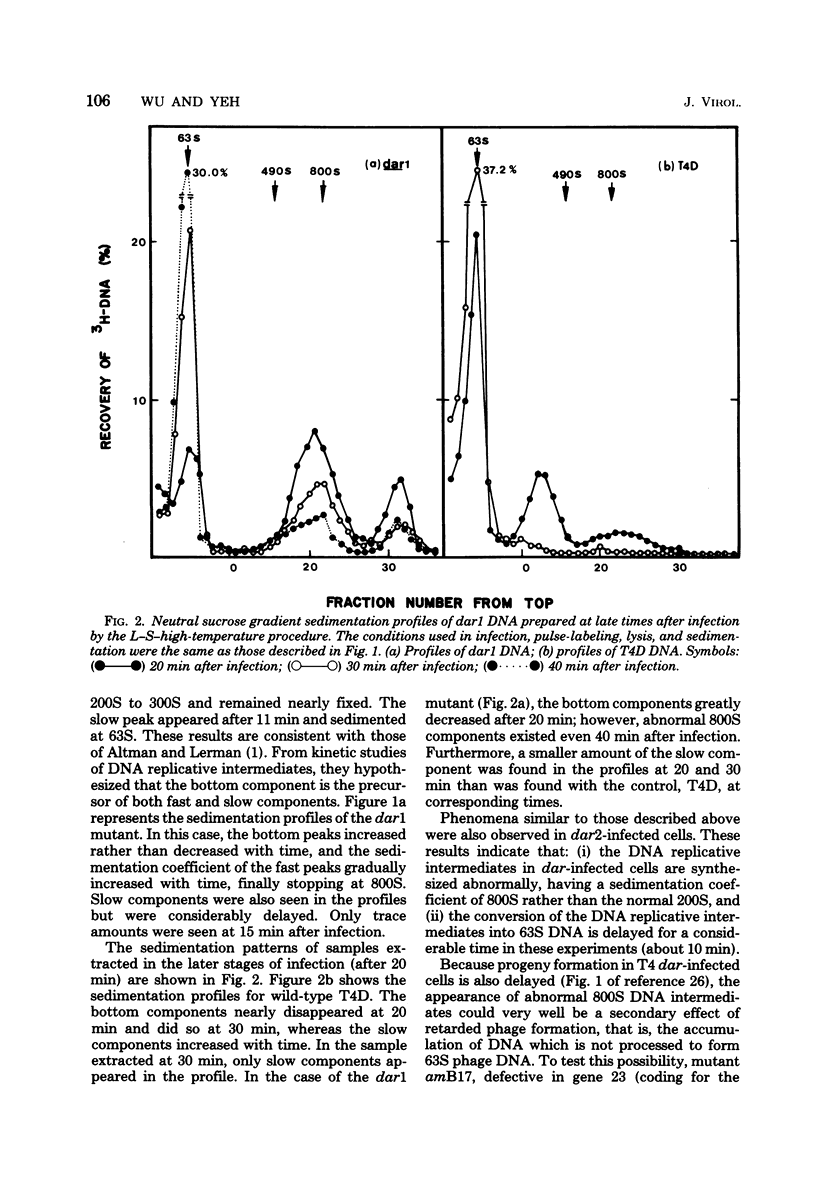
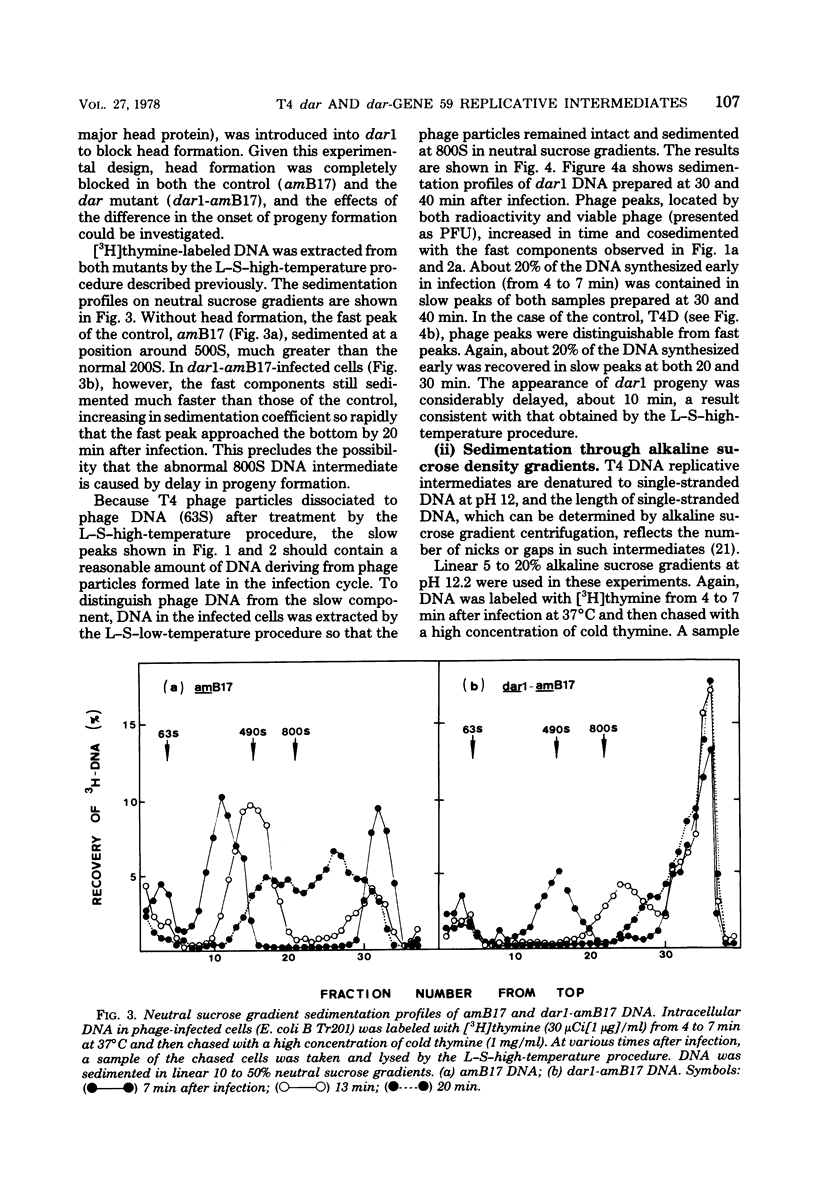
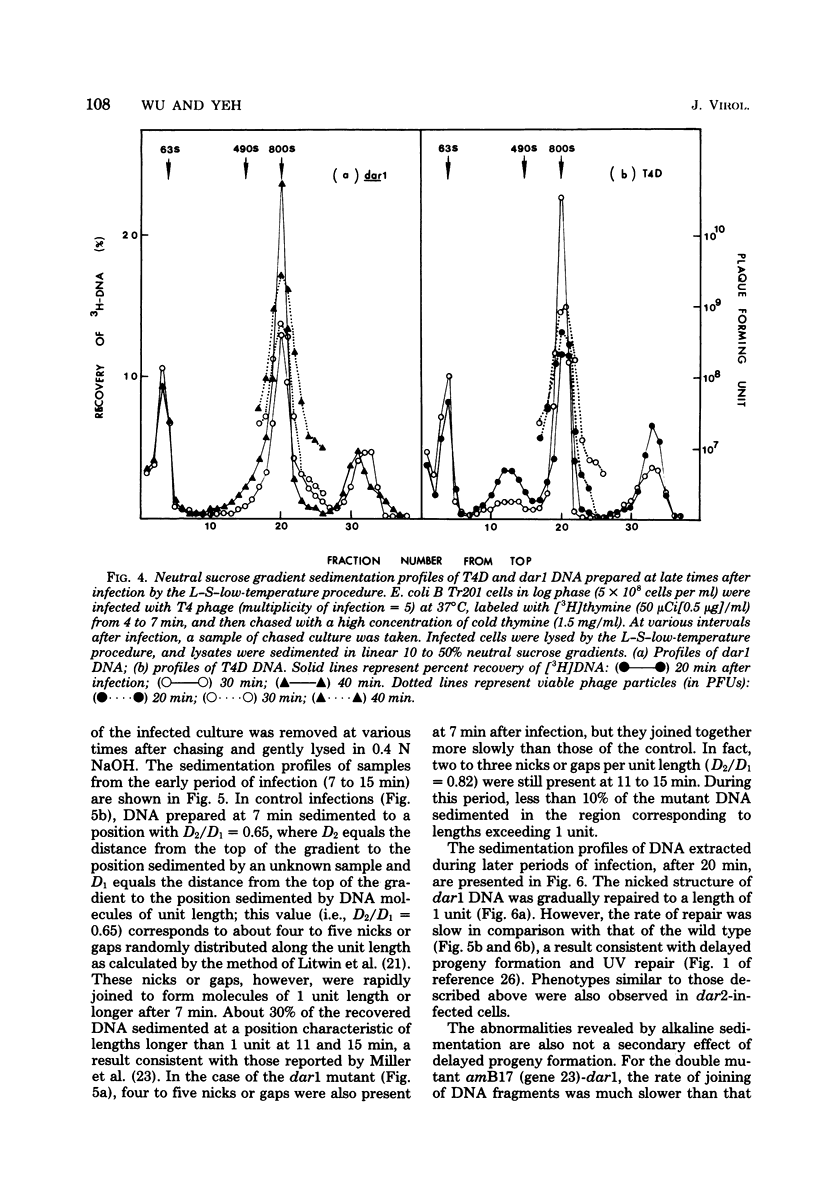
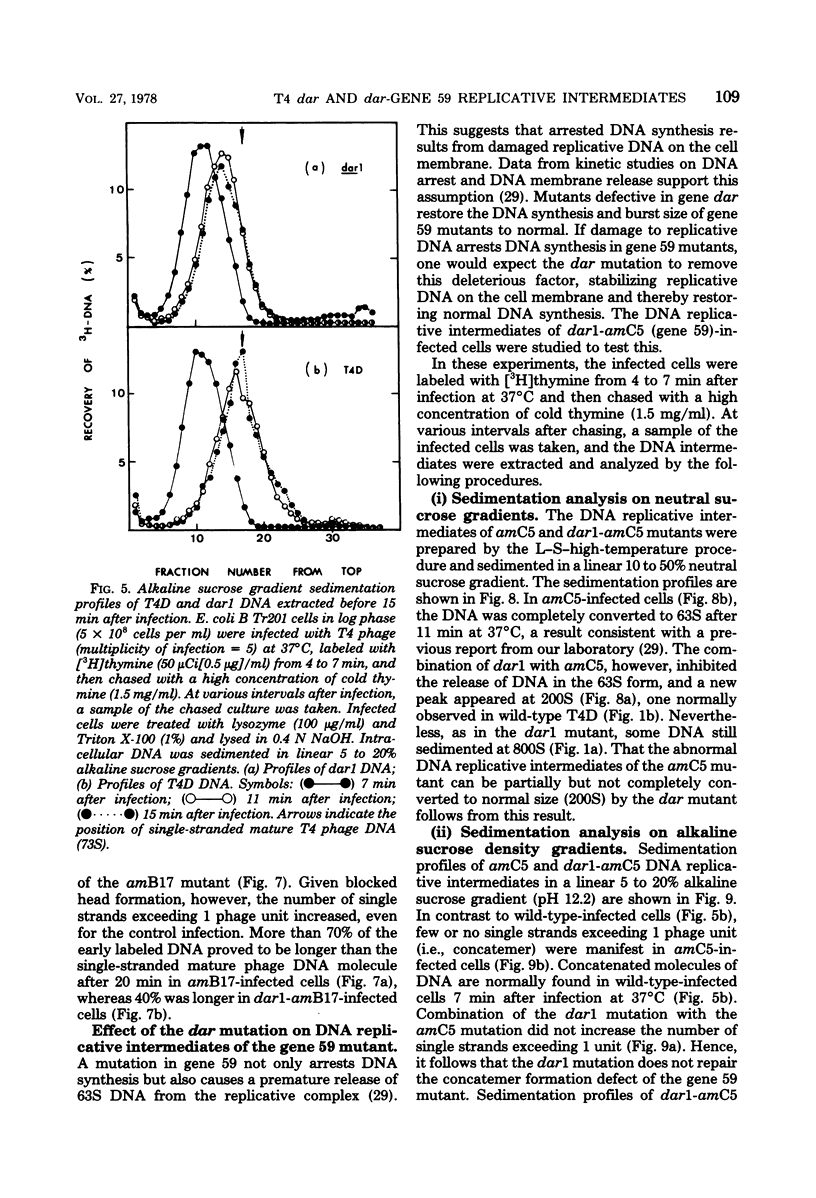
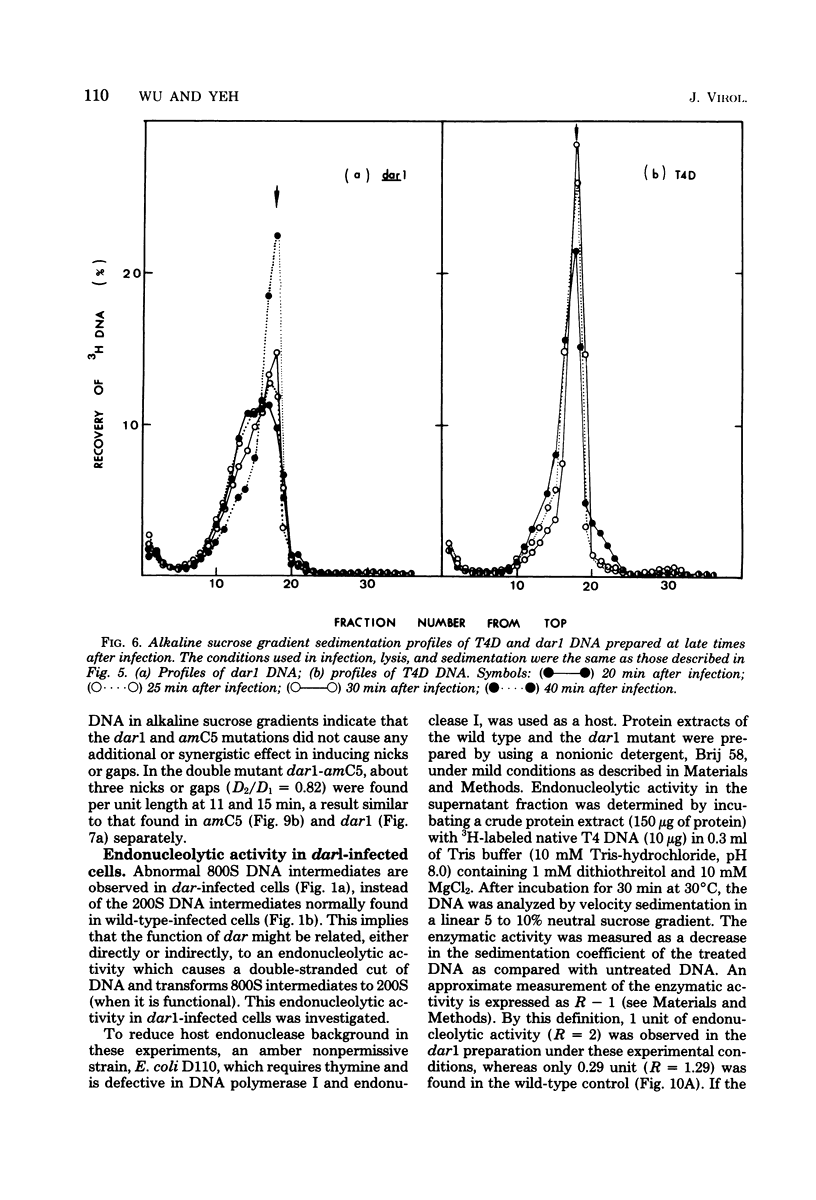
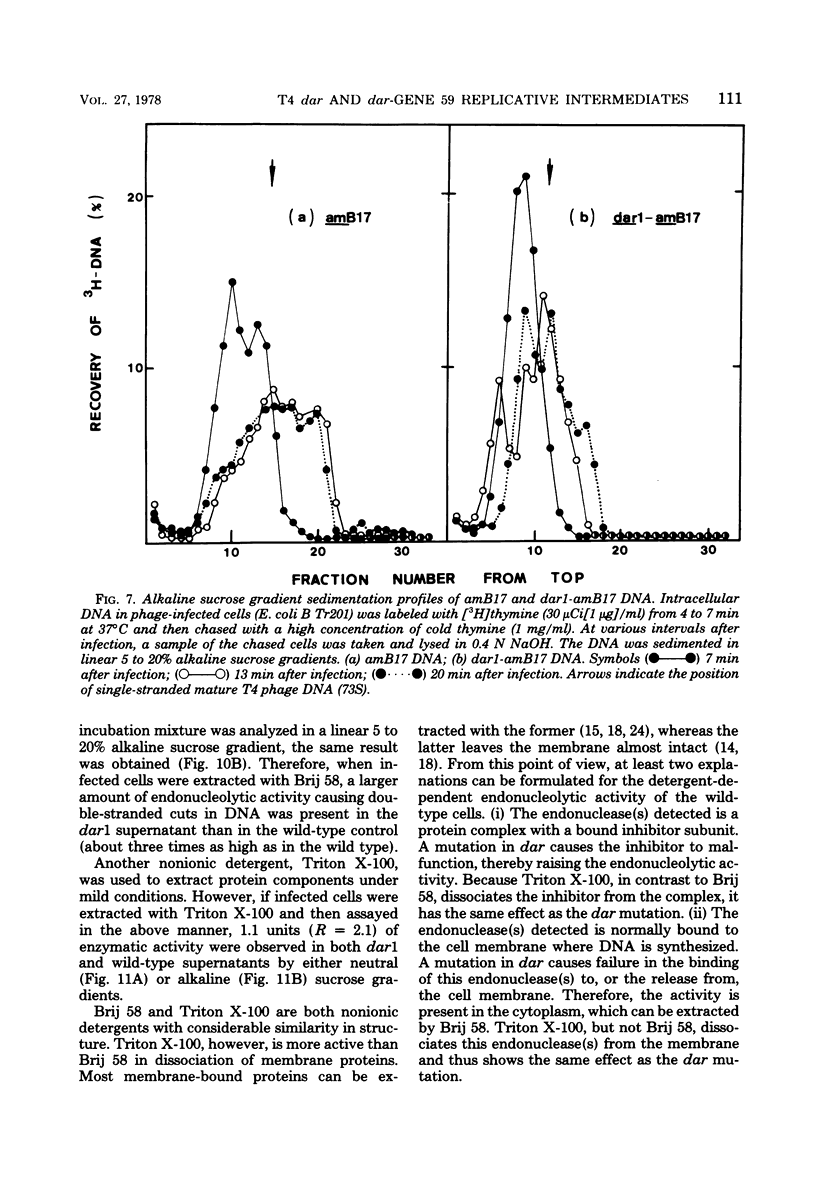
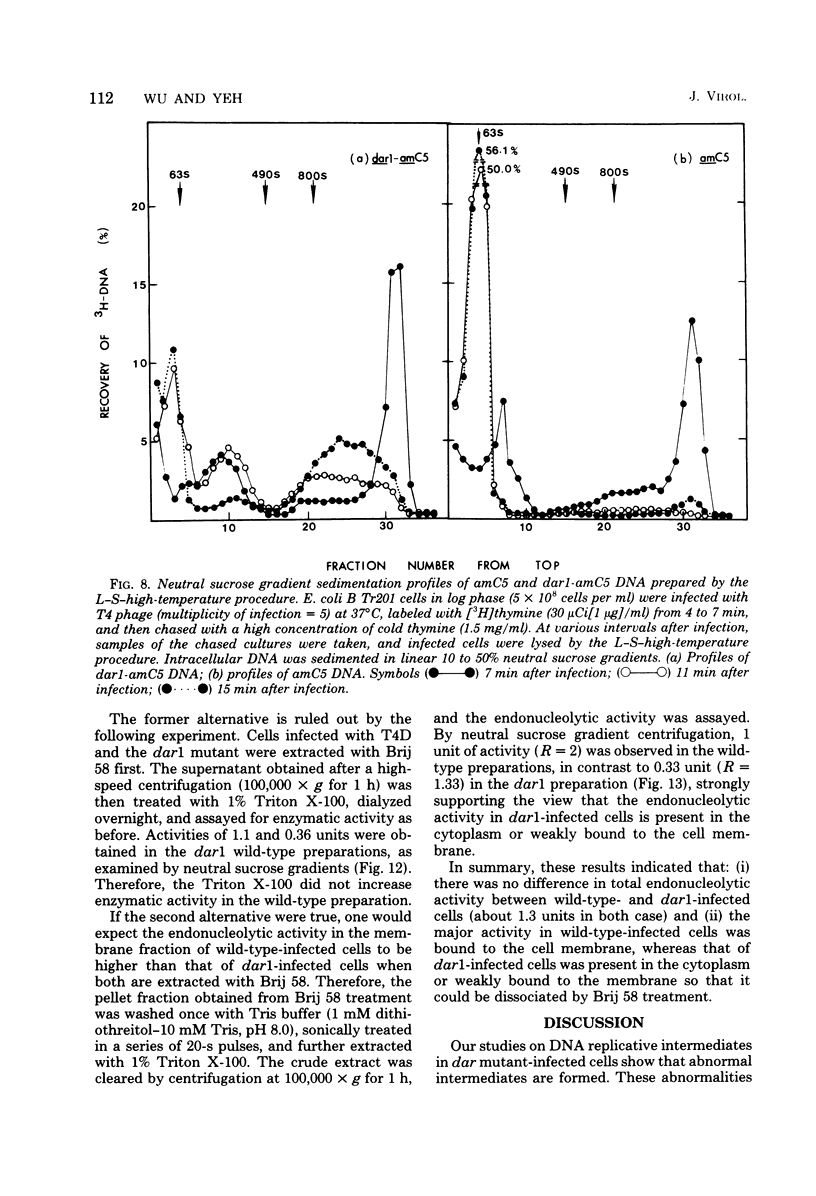
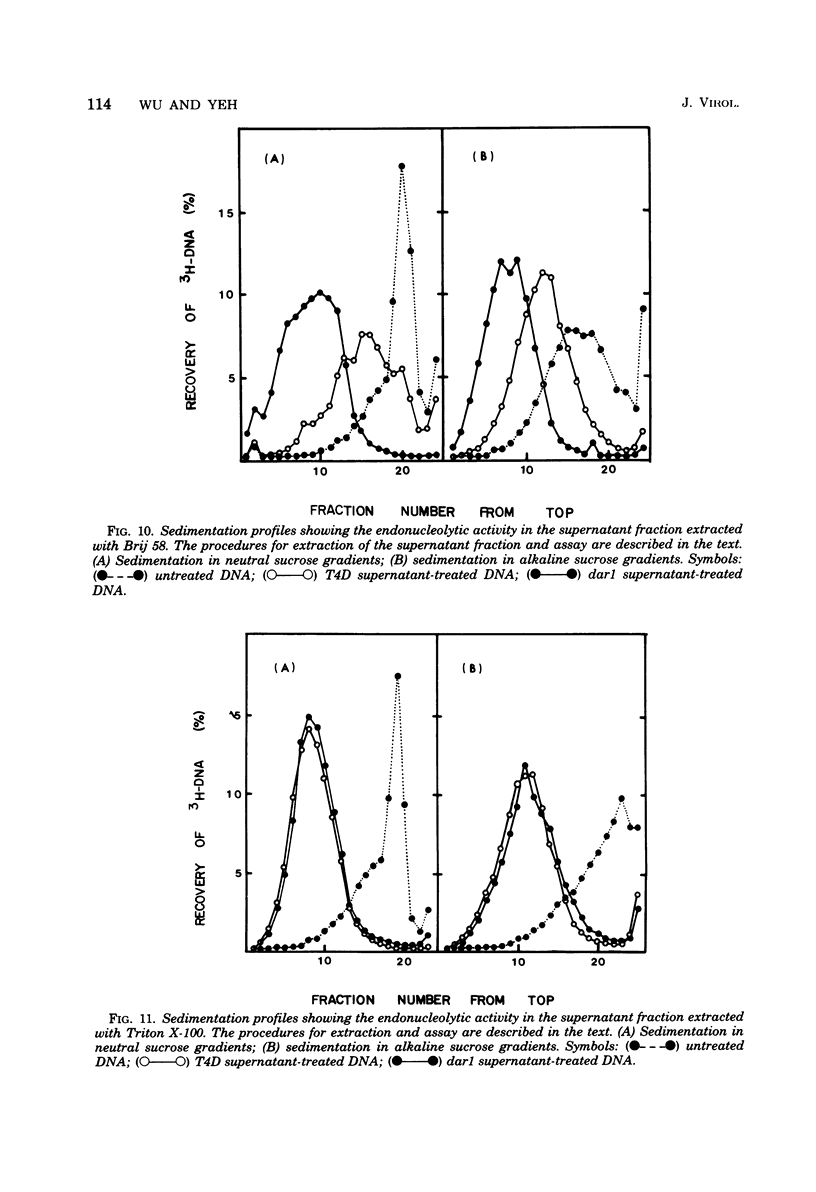
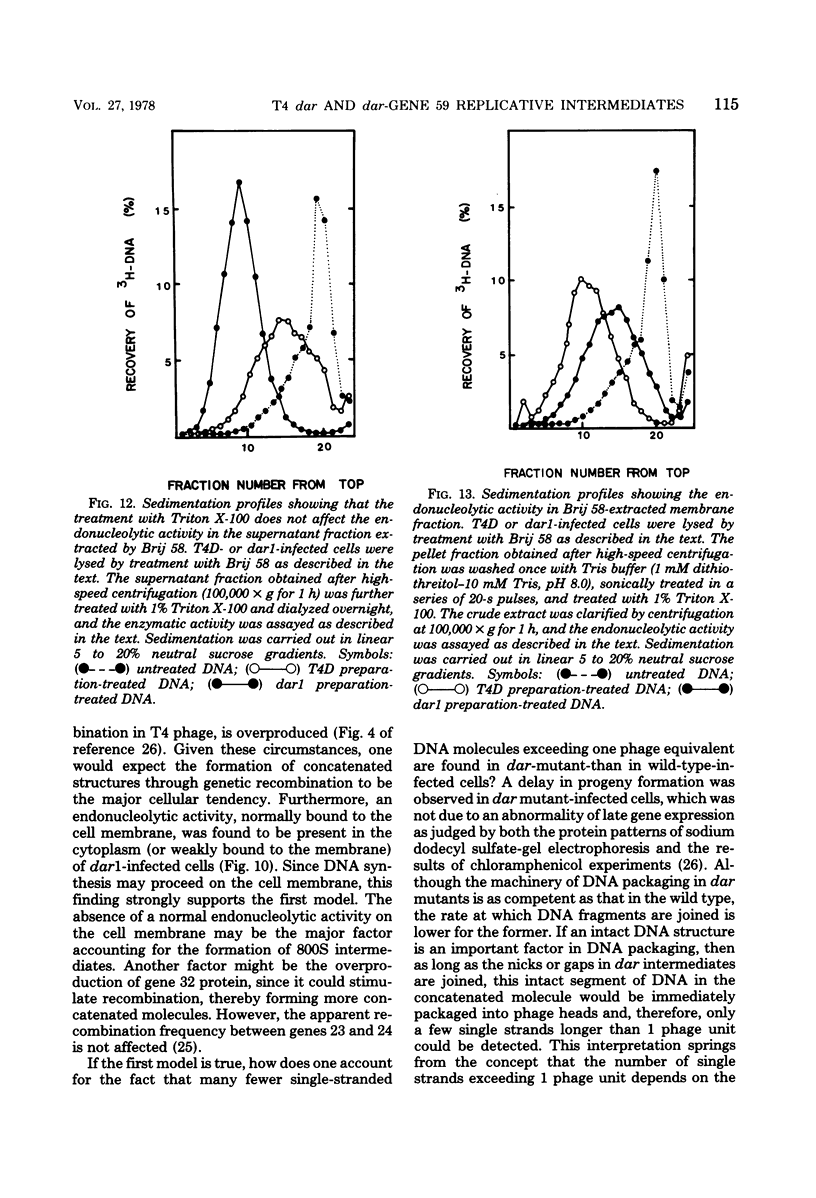
A mutation in the dar gene of phage T4 restored the arrested DNA synthesis caused by the gene 59 mutation. We have studied the DNA replicative intermediates in cells infected with a dar mutant and a dar-amC5 (gene 59) mutant by velocity sedimentation in neutral and alkaline sucrose gradients. In T4 dar-infected cells, compared to the wild type, three kinds of abnormalities were observed in DNA replication (i) There were unusually rapidly sedimenting intermediates (800S). (ii) When centrifuged in alkaline gradients, there was less single-stranded DNA exceeding 1 phage unit. (iii) The rate of repair of DNA intermediates was slower. It has been proposed by others that the 200S DNA replicative intermediates are required for DNA packaging, but our results showed that the 800S DNA of dar does not have to be converted into the 200S form to undergo conversion to mature viral DNA. Therefore, 200S DNA may not be an obligatory intermediate for mature viral DNA formation. In amC5 (gene 59)-infected cells, the DNA was completely converted 2 to 3 min after intiation of replication to the biologically inactive 63S DNA, and DNA synthesis was concomitantly arrested. However, in dar-am-C5 (gene 59)-infected cells, the formation of abnormal 63S DNA did not occur and 200S DNA appeared instead. An endonucleolytic activity, normally associated with the cell membrane and capable of making double-stranded cuts, was found in the cytoplasm of T4 dar-infected cells. Because the total activity of this endonuclease is the same for both wild-type T4D and the dar mutant, it seems unlikely that the dar protein has endonucleolytic activity itself. However, the finding does explain the abnormal sedimentation of dar DNA intermediates (800S) as well as the proposed suppression mechanism of the gene 59 mutation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H. Coiled rings of DNA released from cells infected with bacteriophages T7 or T4 or from uninfected Escherichia coli. J Virol. 1974 Jun;13(6):1346–1355. doi: 10.1128/jvi.13.6.1346-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H., Bernstein C. Circular and branched circular concatenates as possible intermediates in bacteriophage T4 DNA replication. J Mol Biol. 1973 Jul 5;77(3):355–361. doi: 10.1016/0022-2836(73)90443-9. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggelmann H., Pulitzer J. F., Geiduschek E. P. Relationship between gene 55 function, late transcription and an intermediate in the replication of bacteriophage T4 DNA. J Mol Biol. 1970 Apr 28;49(2):509–514. doi: 10.1016/0022-2836(70)90260-3. [DOI] [PubMed] [Google Scholar]
- Doermann A. H. T4 and the rolling circle model of replication. Annu Rev Genet. 1973;7:325–341. doi: 10.1146/annurev.ge.07.120173.001545. [DOI] [PubMed] [Google Scholar]
- DuPraw E. J., Rae P. M. Polytene chromosome structure in relation to the "folded fibre" concept. Nature. 1966 Nov 5;212(5062):598–600. doi: 10.1038/212598a0. [DOI] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. The absence of mature phage DNA molecules from the replicating pool of T-even-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):109–126. doi: 10.1016/s0022-2836(66)80080-3. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W. Solubilization of the membrane bound lactose specific component of the staphylococcal PEP dependant phosphotransferase system. FEBS Lett. 1970 Jun 27;8(5):277–280. doi: 10.1016/0014-5793(70)80286-1. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Knippers R. DNA polymerase II. Nature. 1970 Dec 12;228(5276):1050–1053. doi: 10.1038/2281050a0. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Doermann A. H. Repetitive DNA replication of the incomplete genomes of phage T4 petite particles. Proc Natl Acad Sci U S A. 1975 May;72(5):1734–1738. doi: 10.1073/pnas.72.5.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Kozinski A. W., Litwin S. Molecular Recombination in T4 Bacteriophage Deoxyribonucleic Acid: III. Formation of Long Single Strands During Recombination. J Virol. 1970 Mar;5(3):368–380. doi: 10.1128/jvi.5.3.368-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysiak D., Kaniuga Z. The effect of triton X-100 on the respiratory chain enzymes of a heart-muscle preparation. Eur J Biochem. 1970 May 1;14(1):70–74. doi: 10.1111/j.1432-1033.1970.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. New Late Gene, dar, Involved in DNA Replication of Bacteriophage T4 I. Isolation, Characterization, and Genetic Location. J Virol. 1975 May;15(5):1096–1106. doi: 10.1128/jvi.15.5.1096-1106.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. New late gene, dar, involved in the replication of bacteriophage T4 DNA. II. Overproduction of DNA binding protein (gene 32 protein) and further characterization. J Virol. 1978 Jul;27(1):90–102. doi: 10.1128/jvi.27.1.90-102.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Ma F. J., Yeh Y. C. Suppression of DNA-arrested synthesis in mutants defective in gene 59 of bacteriophage T4. Virology. 1972 Jan;47(1):147–156. doi: 10.1016/0042-6822(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wu R., Wu J. L., Yeh Y. C. Role of gene 59 of bacteriophage T4 in repair of UV-irradiated and alkylated DNA in vivo. J Virol. 1975 Jul;16(1):5–16. doi: 10.1128/jvi.16.1.5-16.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Yeh Y. C. DNA arrested mutants of gene 59 of bacteriophage T4. II. Replicative intermediates. Virology. 1974 May;59(1):108–122. doi: 10.1016/0042-6822(74)90209-8. [DOI] [PubMed] [Google Scholar]


