Abstract
Background
The present study investigated the relationship between cardiorespiratory fitness and executive functioning in pediatric brain tumor survivors who received cranial radiation. This population is known to show executive dysfunction and lower rates of aerobic exercise compared to peers.
Procedure
Nine adolescent survivors of pediatric posterior fossa tumor completed an n-back working memory task during a functional MRI scan, as well as cardiorespiratory fitness testing on a cycle ergometer.
Results
Neuroimaging findings indicated typical activation patterns associated with working memory, mainly in the frontal-parietal network. Higher cardiorespiratory fitness was related to better performance on a behavioral measure of working memory and more efficient neural functioning.
Conclusions
This study provides preliminary evidence that cardiorespiratory fitness may be related to executive functioning, particularly working memory, in pediatric brain tumor survivors. Descriptions of the brain regions recruited for working memory by pediatric brain tumor survivors may be used to inform future interventions or indicators of treatment efficacy.
Keywords: Brain Neoplasms, Neuropsychology, Executive Function, Physical Fitness, Magnetic Resonance Imaging
Introduction
Brain tumor is the second most common type of childhood cancer, accounting for approximately 20% of all diagnoses [1]. Tumors occuring beneath the posterior fossa are the most common brain tumors in childhood [2]. The advent of multimodal treatment in pediatric posterior fossa tumor has significantly increased survival rates in the past few decades, placing greater importance on issues related to survivorship [2]. Late effects from the tumor and treatment may be multifaceted and severe, and may include cognitive dysfunction particularly in the area of working memory [3,4]. Cranial radiation therapy (CRT) directly impacts the neuroanatomical structures underlying core executive functions, such as working memory, which over time negatively impacts IQ and academic achievement scores [5,6]. In addition, damage to the cerebellum from posterior fossa tumor may directly impact executive functions, (e.g., cerebellar cognitive affective syndrome) [7]. Deficits in executive functions affect many aspects of life, including social, adaptive, academic, and career outcomes.
While much literature documents the neurocognitive sequelae associated with pediatric brain tumor, there are relatively few studies attempting to remediate these deficits. Results of pharmacological and cognitive interventions have been equivocal, particularly in the domain of executive functioning [4]. An as-yet unexplored avenue for improving executive functioning in survivors of pediatric brain tumor may be physical exercise. Functionally, cardiorespiratory fitness is typically indicated by peak oxygen uptake, or VO2max [8]. Recent data indicate that aerobic exercise has been associated with improved cognitive outcomes in studies with both children and aging adults [9,10]. Cross-sectional studies have found correlations between cardiorespiratory fitness and higher cognitive functioning in children and adolescents [11-14]. The cognitive benefits of exercise may be particularly relevant for youth with disability, such as survivors of pediatric brain tumor, as they often experience more barriers to physical activity than typically developing peers [15]. However, currently the relationship between cardiorespiratory fitness and executive functions in this population remains unclear.
Historically, the prefrontal cortex has been considered the epicenter of executive functions such as working memory [16]. The n-back working memory task is a classic paradigm in which the subject is asked to monitor a series of stimuli and indicate whether the current stimulus presented is the same as the one seen n trials previously (n is usually 0, 1, 2 or 3) [17]. Studies with adults, adolescents, and children as young as 8 years have consistently found robust patterns of frontal-parietal activation to be significant in accomplishing n-back working memory tasks [18-20].
The present study utilized fMRI methods to investigate neural responses to an n-back working memory task in adolescent survivors of pediatric posterior fossa tumor who received CRT, and to investigate relationships between executive functions and cardiorespiratory fitness in this population. We hypothesized that our sample would show essentially typical patterns of activation in response to this task, including frontal and parietal activation [18]. We also hypothesized that higher cardiorespiratory fitness in our sample would be associated with better scores on a behavioral measure of executive functioning and with more “typical” (i.e. similar to that reported in the literature) patterns of neural activation in response to the n-back task.
Methods
Participants
Survivors of posterior fossa tumor were recruited consecutively from the neuro-oncology clinic at Children’s Hospital of Alabama. Eligible survivors were identified through medical records and approached for recruitment either during regular clinic follow-up visits or by phone.
Inclusion criteria for participation included (a) Posterior fossa tumor survivors at least two years post-completion of medical therapy; (b) Received cranial radiation therapy as part of treatment; (c) Between the ages of 11-18 years; (d) Full-Scale IQ > 70, so as to be able to comprehend and fully participate in the working memory task; (e) Right-handed, due to the neuroimaging component; (f) English speaking; and (g) Lansky score > 70, in order to ensure their ability to pedal the cycle ergometer [21]. Additionally, as this study involved fMRI, exclusionary criteria included any individuals with metal or other implants that were unsafe for the scanner, or participants with claustrophobia. Participants received a small monetary compensation.
In childhood cancer literature, children are generally considered “cured” when they are two years or more post-completion of medical therapy with no recurrence or progression of disease. However, cognitive “late effects” (secondary to diagnosis and treatment) continue to emerge up to 10 years post-completion of therapy and are generally characterized by a failure to keep up with same-age peers over time, not a loss of already-acquired knowledge [6]. Therefore, we elected to set our criterion for time since treatment as two years post-completion of therapy so that cognitive “late effects” (specifically in working memory) would have had ample time to manifest.
Demographic and Psychological Measures
IQ screening was performed using the Wechsler Abbreviated Scale of Intelligence (WASI) [22]. Handedness was assessed through self-report, and confirmed using the Edinburgh Handedness Inventory [23]. Lansky scores were assessed through examiner observation. The Lansky score is a play performance scale in children who have or have been treated for cancer, indicating their functional abilities (e.g., to sit down on the floor, walk independently, etc.) [21]. Each participant’s parent or caretaker completed a demographics questionnaire as well.
fMRI Scanning
Data Acquisition
Brain activation and synchronization associated with working memory and increased cognitive demand were examined using fMRI. Structural images were first acquired during an anatomical scan using high resolution T1-weighted scans using a 160 slice 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) volume scan with a TR = 200 ms, TE = 3.34 ms, flip angle = 7, FOV = 25.6cm, 256 × 256 matrix size, and 1 mm slice thickness. To record functional imaging data, a single-shot gradient-recalled echo-planar pulse sequence was used which offers the advantage of rapid image acquisition (TR = 1000 ms, TE = 30 ms, flip angle = 60 degrees, FOV = 24 cm, matrix 64 × 64). This sequence covers most of the cortex (17 slices, 5mm thick, with a 1 mm gap were acquired in an oblique-axial orientation) in a single cycle of scanning (1 TR) with an in-plane resolution of 3.75 × 3.75 × 5mm. All tasks were completed in a single fMRI session per participant lasting about 60 minutes conducted on a Siemens 3T Allegra MRI scanner (Siemens Corporation, Erlangen, Germany). While in the scanner, E-Prime 1.2 (Psychology Software Tools, Pittsburgh, PA) was used to present the stimuli. An IFIS interface (Integrated Functional Imaging System, Invivo Corporation, Orlando, FL) projected the data onto a screen behind the participant’s head, who viewed it through a mirror. Responses to the stimuli were recorded using fiber-optic button boxes, which indicated performance accuracy and response time.
Experimental Stimuli
During the functional imaging scan, participants completed an n-back working memory task, a paradigm that has been used in a multitude of previous studies with both children and adults [20,24]. In this task, a randomized series of letters of the alphabet was presented, and participants were asked to press a button during three separate conditions: Whenever they saw the letter “x” (0-back condition), whenever they saw the same letter twice in a row (1-back condition), or whenever the letter they saw was the same as two before (2-back condition). Presentation of conditions was counterbalanced such that participants performed each condition twice, but in reverse order (i.e., 0-back, 1-back, 2-back, 2-back, 1-back, 0-back). Participants were given ample opportunity to practice the task on a laptop computer prior to entering the scanner, and were given one truncated practice run of each condition while inside the scanner before beginning the task.
Cardiorespiratory Fitness Testing
Maximum oxygen uptake, or VO2max, testing was completed on a Monark stationary cycle ergometer. A standardized protocol was employed to help each participant achieve his or her maximum aerobic capacity [25]. We chose to use the cycle ergometer instead of the more traditional treadmill test because of the problems in balance often experienced by pediatric posterior fossa tumor survivors [26]. Heart rate data was captured using a POLAR Vantage XL heart rate (HR) monitor (Gays Mills, WI, USA). Oxygen uptake and carbon dioxide production were measured continuously using a Physiodyne Instrument Corporation, MAX-II Cart (Quogue, NY, USA). Gas analyzers were calibrated with certified gases of known concentrations. Participants were encouraged to maintain work output for as long as they could and the test was terminated when participants could no longer maintain the prescribed work level.
Standard criteria for HR, respiratory exchange ratio (RER; which is the ratio of carbon dioxide output to oxygen intake), and plateauing were used to assess achievement of VO2max [27]. Specifically, participants had to meet at least two of the following three criteria to have achieved VO2max: 1. Achieve a HR > 85th percentile of their age predicted maximum; 2. RER > 1.1 (indicating that more carbon dioxide was being exhaled than oxygen was being inhaled); and 3. Observable plateau of VO2 intake (meaning that VO2 values cease increasing over a period of about 30 seconds or more, indicating the participant has reached a maximum level).
Cardiorespiratory fitness variables obtained for behavioral data analyses included VO2max/FFM, or maximum oxygen uptake in relation to fat-free body mass (eliminating the potentially confounding variable, fat mass), and maximum ventilation (VEmax), a measure of lung functioning. Specifically, VEmax is measured in liters per minute, and reflects the volume of air moved in and out of the lungs per minute. VEmax was added as an additional variable of interest because a previous study found greater lung capacity to be related to better working memory performance in cohorts of healthy young, middle-aged, and older adults [28].
Procedure
All procedures were completed in a single session in the following order: IQ screening, then fMRI scan, then cardiorespiratory fitness testing. Fitness testing was always completed after the IQ and fMRI measures because of prior literature indicating that acute exercise may have a temporary beneficial effect on cognitive abilities [29].
Data Analysis
Behavioral and cardiorespiratory fitness data were analyzed using SPSS version 11.5 for Windows. Data were examined to ensure normality and that no outliers were influencing the results. Scores on the WASI were standardized by age. Because of the smaller sample size and thus decreased statistical power, analyses were limited to partial correlations and paired t-tests. Age at time of evaluation was used as a covariate because it was theoretically relevant to both amount of neural activation and working memory abilities [20]. Preliminary bivariate correlations between working memory variables and IQ were completed, and IQ was not found to be related to reaction time or voxel count activation during the working memory task; therefore, due to concerns with statistical power, IQ was not utilized as a covariate in statistical analyses.
The fMRI data were pre- and post-processed, and statistically analyzed using SPM8 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK). Images were corrected for slice acquisition timing, motion corrected, spatially realigned, normalized to the Montreal Neurological Institute template and spatially smoothed with an 8-mm Full Width Half Maximum filter. Statistical analyses were performed on individual data using the general linear model, while group analyses were performed using a random-effects model. Regions of interest (ROIs) with statistically significant activation were identified using a t-statistic on a voxel by voxel basis. To correct for multiple comparisons, a spatial clustering operation was performed in AFNI using AlphaSim with 10,000 Monte Carlo simulations taking into account the entire functional matrix, with a map-wise false-positive probability of p<0.05.
Results
Descriptive and Behavioral Data
All participants who were approached agreed to participate in the study. Fourteen participants were eligible to participate and provided informed assent along with their caregivers’ informed consent in accordance with the protocol approved by the Institutional Review Board. Four participants wore oral braces, which, although safe for the scanner, rendered the imaging unusable. One participant could not begin the cardiorespiratory fitness testing due to a strong gag reflex, as he was unable to wear the mouthpiece. Thus, nine participants completed the study protocol successfully (Table I). All participants who began the fitness test achieved a VO2max score during cardiorespiratory fitness testing (Table I). Details of the feasibility of physical fitness testing and the physical fitness of this cohort compared to normative data are currently in press (Wolfe et al., in press). IQ screening on the WASI indicated a wide range of cognitive abilities (Table I). One participant had a history of cerebellar mutism. Average time since treatment (9.88 years) was much longer than the two years that we required for participation in this study. This is related to the fact that our cohort included only pre-teen and teenage survivors, while the typical age of onset for posterior fossa tumors is around 3-5 years of age [2]. The relatively longer time since treatment in our cohort may have increased the chances that any cognitive late effects in the domain of working memory that were going to manifest for these survivors would be detectable.
Table I.
Participant characteristics
| Demographic | Mean (SD) | Min | Max |
|---|---|---|---|
| Age (years) | 14.89 (1.9) | 11.50 | 17.25 |
| Age at brain tumor diagnosis (years) | 5.00 (2.7) | 1.33 | 8.00 |
| Time since treatment (years) | 9.88 (3.4) | 6.41 | 15.35 |
| Male/Female | 8/1 | -- | -- |
| Caucasian/African-American | 8/1 | -- | -- |
| Medulloblastoma/ependymoma | 6/3 | -- | -- |
| Received whole-brain radiation | 7 | -- | -- |
| Received involved field radiation | 2 | -- | -- |
| Average radiation dose in Gy§ | 54.62 (0.9) | 54.00 | 55.80 |
| Received adjuvant chemotherapy | 7 | -- | -- |
| Received surgical resection | 9 | -- | -- |
Abbreviations: SD = standard deviation; min = minimum value; max = maximum value; Gy = Gray.
Radiation dose reported is total dose including boost to posterior fossa.
Analysis of performance accuracy on the fMRI task across subjects indicated their active participation in the task, as every participant obtained > 50% accuracy during each condition. Average reaction times increased across conditions as the task demand (working memory load) increased. A paired sample two-tailed t-test revealed significant changes in reaction time from 0-back to 1-back, t(10) = −2.67, p<0.05, and from 0-back to 2-back, t(10) = −2.31, p<0.05, with reaction times becoming longer as difficulty increased.
Neural Activation Patterns
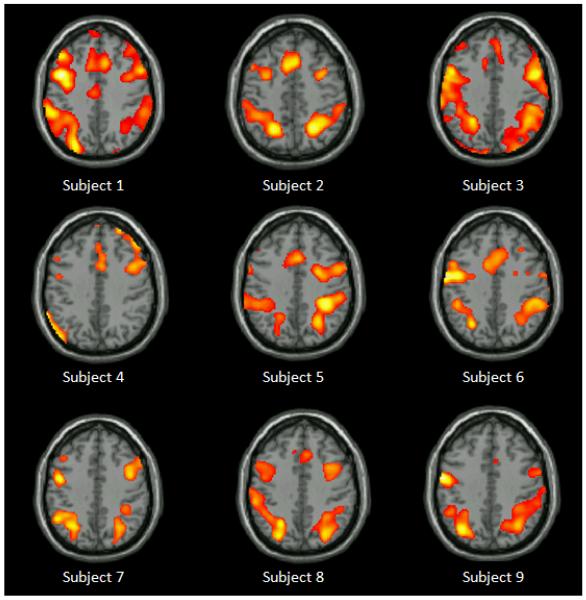
Group analysis of activation for all working memory conditions combined after subtracting out baseline activation ([0back + 1back + 2back] − fixation) showed patterns of frontal-parietal activation (p<0.001, with cluster threshold = 72 contiguous voxels determined by Monte Carlo Simulation using AlphaSim). Specifically, seven clusters of significant activation were noted in: right inferior frontal gyrus (RIFG), supplementary motor area (SMA), left insula (LINS), right insula (RINS), left inferior parietal lobule (LIPL), right inferior parietal lobule (RIPL), and right middle occipital gyrus (RMOG). Given our small sample size, we have displayed individual activation data in Figure 1 to demonstrate the consistency of activation across subjects, and to show that no particular outlier influenced these results.
Figure 1.
Individual activation for each subject during the n-back working memory task compared with fixation baseline (0-back + 1-back + 2-back − Fixation). Clusters of activation are noted primarily in frontal and parietal areas across subjects.
Previous literature has implicated IFG and IPL in completion of working memory tasks (see [30] for a meta-analysis); and these two areas may mediate different aspects of working memory (IFG: phonological storage; IPL: visual rhyming) [30]. Therefore, our results suggest a grossly typical working memory cortical network in this group of pediatric brain tumor survivors.
Executive Functioning and Cardiorespiratory Fitness
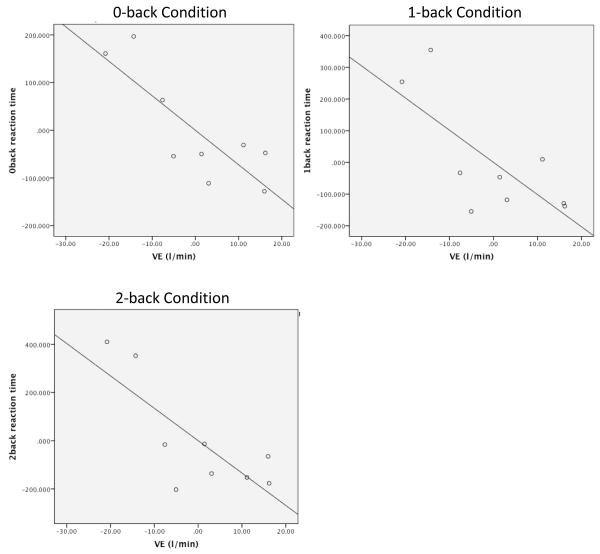
Behavioral data including reaction times were collected from the working memory task. Participants’ VEmax, or lung functioning, was related to reaction time across 0-back (pr = −0.74, p<0.05), 1-back (pr = −0.69, p<0.05), and 2-back (pr = −0.71, p<0.05) conditions using two-tailed partial correlations after controlling for age, such that participants with greater lung functioning showed faster reaction times in all conditions of the working memory task (Figure 2). Reaction times were not related to VO2max/FFM values.
Figure 2.
Partial Correlations between 0-back, 1-back, and 2-back condition reaction times and VE max after accounting for age at evaluation. Abbreviations: VE = Ventilation (a measure of respiratory functioning); l/min = liters per minute (units of VE).
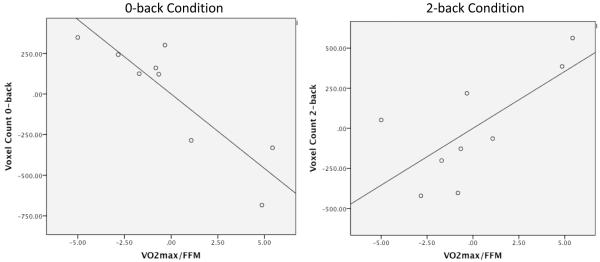
As an index of brain response, the number of activated voxels was counted from ROIs mentioned above across all participants. An interaction was found such that higher cardiorespiratory fitness scores correlated with lower activation in the 0-back condition (vs. baseline) across ROIs (pr = −0.88, p<0.01) after accounting for age at the time of participation in the study. However, in the 2-back condition (vs. baseline), higher cardiovascular fitness scores correlated with greater activation across ROIs (pr = 0.71, p<0.05) after accounting for age (Table III). VO2max/FFM scores did not correlate with voxel counts in the 1-back condition (vs. baseline). Thus, the activation patterns of higher-fit participants appeared to be more efficient in that they showed less brain activation during the easier task, and were able to recruit additional voxels during the more difficult task (Figure 3). This finding indicates that cardiorespiratory fitness may be associated with more efficient neural processing in survivors of pediatric brain tumor.
Table III.
Partial correlations between working memory and fitness data after accounting for age at evaluation
| VO2max/FFM | VEmax | |
|---|---|---|
| 0-back Reaction Time | −.30 | −.82* |
| 1-back Reaction Time | −.22 | −.73* |
| 2-back Reaction Time | −.16 | −.77* |
| 0-back Voxel Count | −.88** | −.02 |
| 1-back Voxel Count | −.07 | −.28 |
| 2-back Voxel Count | .71* | .37 |
Abbreviations: VO2max /FFM = peak oxygen uptake calculated using fat-free mass; VEmax = maximum lung ventilation.
p < 0.05;
p < 0.01
Figure 3.
Partial correlations between VO2max/FFM and voxel count activation in 0-back and 2-back conditions, after accounting for age at evaluation. Participants with greater fitness showed a more efficient neural pattern, activating fewer voxels to accomplish the easier, 0-back condition, and more to accomplish the more difficult, 2-back condition. Abbreviations: VO2max/FFM = Peak oxygen uptake after accounting for fat mass.
Discussion
The present study is unique given its multidimensional investigation spanning behavioral and neural aspects of working memory along with cardiorespiratory fitness in pediatric posterior fossa tumor survivors. Future research should explore the direct effects of cardiovascular exercise on cognitive and neural functioning in this population. Given our small sample size, results should be viewed similarly to those of a pilot study, and will hopefully spur further studies in this area.
Perhaps the most interesting finding of our study was that higher cardiorespiratory fitness was associated with better working memory across a couple of measures. Greater VEmax, an index of lung functioning, was related to quicker responding across 0-back, 1-back, and 2-back conditions. Lung functioning has been shown to relate to several measures of cognitive functioning, including working memory, in healthy adults across the lifespan after controlling for demographic and health-related variables [28].
Peak cardiorespiratory fitness after accounting for fat mass (VO2max/FFM) was related to more efficient neural response, such that higher fit participants showed fewer activated voxels across ROIs in response to the 0-back condition, and greater number of voxels activated during the 2-back condition, with no differences in overall accuracy. It was not clear why reaction time was not related to cardiorespiratory fitness, or why voxel count was not related to lung functioning, in our study. Although the correlation coefficients for these relationships were in the expected directions, they did not reach significance and thus cannot be interpreted. It is possible that these relationships might have reached significance had our sample size been larger. Alternatively, perhaps an additional, not-studied, variable confounded those analyses. Coefficients of variability indicated that over 70% of variance in voxel counts across 0-back and 2-back conditions was accounted for by aerobic fitness, and that over 60% of variance in reaction time was explainable by lung functioning. These strong relationships are remarkable given the myriad of factors that can affect VO2max, lung functioning, reaction times, and neural activation measures.
Cardiorespiratory fitness is a function of both genetic predisposition as well as activity level. It is possible that a common genetic predisposition underlies the relationship between aerobic fitness and cognitive function, i.e., those individuals who have a genetic predisposition towards higher aerobic fitness also have a genetic predisposition towards higher cognitive functioning. Alternatively, a more active lifestyle may have enhanced both aerobic fitness and cognitive functioning for some participants. Given the very high coefficients of variability reported, it is quite possible that both lifestyle and genetic factors may have influenced the reported relationships between working memory and cardiorespiratory fitness. Regardless, these results warrant future studies involving exercise training with this population examining working memory, and more broadly, executive functioning, outcomes.
This fMRI study also found that pediatric survivors of posterior fossa tumor showed typical activation patterns in response to n-back working memory demands, recruiting mainly frontal and parietal lobe areas. This is consistent with previous studies of working memory demand in healthy adults and youth [18]. Therefore, our findings indicate that survivors of pediatric posterior fossa tumor recruit similar areas as typically developing individuals to perform working memory tasks, even though they are at increased risk for cognitive deficits in the domain of working memory. These areas may be employed in future studies, for example linking neurocognitive deficits to neural functioning or assessing the direct effects of working memory interventions on neural functioning.
Limitations
While we recruited every eligible survivor at our medical center, our sample size is small and thus the results should be interpreted rather cautiously. Also, as this was a cross-sectional study, we cannot assume that higher cardiorespiratory fitness caused better working memory in this sample. For example, it might be the case that a survivor’s better working memory assisted him in completing homework more quickly which gave him more time to exercise, thus resulting in higher aerobic fitness. Alternatively, a third variable may better explain this relationship, such as depression, which might negatively impact both working memory and motivation to exercise. Finally, because of the variety of size and location of cerebellar lesions in our participants, we were unable to analyze cerebellar activation. Our report of neural areas utilized to complete a working memory task in this population may be incomplete, as we did not examine the cerebellum or subcortical structures in our analyses.
In conclusion, results of the present study are the first to document neural patterns of working memory, an often-found area of deficit, in pediatric posterior fossa tumor survivors who received CRT. Overall, the brain regions our participants recruited to perform this task were largely consistent with functional neuroimaging literature documenting neural responses to n-back and other working memory tasks in typical adult, adolescent, and child populations. In our sample, cardiorespiratory fitness variables were related to indices of response time and neural activation. While our small sample size and lack of control group limit our ability to generalize these findings to pediatric brain tumor survivors as a whole, we have provided rationale for the next step in this line of research, which would involve future intervention studies to investigate the efficacy of exercise as a method of improving executive functioning in this population.
Table II.
Descriptive Data
| Measure | Mean (SD) | Min | Max |
|---|---|---|---|
| WASI Full-Scale IQ | 85.30 (10.8) | 70 | 109 |
| VO2max/FFM (ml·kg FFM-1·min-1) | 33.87 (3.7) | 29.26 | 40.42 |
| VEmax (l/min) | 51.05 (13.6) | 33.16 | 69.57 |
| BMI | 19.20 (3.38) | 14.50 | 25.50 |
| Fat free mass (kg) | 39.70 (9.33) | 26.22 | 55.43 |
| 0-back Accuracy | 100% (0) | 100% | 100% |
| 0-back Reaction Time (ms) | 524.91 (117.0) | 382.95 | 741.70 |
| 1-back Accuracy | 94% (16.5) | 50% | 100% |
| 1-back Reaction Time (ms) | 590.27 (186.7) | 403.70 | 980.80 |
| 2-back Accuracy | 73% (18) | 50% | 100% |
| 2-back Reaction Time (ms) | 657.21 (235.0) | 411.67 | 1121.80 |
| 0-back Voxel Count | 1096.47 (356.6) | 416.20 | 1475.10 |
| 1-back Voxel Count | 1096.25 (224.6) | 752.50 | 1452.60 |
| 2-back Voxel Count | 1038.46 (378.8) | 457.50 | 1478.30 |
Abbreviations: SD = standard deviation; min = minimum value; max = maximum value; WASI = Wechsler Abbreviated Scale of Intelligence; VO2max/FFM = peak oxygen uptake calculated using fat-free mass; VEmax = maximum lung ventilation; BMI = body mass index (standardized by age and gender); kg = kilograms; ms = milliseconds.
Acknowledgements
We would like to thank Hrishikesh Deshpande and David Bryan for their assistance with collecting and processing data. This research was funded by a training grant from the National Cancer Insitute (grant number 5R25CA047888-22). Ms. Wolfe receives financial support from this grant.
Footnotes
No other authors have any financial disclosures.
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson NE. Late complications in childhood central nervous system tumour survivors. Curr Opin Neurol. 2005;16:677–683. doi: 10.1097/01.wco.0000102623.38669.e5. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe KR, Madan-Swain A, Kana RK. Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Dev Neuropsychol. 2012;37:153–175. doi: 10.1080/87565641.2011.632462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SL. Neurodevelopmental impacts on children treated for medulloblastoma: a review and proposed conceptual model. Dev Disabil Res Rev. 2008;14:201–210. doi: 10.1002/ddrr.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 8.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 4th edition Lea and Febiger; Philadelphia: 1996. [Google Scholar]
- 9.Tomporowski PD, Davis CL, Miller PH, et al. Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–131. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 11.Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to Stroop task performance in preadolescent children. Med Sci Sports Exerc. 2008;40:166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- 12.Themanson JR, Pontifex MB, Hillman CH. Fitness and action monitoring: evidence for improved cognitive flexibility in young adults. Neuroscience. 2008;157:319–328. doi: 10.1016/j.neuroscience.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberg MAI, Pedersen NL, Toren K, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proceedings of the National Academy of Sciences. 2009;106:20906–20911. doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroth S, Kubesch S, Dieterle K, et al. Physical fitness, but not acute exercise modulates event-related potential indices for executive control in healthy adolescents. Brain Research. 2009;1269:114–124. doi: 10.1016/j.brainres.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 15.Ploughman M. Exercise is brain food: The effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236–240. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of Physiology Section 1: The Nervous System. American Physiological Society; Bethesda: 1987. pp. 373–417. [Google Scholar]
- 17.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- 18.Owen AM, McMillan KM, Laird AR, et al. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson CA, Monk CS, Lin J, et al. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- 20.Casey BJ, Cohen JD, Jezzard P, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 21.Lansky SB, List MA, Lansky LL, et al. The measurement of performance in childhood cancer patients. Cancer. 1987;60:1651–1656. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Koshino H, Carpenter PA, Minshew NJ, et al. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Dzurenkova D, Marcek T, Hajkova M. Specialties of assessment of endurance capabilities in sport active children. Bratisl Lek Listy. 2011;102:424–437. [PubMed] [Google Scholar]
- 26.Schoch B, Konczak J, Dimitrova A, et al. Impact of surgery and adjuvant therapy on balance function in children and adolescents with cerebellar tumors. Neuropediatrics. 2006;37:350–358. doi: 10.1055/s-2007-964904. [DOI] [PubMed] [Google Scholar]
- 27.Rowland TW. Developmental Exercise Physiology. Human Kinetics; Champaign, IL: 1996. [Google Scholar]
- 28.Anstey KJ, Windsor TD, Jorm AF, et al. Association of pulmonary function with cognitive performance in early, middle, and late adulthood. Gerontology. 2004;50:230–234. doi: 10.1159/000078352. [DOI] [PubMed] [Google Scholar]
- 29.Chang YK, Liu S, Yu HH, et al. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Arch Clin Neuropsychol. 2012;27:225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- 30.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cog Aff Beh Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 31.Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:29–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- 32.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]