Abstract
We demonstrated here that 4,5', 8-trimethylpsoralen (trioxsalen) is a valuable probe for the structure of SV40 DNA-histone complexes. Trioxsalen readily penetrated intact cells and, in the presence of 340- to 380-nm light, covalently cross-linked DNA preferentially at the sites available for micrococcal nuclease digestion. Histograms of the lengths of the regions of SV40 DNA protected from cross-linking, as visualized by electron microscopy, indicated a repeating pattern of base pairs in DNA from both infected cells and virus particles. The ability of the trioxsalen probe to act in vivo and to map the location of protected regions may provide a powerful tool for analyzing the role of nucleosomes in the structure of the virus particle and in intracellular complexes such as transcription templates and replication intermediates.
Full text
PDF


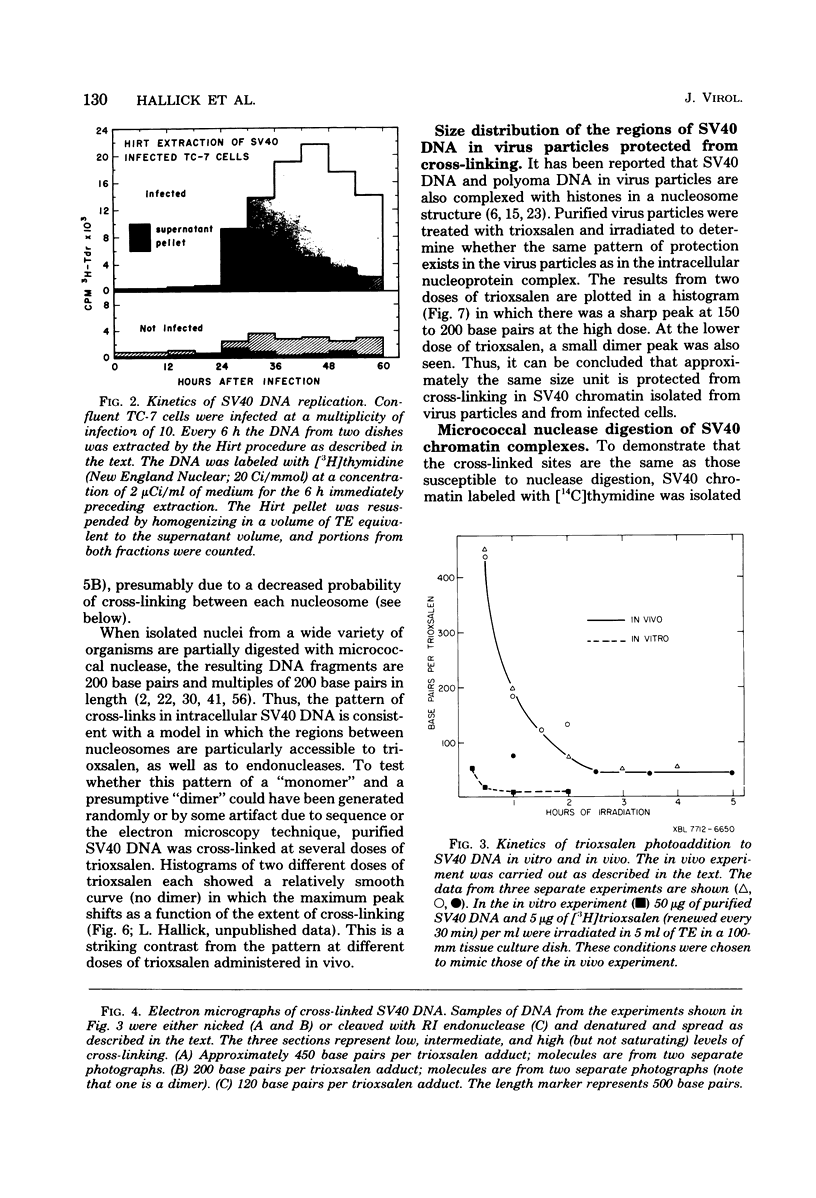
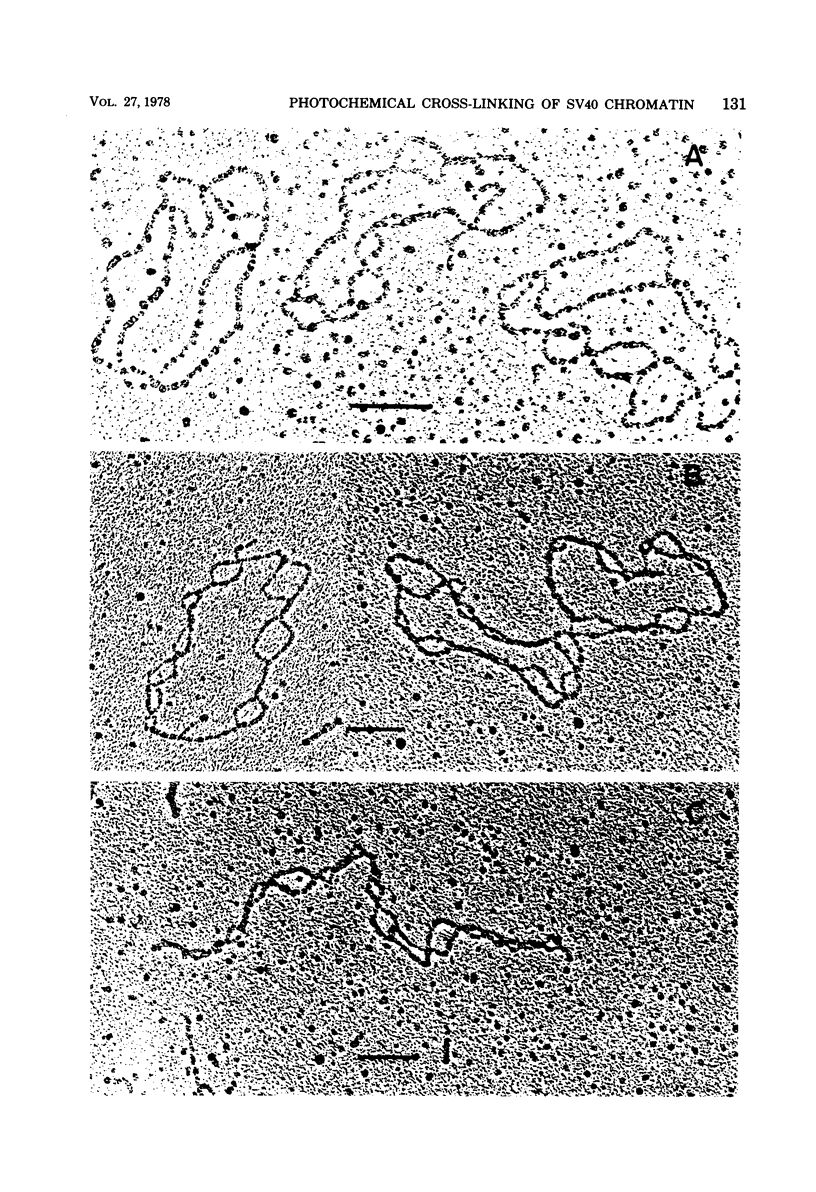
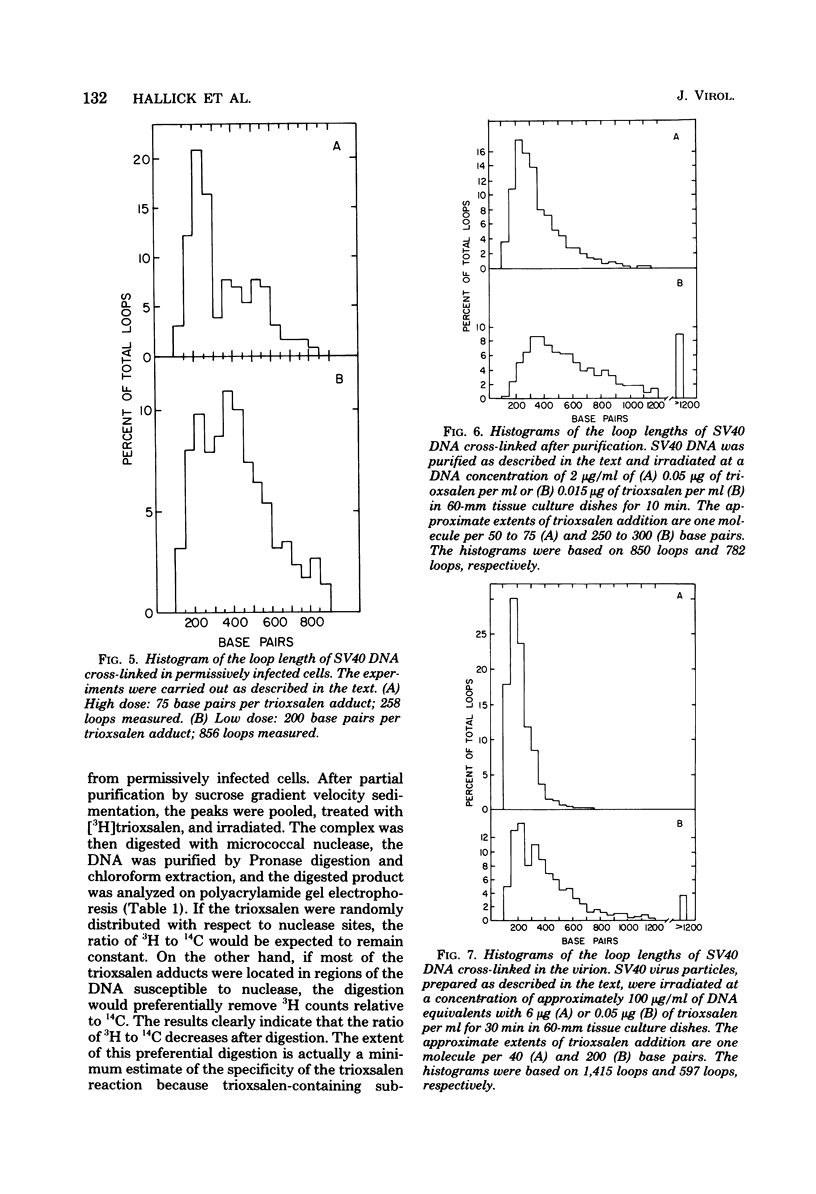


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., May E., Salzman N. P. Characterization of simian virus 40 tsA58 transcriptional intermediates at restrictive temperatures: relationship between DNA replication and transcription. J Virol. 1977 Jun;22(3):702–710. doi: 10.1128/jvi.22.3.702-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne L. A., Hewish D. R., Mobbs J. Mammalian chromatin substructure studies with the calcium-magnesium endonuclease and two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1974 Oct;143(1):67–72. doi: 10.1042/bj1430067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Cremisi C., Pignatti P. F., Croissant O., Yaniv M. Chromatin-like structures in polyoma virus and simian virus 10 lytic cycle. J Virol. 1975 Jan;17(1):204–211. doi: 10.1128/jvi.17.1.204-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. Inter-strand cross-linkages occurring in the photoreaction between psoralen and DNA. FEBS Lett. 1970 Jul 29;9(2):121–123. doi: 10.1016/0014-5793(70)80330-1. [DOI] [PubMed] [Google Scholar]
- Dall'Aqua F., Marciani S., Vedaldi D., Rodighiero G. Formation of inter-strand cross-linkings on DNA of guinea pig skin after application of psoralen and irradiation at 365 nm. FEBS Lett. 1972 Nov 1;27(2):192–194. doi: 10.1016/0014-5793(72)80617-3. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Honda B. M., Baillie D. L., Candido E. P. The subunit structure of chromatin: characteristics of nucleohistone and nucleoprotamine from developing trout testis. FEBS Lett. 1974 Nov 1;48(1):156–159. doi: 10.1016/0014-5793(74)81086-0. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Krauch C. H., Krämer D. M., Wacker A. Zum Wirkungsmechanismus photodynamischer Furocumarine Photoreaktion von Psoralen-(4-14C) mit DNS, RNS, Homopolynucleotiden und Nucleosiden. Photochem Photobiol. 1967 May;6(5):341–354. doi: 10.1111/j.1751-1097.1967.tb08882.x. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F., Mazaitis A. J., Pühler A. Electron microscopy of simian virus 40 DNA configuration under denaturation conditions. J Virol. 1975 Mar;15(3):585–598. doi: 10.1128/jvi.15.3.585-598.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Engel J. D. Subunit structure of chromatin is the same in plants and animals. Nature. 1975 Apr 3;254(5499):449–450. doi: 10.1038/254449a0. [DOI] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A. Proteins in intracellular simian virus 40 nucleoportein complexes: comparison with simian virus 40 core proteins. J Virol. 1975 Mar;15(3):439–448. doi: 10.1128/jvi.15.3.439-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Rodighiero G., Breccia A., Dall'Acqua F., Malesani G. Skin-photosensitizing furocoumarins: photochemical interaction between DNA and -O14CH3 bergapten (5-methoxy-psoralen). Photochem Photobiol. 1966 Sep;5(9):739–745. doi: 10.1111/j.1751-1097.1966.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- PEACOCKE A. R., WALKER I. O. The thermal denaturation of sodium deoxyribonucleate. I. The limits. J Mol Biol. 1962 Nov;5:550–559. doi: 10.1016/s0022-2836(62)80128-4. [DOI] [PubMed] [Google Scholar]
- Pathak M. A., Krämer D. M. Photosensitization of skin in vivo by furocoumarins (psoralens). Biochim Biophys Acta. 1969 Nov 19;195(1):197–206. doi: 10.1016/0005-2787(69)90616-9. [DOI] [PubMed] [Google Scholar]
- Pignatti P. F., Cremisi C., Croissant O., Yaniv M. SV 40 nucleoprotein complexes: structural modifications after isopycnic centrifugation in metrizamide gradients. FEBS Lett. 1975 Dec 15;60(2):369–373. doi: 10.1016/0014-5793(75)80751-4. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Celis J., Kaltoft K., Leer J. C., Nielsen O. F., Westergaard O. Tetrahymena ribosomal RNA gene chromatin is digested by micrococcal nuclease at sites which have the same regular spacing on the DNA as corresponding sites in the bulk nuclear chromatin. Nucleic Acids Res. 1976 Feb;3(2):493–505. doi: 10.1093/nar/3.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., McCarthy B. Location of histones on simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2895–2899. doi: 10.1073/pnas.72.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Crawford L. V. The arrangement of nucleosomes in nucleoprotein complexes from polyoma virus and SV40. Cell. 1977 May;11(1):35–49. doi: 10.1016/0092-8674(77)90315-4. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Crew F., Crawford L. V. Comparison of nuclease digestion of polyoma virus nucleoprotein complex and mouse chromatin. J Virol. 1978 Jan;25(1):175–186. doi: 10.1128/jvi.25.1.175-186.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Tan K. B. Histones: metabolism in simian virus 40-infected cells and incorporation into virions. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2805–2809. doi: 10.1073/pnas.74.7.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Schmatchenko V. V., Bakayev V. V., Chumackov P. M., Georgiev G. P. Compact form of SV40 viral minichromosome is resistant to nuclease: possible implications for chromatin structure. Nucleic Acids Res. 1977 Oct;4(10):3303–3325. doi: 10.1093/nar/4.10.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Williamson R. Properties of rapidly labelled deoxyribonucleic acid fragments isolated from the cytoplasm of primary cultures of embryonic mouse liver cells. J Mol Biol. 1970 Jul 14;51(1):157–168. doi: 10.1016/0022-2836(70)90277-9. [DOI] [PubMed] [Google Scholar]
- van Bruggen E. F., Arnberg A. C., van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. Electron microscopy of chromatin subunit particles. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1365–1370. doi: 10.1016/0006-291x(74)90348-9. [DOI] [PubMed] [Google Scholar]