Abstract
Objectives
To elucidate shear dependent effects of deformation of the endothelial glycocalyx on adhesion of circulating ligands in post-capillary venules, and delineate effect of matrix metalloproteases (MMPs).
Methods
Adhesion of leukocytes (WBCs) and lectin-coated fluorescently labeled microspheres (FLMs, 0.1 μm diameter), to endothelium (EC) of post-capillary venules in mesentery was examined during acute reductions in shear rates (γ̇, hemorrhagic hypotension). Adhesion was examined with or without superfusion with 0.5 μM doxycycline to inhibit MMPs. Thickness of the glycocalyx was measured by exclusion of fluorescent 70 kDa dextran from the EC surface.
Results
During superfusion with Ringers, rapid reductions in γ̇ resulted in a significant rise in WBC adhesion and a two-fold rise in microsphere adhesion. With addition of doxycycline WBC and FLM adhesion increased two-fold under high and low flow conditions. FLM adhesion was invariant with γ̇ throughout the network in the normal (high) flow state. With reductions in γ̇, thickness of the glycocalyx increased significantly, with or without doxycycline.
Conclusions
The concurrent increase in WBC and FLM adhesion with increased thickness of the glycocalyx during reductions in shear suggests that glycocalyx core proteins recoil from their deformed steady state configuration, which increases exposure of binding sites for circulating ligands.
Keywords: Low flow state, hemorrhagic hypotension, leukocyte adhesion, glycocalyx, matrix metalloproteases, doxycycline
INTRODUCTION
The interface between blood and the vascular wall has been established as an important barrier that plays a role in blood-endothelial interactions, transvascular exchange of fluid and solutes and endothelial cell (EC) function [40; 41; 52]. Early studies of the structure of the capillary wall drew attention to a specialized “hematoparenchymal barrier” derived from EC secretions that form the “intercellular cement” between adjacent ECs and the endothelial basement membrane [57]. In view of its predominant polysaccharide constituents, Bennett [4] termed it the “glycocalyx,” as derived from the Latin for “sweet husk.” In view of its labile nature and complement of proteins adsorbed from the blood stream, it has often been referred to as the endothelial surface layer [40]. The function and molecular composition of the glycocalyx has long been viewed as a principal determinant of vascular homeostasis [6]. Studies by electron microscopy have suggested that the fine structure of the glycocalyx consists of a network of glycoproteins on the order of 50 to 100 nm thick, with a characteristic spacing of 20nm that accounts for the resistance to filtration of small molecules [46]. In vivo observations by direct intravital microscopy have revealed an apparent thickness of the glycocalyx, estimated by the exclusion of erythrocytes and macromolecules [51], on the order of 400–500 nm. This dimension significantly exceeds that obtained in either fixed specimens or cultured cells and has been shown to retard the flow of fluid parallel to the EC surface [13; 39; 44].
The structure of the endothelial glycocalyx has been elucidated by biochemical and histochemical analyses. The major components have been identified as an array of transmembrane and GPI-membrane anchored proteins (syndecans and glypicans, respectively) decorated with the glycosaminoglycans (GAGs) heparan sulfate, chondroitin sulfate and hyaluronan [40; 41]. Interest in the mechanical properties of the glycocalyx has evolved from multiple perspectives: (1) Its role in affecting fluid flow on the EC surface [10; 19; 25], (2) the transmission of shear stresses to mechanosensors on the EC [42; 49; 50; 52; 53], (3) its ability to affect the passage of blood cells through narrow capillaries [7–9; 43], and (4) its effect on the mechanics of blood cell adhesion [36; 56]. Modeling of the structure of the glycocalyx has suggested that its structural rigidity results from oncotic forces that hold its constituents together [42], electrostatic interactions amongst the predominantly negatively charged GAGs [47], and flexural rigidity of the core proteins that support the GAG matrix [19; 53].
Several experiments have explored the structure and function of the glycocalyx in light of the effect of fluid shear stresses imposed by the blood stream. Studies on cultured ECs have shown that a three-fold increase in the amount of hyaluronan incorporated into the glycocalyx occurs in response to fluid shear stresses compared to static conditions [16]. Modulation of glycan synthesis by fluid shear stress may also lead to a thinner glycocalyx [15; 17]. Increased synthesis of GAGs by cultured monolayers of ECs occurs with prolonged exposure to high shear stresses of 15 or 40 dyn/cm2 [3]. In contrast, prior studies revealed a decrease in proteoglycan synthesis when ECs were cultured under low levels of shear stress [18]. In vivo studies of the accumulation of glycans on the surface of post-capillary venules during a one hour period of ischemia demonstrated a 15–40% increase in glycan content on the surface of the EC [37]. These results led to the suggestion that the composition of the EC glycocalyx represents a balance between the continued biosynthesis of new glycans and the shear dependent removal (shedding) of existing glycans. It has been shown that proteolytic cleavage of the syndecan ectodomain results from the convergence of multiple intracellular pathways that activate a cell surface metalloproteinase [12]. A likely candidate for effecting the shedding of glycans in response to shear stresses and EC activation has been identified as a member of the family of matrix metalloproteases [33; 38]. Thus, the role of shear stresses in affecting the composition and function of the glycocalyx remains to be fully described.
The current studies were undertaken to explore the role that shear stresses (τ) play in affecting the adhesion of ligands specific for glycans on the surface of the EC. To this end, lectin coated fluorescently labeled microspheres (FLM, 0.1 μm diameter) were infused into the systemic circulation of the rat to achieve a steady state circulating concentration. Previous in vitro studies have demonstrated that the adhesion of these FLMs to immobilized chondroitin sulfate could not be disrupted by increasing shear stresses from 0 to 100 dyn/cm2 [32]. The number of FLMs adhered per 100 μm length of post-capillary venule were monitored during reductions in wall shear rates from prevailing steady state values. Wall shear rates (γ̇) were used as a measure of the levels of τ acting on the glycocalyx under the assumption that τ =ηγ̇ where η is the effective viscosity of the blood stream. Acute reductions in γ̇ were induced by withdrawing blood from an indwelling catheter in a carotid artery and producing a state of hemorrhagic hypotension. The variation in FLM adhesion was obtained under normal (control) superfusion of the tissue with Ringers solution and with the addition of 0.5 μM doxycycline. Previous studies revealed that inhibition of matrix metalloprotease activity with doxycycline inhibited shedding of glycans from the EC [38] and also increased the adhesion of FLMs and leukocytes (WBCs) to the endothelium [33]. In addition to the adhesion of FLMs, WBC adhesion was measured, and structural responses were examined in the context of changes in the thickness of the glycocalyx with reductions in wall shear rate. Taken together, these experiments suggest that reductions in shear stresses on the surface of the EC cause a relaxation of the glycocalyx, which in turn increases its thickness and induces conformational changes of its constituents that increase its ability to capture circulating ligands on FLMs and WBCs.
MATERIALS AND METHODS
Animal Preparation
All animal studies conformed to the Guiding Principles in the Care and Use of Animals established by the American Physiological Society and all protocols have been approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University.
Male Wistar rats, weighing 250–400g were anesthetized with Inactin (120 mg/kg, i.p), tracheostomized, and allowed to breathe under spontaneous respiration. The right jugular vein and its paired carotid artery were cannulated with polyethylene tubing (PE-50). Supplemental anesthetic was administered via the jugular catheter, as needed, to maintain a surgical plane of anesthesia. The carotid catheter was connected to a strain-gage pressure transducer to monitor central arterial pressure, which averaged a nominal 125 mmHg. Core temperature was monitored by a rectal probe and was maintained between 36–37 °C with the aid of a heating pad.
Intravital Microscopy
The intestinal mesentery was exteriorized through a midline abdominal incision and placed on a glass pedestal to permit viewing under brightfield microscopy by either trans- or incident-illumination. The tissue was superfused with HEPES-buffered Ringer’s solution (pH = 7.4) at a temperature of 37.0 °C. Fluorescence microscopy was performed under incident illumination (Hg arc lamp) using a dichroic mirror and filters appropriate for fluorescein excitation and emission spectra. Post-capillary venules were selected for microscopic observation under bright field or fluorescent epi-illumination, using a Zeiss 40x/0.75NA water immersion objective. Visual recordings of post-capillary venules were digitized with a PCO 1600 digital CCD camera (PCO Imaging, Germany) at a spatial resolution of 1600×1200 pixels with a depth of 14 bits for subsequent analysis. The effective magnification yielded a pixel spacing of 17.4 pixels/μm and a width of the video field of 92 μm. Transmitted light images were made with tungsten illumination.
Alterations in the structure and glycan content of the endothelial (EC) surface layer were elucidated by the binding of fluorescently labeled microspheres (FLMs) coated with the lectin Bandeiraea Simplificifolia (BS1; Sigma). A bolus of coated FLMs was infused at 2 × 1012spheres/ml/kg via the jugular vein to obtain a circulating concentration of 106FLM/mm3. The circulating concentration of FLMs was maintained at that level by intravenous infusion of 2 × 1010 spheres/kg/min via the jugular vein using a syringe pump (Harvard Apparatus, Holliston, MA, model PHD-2000). Invariance of the circulating concentration of FLMs was verified by measurement of FLM concentration in blood samples withdrawn from the carotid artery, and examined with a hemocytometer under fluorescence microscopy. Images of fluorescently labeled microspheres (FLMs) adhered to the endothelium in a 100 μm length of venule were recorded while focusing above and below the microvessel diametral plane. The number of FLMs adhered per 100 μm were counted during off-line video analysis. In vitro calibration studies of the adhesion of FLMs to glass surfaces to which various concentrations of chondroitin sulfate were covalently linked revealed that the number of bound FLMs was proportional to GAG concentration, and was invariant with wall shear stresses over a physiological range of 1 to 50 dynes/cm2 [32].
Microsphere Preparation
Methods for the preparation of the lectin coated microspheres have been described in detail previously [36] and are summarized as follows. Fluorescent (yellow-green) carboxylate-modified polystyrene microspheres, 0.1 μm in diameter (FLMs, Fluospheres; Molecular Probes, Eugene, OR), were labeled by covalent linkage (carbodiimide reaction) of the lectin Bandeiraea Simplificifolia (BS1, Sigma, St Louis, MO) which preferentially binds to galactose residues on the endothelial cell surface. The protein content on the FLMs was determined by assay of the uptake of lectin from the labeling solutions using a spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD) and found to average 2×104 lectin molecules per microsphere.
Hemodynamic Measurements
To obtain a measure of hemodynamic shear rates on the EC surface, red cell velocities and microvessel diameters were measured in postcapillary venules ranging in width from 10 to 50 μm using a Zeiss water-immersion 40x/0.75 NA objective under transillumination. The image of a microscopic field was switched to project onto a CCD camera (Panasonic, WV-51CCD) for an effective width of the video field of 100 μm. Red cell velocity (VRBC) along the centerline of post-capillary venules was measured with the two-slit photometric technique using a self-tracking correlator (IPM, San Diego). The mean velocity of blood (VMEAN) was calculated from the relationship VMEAN = VRBC/1.6 [34]. Vessel diameter (D) was measured by the video image shearing technique using an image shearing monitor (IPM, San Diego). Wall shear rates were estimated from the Newtonian-flow relationship for flow in a cylindrical tube, viz. γ̇ = 8V MEAN / D.
Glycocalyx Thickness
Thickness of the EC glycocalyx was estimated using an enhancement of the method of Henry and Duling [20], as described previously [14]. In brief, a bolus of 2 % (in 0.15 ml PBS) of fluorescein isothiocyanate (FITC) labeled 70kDa dextran (Dx70, Sigma) was given i.v. (jugular vein) and fluorescence was allowed to reach a steady state level, as observed in individual microvessels under incident fluorescence microscopy. The radial profile of fluorescence intensity at the EC surface was digitized and fit by a sigmoidal curve using a least squares method. The location of the inflection point in the radially decreasing dye intensity curve was taken as the outer edge of the glycocalyx. The inflection point was found to coincide with the edge of a knife-edge target viewed under transmitted light. The surface of the EC was taken as the outer edge of the dark refractive band present near the surface of each microvessel under trans-illumination. The spatial difference between the inflection point and surface of the EC was taken as the thickness of the glycocalyx. All image processing and measurements used in the acquisition and analysis of the radial intensity profiles were done using ImageJ (NIH, Bethesda, MD).
Experiment Protocols
To quantitate the adhesion of FLMs and WBCs as a function of wall shear rates, hemorrhagic hypotension was induced by rapidly withdrawing blood from the carotid catheter while monitoring central arterial pressure and venular VRBC. Blood was withdrawn (typically 5–10 ml) over a 5–10 min period until VRBC fell by 50%. Red cell velocities were maintained at that level over a 30 min period by either withdrawing additional blood as needed or returning blood to the circulation via the carotid artery. To examine the effect of MMP inhibition on the relationship between shear rate and FLM and WBC adhesion, separate experiments were conducted with the addition of doxycycline to the superfusate at a concentration of 0.5 μM. For these experiments, the mesentery was irrigated with this mixture for 30 min prior to measurement of adhesion and red cell velocities under initial and low shear rate (hypotensive) conditions. In both cases of control and doxycycline superfusion, red cell velocity, FLM and WBC adhesion were sampled in 3–4 venules of each tissue to obtain a sampling under initial conditions and during a 25 min period following withdrawal of blood and onset of hemorrhagic hypotension.
Statistics
Statistical analyses of trends in the data were performed using SigmaStat (Systat, Inc. San Jose, CA) with either Student’s t-test for paired measurements or the Student-Newman-Keuls method for ANOVA of multiple comparisons.
RESULTS
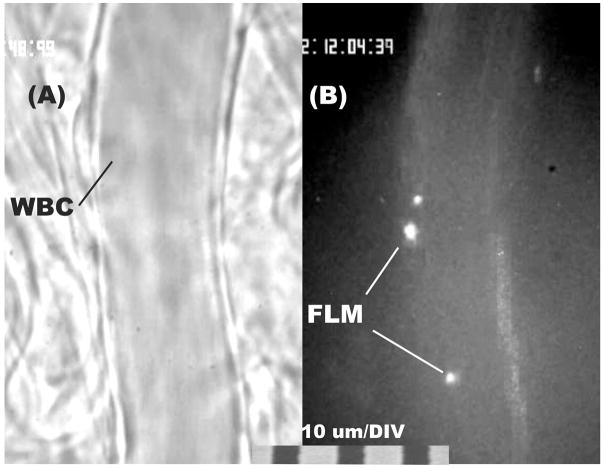
To illustrate the adhesion of WBCs and FLMs to the endothelium, shown in Fig. 1 is the image of a post-capillary venule (nominal diameter = 20 μm) in the normal flow state. Average values for hemodynamic values, WBC and FLM adhesion are summarized in Table 1. Typically, WBC adhesion in the normal flow state averaged less than 1 cell per 100 μm of venule length (Fig. 1A). As shown in Fig. 1B, adhesion of FLMs to the endothelium averaged on the order of 10 microspheres per 100 μm. Since only a fraction of these could be seen at any given depth of focus it was necessary to sample the visual field along the z-axis to count all microspheres adhered along the venule length.
Figure 1.
Illustration of adhesion of WBCs (panel A, transmitted light) and lectin coated fluorescently labeled microspheres (FLM, panel B, fluorescence epi-illumination) to the luminal surface of post-capillary venules in mesentery of the rat. Adhesion was characterized in terms of the number of WBCs or FLMs adhering per 100 μm length of venule. The total number adhered was sampled by focusing the microscope up and down.
Table 1.
Initial Values Prior to Onset of Low Flow State
| Control | Doxycycline | |
|---|---|---|
| Number Venules | 20 | 20 |
| Diameter (μm) | 33.5 ± 7.2 | 30.1 ± 8.1 |
| Diameter Range (μm) | 17.0 – 50.0 | 17.6 – 42.4 |
| Red Cell Vel, VRBC (mm/s) | 2.9 ± 1.34 | 2.8 ± 1.5 |
| Shear Rate, γ̇(sec−1) | 432.0 ± 192.3 | 478.0 ± 270.3 |
| WBC Adhesion/100 μm | 0.65 ± 0.09 | 4.4 ± 3.2* |
| FLM Adhesion/100 μm | 12.2 ± 3.6 | 10.8 ± 5.0 |
Values given as mean ± SD.
Significant rise compared to control, p < 0.001.
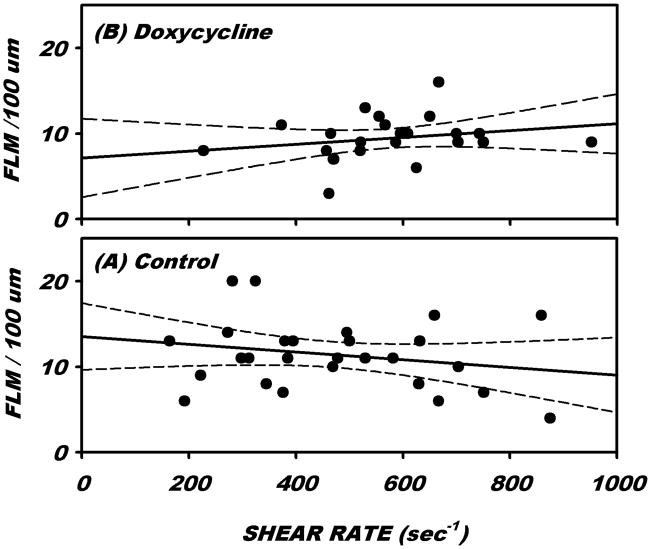
Under normal flow conditions, the number of FLMs adhered to the EC was invariant with prevailing wall shear rate, γ̇. Shown in Fig. 2 is the distribution of FLM adhesion as a function of γ̇ under control conditions (Ringers superfusion only) and with the addition of doxycycline (0.5 μM). Linear regressions revealed a slope that was not significantly different from 0 over a broad range of γ̇ from 100 to 900 sec−1, p = 0.272 and 0.242, respectively, t-test,. FLM adhesion averaged 12.2 per 100 μm of venule length (Table 1) for an average γ̇ of 432 sec−1. Following superfusion of the tissue with doxycycline, average FLM adhesion and γ̇ were not significantly different from those with Ringer’s solution alone (Table 1).
Figure 2.
Adhesion of fluorescently labeled microspheres (FLMs) as a function of prevailing steady state wall shear rates under (A) control conditions (superfusion with Ringers solution only) and (B) with the addition of 0.5 μM doxycycline. Each case revealed no significant trend over a broad range of wall shear rates ranging from 200 to 900 sec−1.
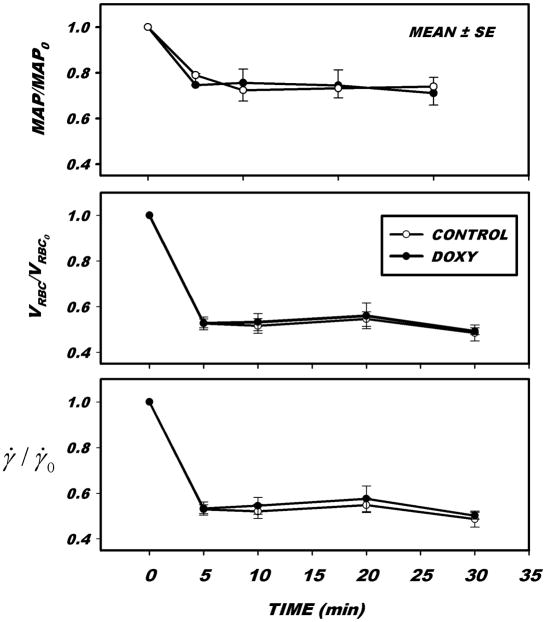
The time course of changes in hemodynamic parameters during hemorrhagic hypotension is illustrated in Fig. 3, for both control (Ringer’s solution alone) and with the addition of doxycycline in the superfusate. A 30% reduction in mean arterial pressure was sufficient to reduce VRBC by 50%. Further reductions were found to be impractical since they presented difficulties in maintaining a sustained level of flow over a prolonged period. As indicated, this reduction in VRBC resulted in a similar reduction in γ̇ which could be maintained over a 30 min period. The addition of doxycycline to the superfusate did not affect the time course of hemodynamics in the low flow state.
Figure 3.
Hemodynamic trends during induction of a low flow state by hemorrhagic hypotension. Following a control period, blood was withdrawn from a carotid catheter while measuring mean arterial pressure (MAP) and red cell velocity (VRBC) in post-capillary venules. A sufficient volume of blood was withdrawn to reduce VRBC by 50%, which was maintained at that level by either further withdrawal or return of blood. Calculated wall shear rates (γ̇, estimated by a Newtonian flow approximation) fell, on average 50% in the monitored venule. The decline in VRBC and γ̇ was heterogeneous throughout the network of small venules, which resulted in a decrease in γ̇ as great as 80% in some microvessels. Initial values prior to the onset of hemorrhagic hypotension are given in Table 1.
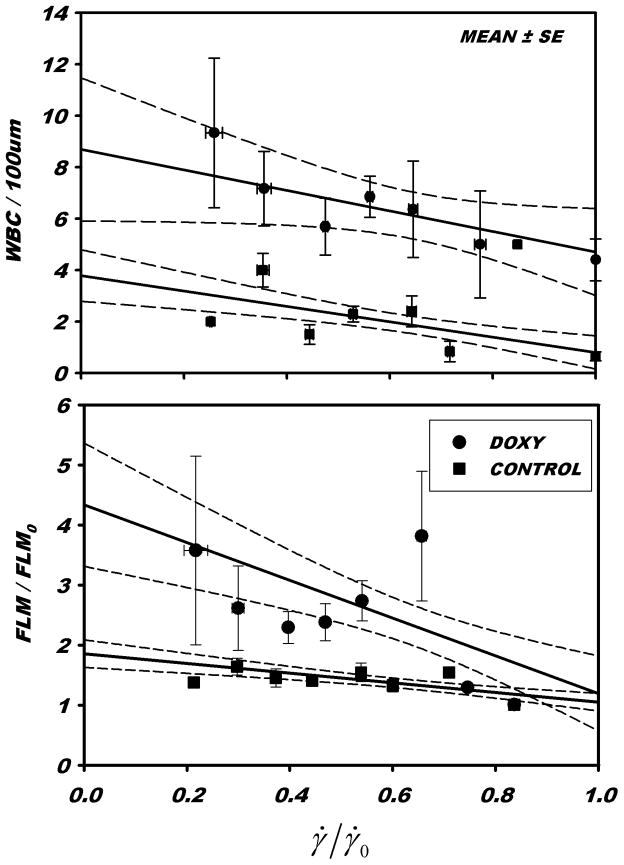
Presented in Fig. 4 is the variation of FLM and WBC adhesion with reductions in shear rate. For each venule, FLM adhesion andγ̇ were normalized with respect to their initial values obtained in the normal flow state prior to onset of the hemorrhagic hypotension. WBC adhesion per 100 μm of venule length was not normalized because many venules had little or no adhesion during the control period. Under control conditions (Ringers superfusate alone) WBC adhesion rose significantly as shear rates were reduced to 25% of control, p<0.0001. With the addition of doxycycline to the superfusate, WBC adhesion rose significantly during normal flow (p<0.001, Table 1), and was uniformly elevated for all shear rates. Adhesion rose significantly with reductions in shear rate to 25% of control, p = 0.05. The slopes of the regression lines for WBC adhesion with reductions in shear rate for normal and doxycycline superfusates were not significantly different, p=0.171. During the shear rate reductions, under control conditions and with the addition of doxycycline, FLM adhesion rose significantly, p < 10−5. The slopes of the rise in FLM adhesion were not significantly different with or without doxycycline, p = 0.749. Under control conditions, the slope of the WBC adhesion regression was not significantly different from that of the FLM adhesion, p = 0.936. With the addition of doxycycline, the slopes of WBC and FLM vs. γ̇/γ̇0 were not significantly different, p=0.149.
Figure 4.
The change in WBC and lectin coated microsphere (FLM) adhesion with acute reductions in shear rate (γ̇) for control conditions (Ringers superfusion) and with the addition of 0.5 μM doxycycline, normalized with respect to normal high flow (pre-hemorrhage) shear rate, γ̇0. The overall effect of doxycycline on WBC adhesion was to increase WBC adhesion (noted in Table 1). As γ̇/γ̇ was reduced, WBC adhesion rose significantly, with or without doxycycline, t-test on slope, p < 0.05. The rate of increase (slope) of WBC adhesion with reductions in shear with or without doxycycline were not significantly different, p = 0.171. The slope of the FLM adhesion lines, with and without doxycycline, were each significantly different from zero, p < 10−5, but were not significantly different from one another, p = 0.749.
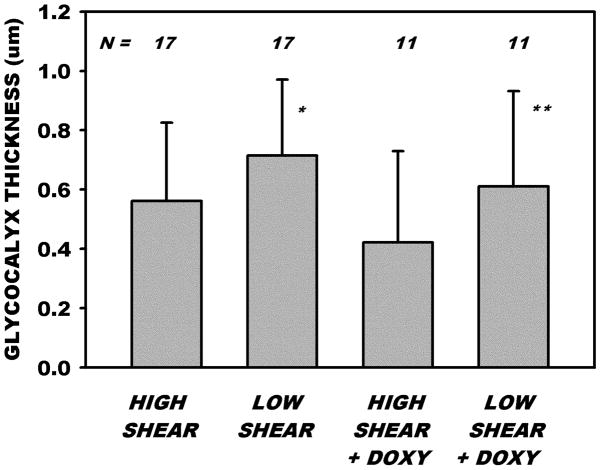
Measurements of changes in thickness of the glycocalyx with reductions in shear rate revealed (Fig. 5) that thickness increased significantly from 0.561 ± 0.265 SD to 0.715 ± 0.255 μm, p <0.027, paired t-test. During both normal (high shear) and low flow states, thickness was statistically invariant with time during the 30 min period of sampling. A linear regression of thickness vs. time revealed a correlation coefficient of r2 = 0.022, p=0.401. With doxycycline added to the superfusate (0.5 μM) thickness rose significantly from 0.422 ± 0.307 SD in the high flow state to 0.610 ± 0.321 SD μm in the low flow state, p < 0.035, paired t-test. With doxy, thickness also did not vary with time during the sampling period, r2=0.001, p=0.833. On a percentage basis, thickness increased 26% and 36% for Ringers superfusion alone and with the addition of doxy, respectively, and the differences between the two treatments were not significant, p = 0.301. The initial thicknesses of the glycocalyx at high shear was not significantly different with or without doxy, p = 0.212, nor different at low shear, p = 0.349.
Figure 5.
Measurements of the thickness of the glycocalyx under normal flow (high shear) conditions and following reduction of γ̇ by hemorrhagic hypotension. For 17 venules, thickness of the glycocalyx rose significantly from 0.5 to 0.7 μm with reduction in γ̇ from 594 ± 253 SD to 376 ± 270 SD sec−1. A similar rise in thickness occurred in the presence of doxycycline for 11 venules with reductions in γ̇, as shear rates were reduced from 600 ± 351 SD to 350 ± 309 SD sec−1. * and ** indicate significantly greater compared to respective high shear values, p < 0.01 and p< 0.05, respectively. At high shear rates (pre-hemorrhage values), thicknesses were not significantly different with or without doxycycline, nor were they different at low shear rates.
DISCUSSION
Increased adhesion of WBCs to the EC in response to reductions in flow and wall shear rate (stress) is characteristic of receptor mediated adhesion [2; 23; 30]. Quantitative studies of the rolling velocity and number of adherent WBCs on either artificial surfaces coated with receptors for specific ligands [1; 28; 29], or monolayers of cultured endothelial cells [3; 22; 27], have suggested that adhesiveness is governed by regulation of the affinity and avidity of integrin molecules on the WBC and EC [24; 26; 35; 54]. However, recent studies suggest that the endothelial glycocalyx may serve as a barrier to WBC-EC adhesive contact, since the length of adhesion receptors on the EC range from 20 nm for the β2 integrin ligands to 30–40 nm for E- and P-selectins [45], which are well within the depths of the glycocalyx. Further, the composition and structure of the glycocalyx may change by shedding of glycans in response to inflammatory stimuli, such as either the chemoattractant fMLP [14] or the cytokine TNF-α [21]. This shedding has been shown to promote exposure of adhesion receptors on the endothelial surface [5; 36]. It is generally recognized that the effectors of the shedding process may be a member of the matrix metalloproteinase family (MMPs) [12], which cleave the core proteoglycans, or a lyase such as heparanase [55] that cleaves the GAG chains. Although the precise effector of shedding and sequence of signaling events remain to be determined, recent studies suggest that inhibition of MMP activity using sub-antimicrobial doses of doxycycline may inhibit shedding of glycans from the EC and WBC adhesion in response to chemoattractants [38]. Within this framework, the present study has aimed to elucidate the shear dependency of WBC adhesion and a non-specific binding of a lectin (BS-1) to glycans on the EC surface with reductions in wall shear rates and topical application of doxycycline.
In the case of lectin coated microspheres, a significant two-fold rise in adhesion with acute reductions inγ̇ (Fig. 4) occurs over a range in γ̇ for which FLM adhesion is invariant with shear rate in the steady state (Fig. 2). These differences suggest that relaxation of the glycocalyx occurs with acute reductions in γ̇ and shear stresses acting on the endothelial surface layer. This relaxation may be manifest by an unfurling of its constituents and their extension into the venule lumen, as evidenced by the increased thickness of the glycocalyx characterized by the exclusion of FITC-Dx70 (Fig. 5).
The present measurements of thickness of the glycocalyx in post-capillary venules agree within an order of magnitude with prior measurements capillaries of other tissues, although not with the effects of variations in shear rate. As shown by Vink and Duling [51], the exclusion zone of Dx-70 remained constant at about 350–400 nm in capillaries (hamster) where red cells maintained an average velocity ranging from 25 to 200 μm/s. These thickness values are consistent with those observed in capillaries of the rat mesentery (348 nm, [31]) although much less than those measured in venules (560 nm, Fig. 5). Assuming that red cell velocity in a 5 μm diameter capillary is related to mean velocity by VRBC = VMEAN×1.3 [48], then γ̇ (= 8VMEAN/D) would be on the order of 24–190 sec−1. While this range of shear rates lies within the low end of the range measure here, it corresponds to a naturally occurring range of shear rates to which the glycocalyx could adapt, as shown in Fig. 2 for the case of constant microsphere adhesion. Neither sets of data include thickness measurements during a zero flow state. In the present studies, thickness measurements under zero flow were precluded by rapid photobleaching of the FITC labeled Dx-70 which prevented acquisition of meaningful measurements.
The current measurements of increased glycocalyx thickness with reductions in shear raise the question of the reversibility with a subsequent increase in shear. Due to the long duration of these experiments and the substantial WBC-EC adhesion incurred in the low flow state, it was technically challenging to reverse the effects of the induced low flow state. Irreversible adhesion of WBCs within the time scale of the experiment altered patterns of network perfusion that precluded return to high shear rate in many vessels with reintroduction of blood volume. Hence it was not possible to systematically evaluate reversal of the effects of low shear with the current protocols.
Modeling studies of the deformation of the glycocalyx have aimed to examine multiple factors that determine its thickness in response to fluid shear stresses or the passage of red cells or white cells through a capillary. These have included: (1) the hydraulic resistance afforded by the fiber matrix of proteoglycans and GAGs [11], (2) the structural rigidity of the principal proteins [19], (3) the resistance to compression of the layer afforded by the colloid osmotic pressure produced by its constituents [43], and (4) the effects of electrostatic interactions that may drive recovery from deformation of the glycocalyx [9]. The increase in thickness attendant to acute shear stress reductions observed here is consistent with a mechanical relaxation of the glycocalyx. This relaxation corresponds to a hypothetical elastic recoil of the core proteins attendant to reductions in fluid shear stresses, as modeled by Han et al. [19]. As suggested by the simplified model of the structure of the endothelial surface layer employed therein, the concentration of core proteins (c) on the EC surface may vary with thickness (δ ) during their elastic recoil such that c = c0δ0 / δ, where ()0 represents initial values at high shear. Thus, as fluid shear stresses are rapidly reduced, the height of the glycocalyx will increase and give rise to an effective decrease in c. It is conceivable that the diminished concentration of constituents may facilitate increased penetration of circulating ligands on the WBC or lectin coated microspheres, and lead to their increased adhesion, as shown in Fig. 4. It is also conceivable, albeit not as clearly delineated by molecular models, that relaxation (recoil) of proteoglycans and GAG chains on the EC surface my result in increased exposure of binding sites as conformational adjustments occur with diminished shear stresses. Modeling of the large deformations incurred during elastic recoil in a direction normal to the EC surface [19] suggest that the proteins unfurl in a biphasic pattern. During recovery from compression of the surface layer by the passage of a WBC, the surface layer unfurls with the proteins remaining matted down with their ends parallel to the EC membrane until the layer reaches 36% of its undeformed thickness. In the second phase, further relaxation opens up the spaces between the core proteins as they attain a position normal to the EC surface during the remaining 64% of undeformed thickness. Given the 26–36% increase in thickness (Fig. 5) found in response to sustained reduction of fluid shearing stresses, if maximal extension of the core proteins is reached in the low flow state, it is possible that the deformations incurred correspond to this second phase that is characterized by greater exposure of the length of the core proteins.
It is interesting to note that the time scale of the imposed alterations in the thickness of the glycocalyx are strikingly different. In the modeling exercise of Han et al. the entire problem was set up to simulate the rapid recovery of the glycocalyx from a crushing deformation imposed by a WBC as it squeezed through a narrow capillary, and the recovery time was typically on the order of 0.4 sec. In contrast, the present study focuses on the relaxation of the glycocalyx over a 10 min period. Attempts to impose a rapid cessation of flow accompanied by measurements of the thickness of the glycocalyx were confounded by the rapid photobleaching of the fluorophore on the Dx70 molecule during a no-flow period and hence rapid transient deformations were not possible.
The basis for the added effect on adhesion by doxycycline is not as simply delineated. Doxycycline (doxy) is recognized as a broad spectrum inhibitor of matrix metalloproteases and a modest chelator of divalent cations. Superfusion of the mesenteric tissue with doxy (0.5 μM) inhibited the activation of MMPs on the surface of the mesenteric venules, as evidenced by the attenuated cleavage of circulating substrates introduced into the blood stream [38]. Studies contrasting the relative effects of doxycycline and EDTA (a strong chelator of divalent cations) suggest that the effects of doxycycline are not due to its ability to chelate divalent cations [33]. As shown therein, measurement of the adhesive interactions of WBCs with the EC revealed decreased adhesion and increased rolling velocity of WBCs in response to EDTA, suggestive of diminished adhesive forces. In contrast, with doxycycline, WBC adhesion increased and rolling velocity decreased, suggestive of an increased adhesiveness, presumably because of inhibition of sheddase activity that resulted in a net increase in adhesion receptors on the EC. Thus, given that doxycycline is electrically neutral, it appears unlikely that it affects electrostatic interactions that may induce conformational changes of constituent proteins. The significantly greater increase of FLM adhesion with reductions in shear rate compared to that with Ringers solution alone (Fig. 4) may thus represent the effects of increased receptor concentration on the EC arising from MMP inhibition. The similarity of the slopes of WBC adhesion vs γ̇γ̇0 with and without doxy may be due to the greater physical size of the WBC, its deformability and the uniqueness of integrin mediated adhesion compared to the less specific carbohydrate binding of the lectin coated FLMs.
The finding that doxy did not affect the thickness of the glycocalyx, nor its percentage increase with acute reductions in shear (Fig. 5) suggests that shear rate reductions did not alter the composition of the glycocalyx. In contrast, previous measurements following a one hour period of ischemia revealed a 30% increase in accumulation of lectin coated microspheres on the venule wall that was interpreted as an accumulation of glycans synthesized during the ischemic period [37]. However, this accumulation was quickly washed off the vessel wall with reperfusion. Given that the flow reductions imposed here were not as severe, and of lesser duration, it is unlikely that significant changes in composition of the glycocalyx occurred within the 10 min following flow reduction. Thus, the present results may reflect the lessening of molecular deformation due to reduced fluid shear stresses acting on the glycocalyx and not the attenuation of enzymatic processes that affect the structure of the glycocalyx.
In conclusion, the present studies have aimed to elucidate the potential for deformation of the venular glycocalyx to affect adhesion of circulating ligands. To this end, it has been found that during rapid reduction in wall shear rates on the endothelial surface both WBC-EC adhesion and the non-specific binding of lectin coated microspheres rapidly increase. The increase in microsphere adhesion occurs over a range of shear rates similar in magnitude to that of the normal (high) flow state for which microsphere adhesion is invariant with shear rate. This finding suggests that the endothelial surface layer is matted down at high shear and unfurls during rapid flow reductions to increase the number of potential binding sites for circulating ligands. Further, inhibition of normally occurring sheddase activity with the MMP inhibitor doxycycline, enhances the effects of shear rate reductions, presumably by increasing the number of available binding sites. The apparent recoil of the glycocalyx with reductions in shear appears to be supported by the observed increase in its thickness, as evidenced by the exclusion of Dx70 from the EC surface, which does not appear to be affected by doxycycline. While these results are consistent with some aspects of models of the structural rigidity of the glycocalyx, the present study only scratches the surface of a complex problem. Further studies will be necessary to elucidate the molecular dynamics of constituents of the glycocalyx during rapid reductions in shear.
Acknowledgments
Supported by NIH R01 HL39286-20
References
- 1.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arfors KE, Lundberg C, Lindbom L, Lundberg K, Beatty PG, Harlan JM. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- 3.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennett HS, Luft JH, HAMPTON JC. Morphological classifications of vertebrate blood capillaries. Am J Physiol. 1959;196:381–390. doi: 10.1152/ajplegacy.1959.196.2.381. [DOI] [PubMed] [Google Scholar]
- 5.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 6.Copley AL. Hemorheological aspects of the endothelium-plasma interface. Microvasc Res. 1974;8:192–212. doi: 10.1016/0026-2862(74)90094-6. [DOI] [PubMed] [Google Scholar]
- 7.Damiano ER, Duling BR, Ley K, Skalak TC. Axisymmetric pressure-driven flow of rigid pellets through a cylindrical tube lined with a deformable porous wall layer. J Fluid Mech. 1996;314:163–189. [Google Scholar]
- 8.Damiano ER. The effect of the endothelial-cell glycocalyx on the motion of red blood cells through capillaries. Microvasc Res. 1998;55:77–91. doi: 10.1006/mvre.1997.2052. [DOI] [PubMed] [Google Scholar]
- 9.Damiano ER, Stace TM. A mechano-electrochemical model of radial deformation of the capillary glycocalyx. Biophys J. 2002;82:1153–1175. doi: 10.1016/S0006-3495(02)75474-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Weinbaum S. Lubrication theory in highlly compressible porous media: the mechanics of skiiing, from red cells to humans. J Fluid Mechanics. 2000;422:281–317. [Google Scholar]
- 12.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Lipowsky HH. Measurement of solute transport in the endothelial glycocalyx using indicator dilution techniques. Ann Biomed Eng. 2009;37:1781–1795. doi: 10.1007/s10439-009-9743-9. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259:393–400. doi: 10.1111/j.1365-2796.2006.01625.x. [DOI] [PubMed] [Google Scholar]
- 16.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–2. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 17.Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- 18.Grimm J, Keller R, de Groot PG. Laminar flow induces cell polarity and leads to rearrangement of proteoglycan metabolism in endothelial cells. Thromb Haemost. 1988;60:437–441. [PubMed] [Google Scholar]
- 19.Han Y, Weinbaum S, Spaan J, Vink H. Large-deformation analysis of the elastic recoil of fibre layers in a Brinkman medium with application to the endothelial glycocalyx. Journal of Fluid Mechanics. 2006;554:217–235. [Google Scholar]
- 20.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 21.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 22.Hoover RL, Folger R, Haering WA, Ware BR, Karnovsky MJ. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- 23.House SD, Lipowsky HH. Leukocyte-endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- 24.Kinashi T, Katagiri K. Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL. Immunol Lett. 2004;93:1–5. doi: 10.1016/j.imlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol. 1979;237:H481–H490. doi: 10.1152/ajpheart.1979.237.4.H481. [DOI] [PubMed] [Google Scholar]
- 26.Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 28.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 30.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipowsky HH, Gao L, Lescanic A. Shedding of the Endothelial Glycocalyx in Arterioles, Capillaries and Venules and its Effect on Capillary Hemodynamics During Inflammation. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipowsky HH, Haynes CA. Synthesis of an artificial glycocalyx for studies of leukocyte adhesion. Proceedings of the 2005 Summer Bioengineering Conference; Vail, CO. June 22–26; 2005. p. abstract 247851. [Google Scholar]
- 33.Lipowsky HH, Sah R, Lescanic A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am J Physiol Heart Circ Physiol. 2011;300:H415–H422. doi: 10.1152/ajpheart.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 35.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 37.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mulivor AW, Lipowsky HH. Inhibition of Glycan Shedding and Leukocyte-Endothelial Adhesion in Postcapillary Venules by Suppression of Matrixmetalloprotease Activity with Doxycycline. Microcirculation. 2009:1–10. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 39.Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 40.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 41.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Secomb TW, Hsu R, Pries AR. Effect of the endothelial surface layer on transmission of fluid shear stress to endothelial cells. Biorheology. 2001;38:143–150. [PubMed] [Google Scholar]
- 43.Secomb TW, Hsu R, Pries AR. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am J Physiol Heart Circ Physiol. 2001;281:H629–H636. doi: 10.1152/ajpheart.2001.281.2.H629. [DOI] [PubMed] [Google Scholar]
- 44.Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 46.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001;136:239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 47.Stace TM, Damiano ER. An electrochemical model of the transport of charged molecules through the capillary glycocalyx. Biophys J. 2001;80:1670–1690. doi: 10.1016/S0006-3495(01)76139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starr MC, Frasher WG., Jr A method for the simultaneous determination of plasma and cellular velocities in the microvasculature. Microvasc Res. 1975;10:95–101. doi: 10.1016/0026-2862(75)90023-0. [DOI] [PubMed] [Google Scholar]
- 49.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarbell JM, Shi ZD. Effect of the glycocalyx layer on transmission of interstitial flow shear stress to embedded cells. Biomech Model Mechanobiol. 2012 doi: 10.1007/s10237-012-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 52.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 53.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 2009;4:e5181. doi: 10.1371/journal.pone.0005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Chien S, Weinbaum S. Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys J. 2001;80:1124–1140. doi: 10.1016/S0006-3495(01)76090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zweifach BW. Structural makeup of capillary wall. Ann N Y Acad Sci. 1955;61:670–677. doi: 10.1111/j.1749-6632.1955.tb42521.x. [DOI] [PubMed] [Google Scholar]