Abstract
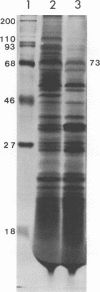
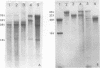
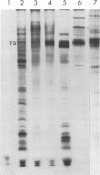
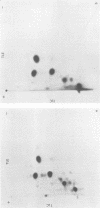
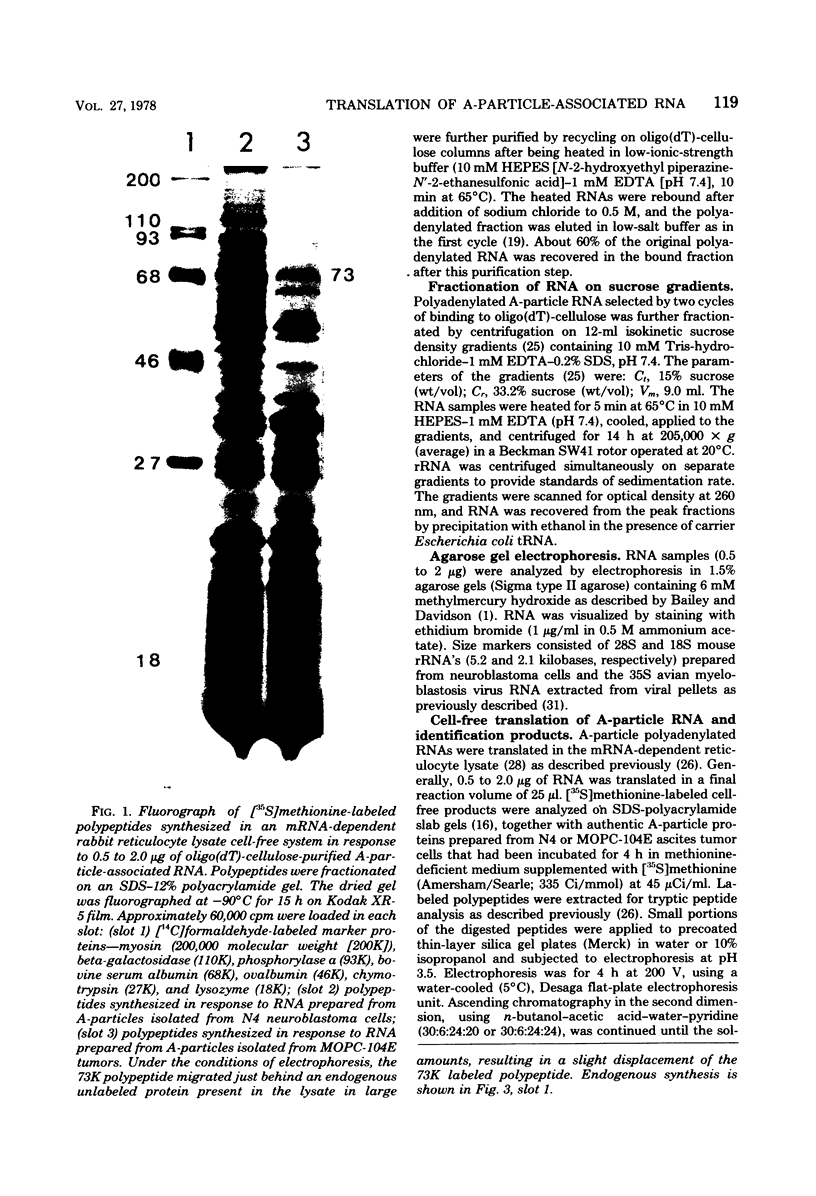
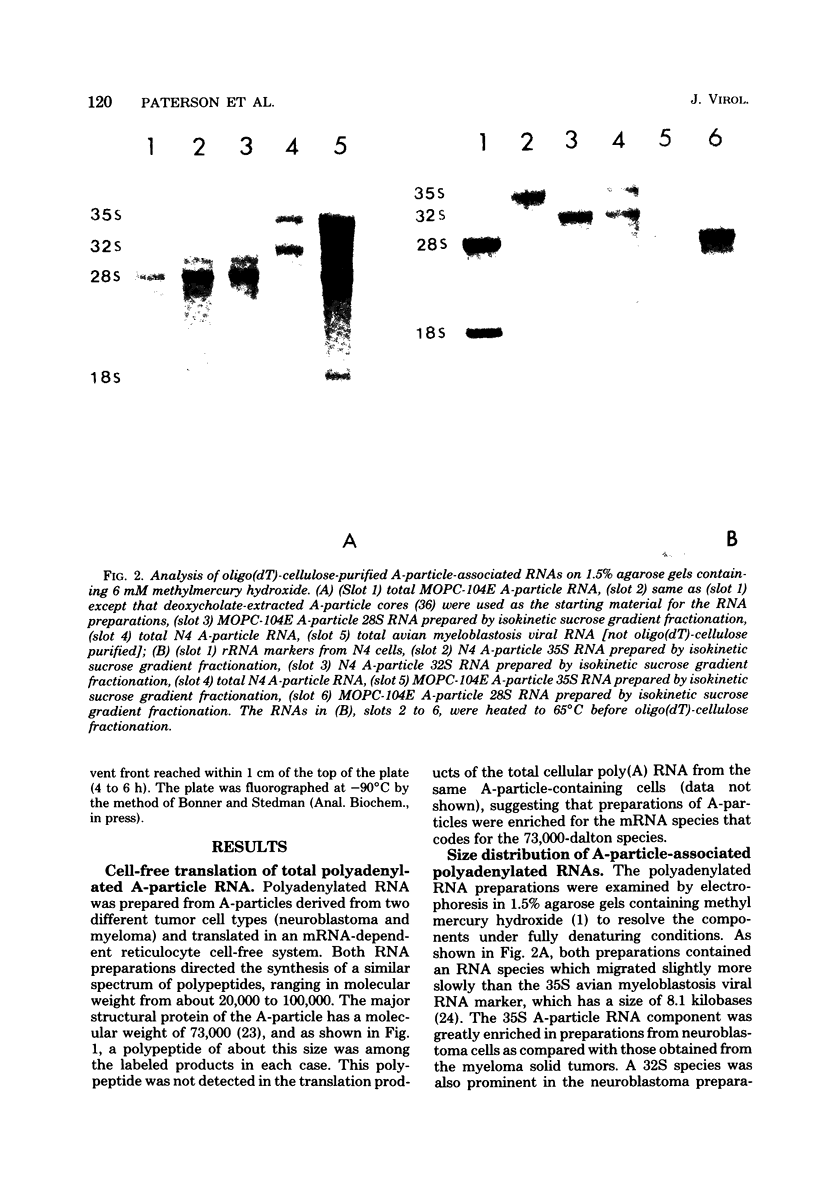
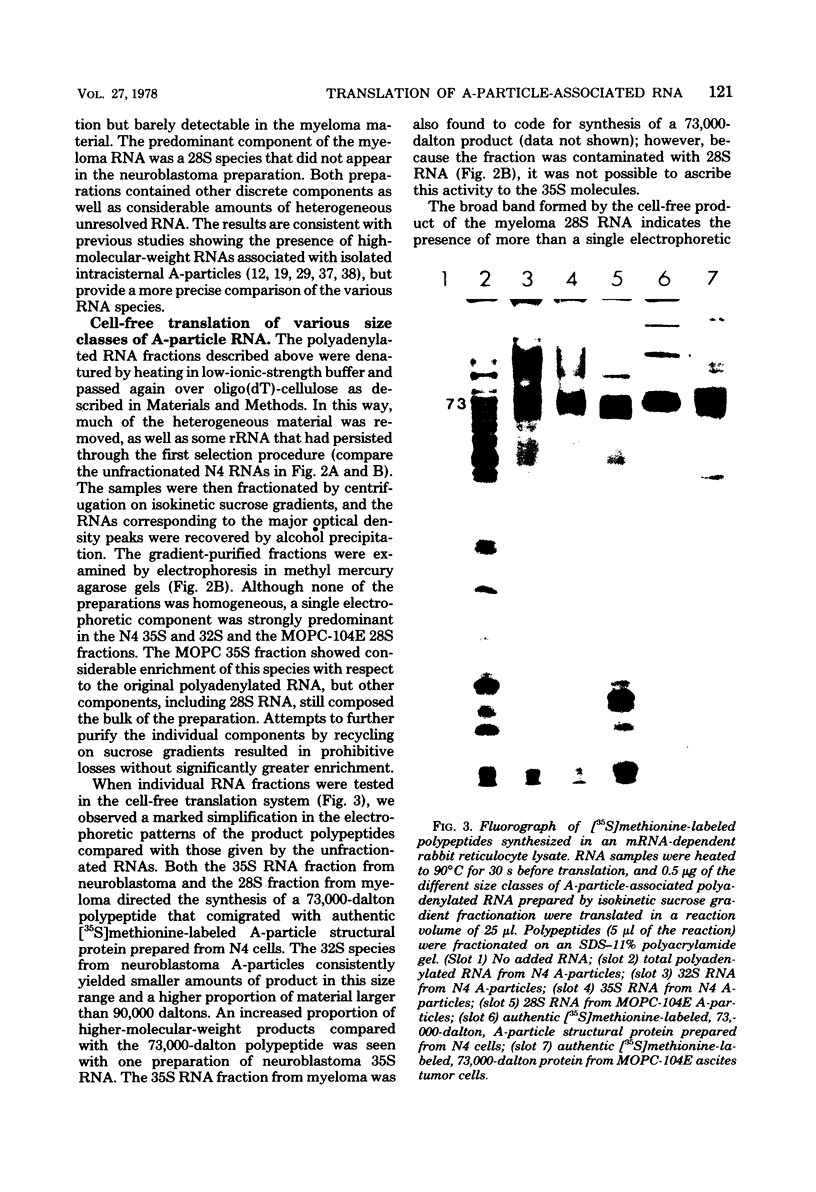
Intracisternal type A particles were isolated from MOPC-104E myeloma grown subcutaneously and from N 4 neuroblastoma cells in culture. Polyadenylated RNA was prepared from the particles and tested in a cell-free translation system derived from rabbit reticulocytes. RNA from the two sources directed the synthesis of multiple polypeptides with similar distributions of electrophoretic mobilities in sodium dodecyl sulfate-containing polyacrylamide gels, including one conponent of the same size as the major A-particle structural protein (73,000 daltons). Analysis of the RNAs by electrophoresis in methyl mercury-containing agarose gels revealed a 35S component common to A-particles from both cell types. This was a major component of the N4 preparations, whereas a 28S species predominated in the case of MOPC-104E. These two RNAs (35S from N4 cells and 28S from MOPC-104E), when isolated on isokinetic sucrose gradients, each directed the synthesis of a 73,000-molecular-weight polypeptide that comigrated on gels with authentic A-particle structural protein. Idnetity of the cell-free product was confirmed by two-dimensional analysis of the [35S]methionine-labeled tryptic peptides. The N4 RNA preparations also contained a major32S component which did not code effectively for the A-particle structural protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Dina D., Beemon K., Duesberg P. The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell. 1976 Oct;9(2):299–309. doi: 10.1016/0092-8674(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Besmer P. RNA metabolism of murine leukemia virus II. Endogenous virus-specific RNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1975 Apr;15(4):836–842. doi: 10.1128/jvi.15.4.836-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Krueger R. G. Intracisternal A particles from FLOPC-1 BALB/c myeloma: presence of high-molecular-weight RNA and RNA-dependent DNA polymerase. J Virol. 1976 May;18(2):745–756. doi: 10.1128/jvi.18.2.745-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K., Orenstein J. M., Wilson S. H. Differential response of type C and intracisternal type A particle markers in cells treated with iododeoxyuridine and dexamethasone. J Virol. 1976 Aug;19(2):709–716. doi: 10.1128/jvi.19.2.709-716.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Synthesis and turnover of intracisternal A-particle structural protein in cultured neuroblastoma cells. J Biol Chem. 1975 Jul 10;250(13):5192–5199. [PubMed] [Google Scholar]
- Lueders K. K., Segal S., Kuff E. L. RNA sequences specifically associated with mouse intracisternal A particles. Cell. 1977 May;11(1):83–94. doi: 10.1016/0092-8674(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Scolnick E. M., Duesberg P. Base sequence differences between the RNA components of Harvey sarcoma virus. J Virol. 1975 Sep;16(3):749–753. doi: 10.1128/jvi.16.3.749-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Baenziger N. L., Dobbertin D. C., Thach R. E. Characterization of DNA polymerase and RNA associated with A-type particles from murine myeloma cells. J Virol. 1975 Feb;15(2):407–415. doi: 10.1128/jvi.15.2.407-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Yau P., Dobbertin D. C., Sweeney T. K., Thach S. S., Brendler T., Thach R. E. Relationships between intracisternal type A and extracellular oncornavirus-like particles produced in murine MOPC-460 myeloma cells. J Virol. 1976 Apr;18(1):344–355. doi: 10.1128/jvi.18.1.344-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Shih M. S., Gilden R. V., Hatanaka M. Sarcoma and helper-specific RNA tumor virus subunits in transformed nonproducer mouse cells activated to produce virus by treatment with bromodeoxyuridine. J Virol. 1974 Nov;14(5):1262–1267. doi: 10.1128/jvi.14.5.1262-1267.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Ralph P., Sarkar S., Cohn M. Leukemia viruses associated with mouse myeloma cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):344–351. doi: 10.1073/pnas.66.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivel N. A., Lueders K. K., Kuff E. L. Structural organization of murine intracisternal A particles. J Virol. 1973 Feb;11(2):329–334. doi: 10.1128/jvi.11.2.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Analysis of high-molecular-weight ribonucleic acid associated with intracisternal A particles. J Virol. 1973 Feb;11(2):287–298. doi: 10.1128/jvi.11.2.287-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Physicochemical analysis of the deoxyribonucleic acid product of murine intracisternal A particle RNA-directed DNA polymerase. Biochim Biophys Acta. 1976 Oct 4;447(2):167–174. doi: 10.1016/0005-2787(76)90340-3. [DOI] [PubMed] [Google Scholar]
- Zaane D. V., Gielkens A. L., Hesselink W. G., Bloemers H. P. Identification of Rauscher murine leukemia virus-specific mRNAs for the synthesis of gag- and env-gene products. Proc Natl Acad Sci U S A. 1977 May;74(5):1855–1859. doi: 10.1073/pnas.74.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]