Abstract
Chronic inflammation is a potentially important pathway through which psychosocial stressors increase risk for cardiovascular disease. However, prior research on stress and inflammation has been conducted almost exclusively in high income, industrialized populations with low levels of infectious disease. In this study we test the hypothesis that psychosocial stressors are associated with elevated concentrations of C-reactive protein (CRP) among young adults in the Philippines (n=1,622), who have grown up in an ecological and epidemiological setting that differs substantially from that of the US. In addition, we apply a developmental, ecological perspective to consider whether microbial and nutritional environments in infancy alter patterns of association between stressors and CRP. Data come from the Cebu Longitudinal Health and Nutrition Survey, a prospective cohort study that began collecting data in 1983-84 when participants were in utero. A series of regression models indicate trends toward significant interactions between perceived stress and environmental factors in infancy, including exposure to animal feces, season of birth, and birth weight. Parental absence in childhood was a significant predictor of CRP in adulthood in interaction with exposure to animal feces in infancy. Positive associations between stressors and CRP were only evident for individuals with lower levels of microbial exposure in infancy, or lower birth weight. These results suggest that early environments influence the development of inflammatory phenotypes in ways that moderate sensitivity to psychosocial stressors in adulthood, and they underscore the value of a comparative, developmental approach to research on social environments, inflammation, and disease.
Keywords: inflammation, high sensitivity C-reactive protein, psychoneuroimmunology, infectious disease, cardiovascular disease, developmental origins of adult disease, human ecological immunology
1. Introduction
Inflammation plays a central role in innate immune defenses against infectious disease (Kumar et al., 2004), but recent research has focused on the role of chronic, low-grade inflammatory processes in the pathophysiology of a wide range of chronic degenerative diseases (Libby et al., 2002; Pearson et al., 2003). In particular, elevated concentrations of C-reactive protein (CRP) have been consistently associated with increased risk for cardiovascular disease (Ridker, 1998), type II diabetes (Pradhan et al., 2001), late-life disability (Kuo et al., 2006), and mortality (Harris 1999; Jenny et al., 2007). A parallel line of research has demonstrated that psychosocial stressors are positively associated with inflammation (Lutgendorf et al., 1999; Maes et al., 1999; Owen et al., 2003; McDade et al., 2006; Miller et al., 2009; Kiecolt-Glaser et al., 2010), pointing toward inflammation as a potentially important pathway through which psychosocial environments shape risk for cardiovascular disease, and other diseases of aging (Black, 2002; Steptoe et al., 2007).
Relatively few studies have pursued a developmental, life course approach to evaluating the long-term effects of early psychosocial stressors on inflammation, with a few notable exceptions. Maltreatment and social isolation in childhood have been associated with elevated CRP in young adults in New Zealand (Danese et al., 2007), while harsh family environments and socioeconomic adversity early in life predict elevated CRP and enhanced inflammatory responses to challenge in the US and Canada (Taylor et al., 2006; Miller et al., 2009; Miller et al., 2010).
A limitation of current research on stress, inflammation, and disease is that it has focused almost exclusively on individuals living in affluent, industrialized settings where the epidemiologic environment is defined by low prevalence of infectious diseases and high prevalence of chronic degenerative diseases of aging. Yet three-fourths of the world’s deaths from coronary heart disease now occur in lower and middle income nations (Gaziano et al., 2010). This fact alone should motivate more international research on the causes and consequences of variation in inflammation, particularly since evidence is accumulating for considerable differences in baseline CRP concentrations across international settings (Vikram et al., 2003; Albert et al., 2004; Araújo et al., 2004; Ye et al., 2007).
In addition, there is a particularly compelling scientific rationale for embracing a more comparative, ecological approach: The human immune system is characterized by considerable developmental plasticity and ecological sensitivity, and nutritional and microbial exposures in infancy are important determinants of variation in aspects of immunity within and across populations (Yazdanbakhsh et al., 2002; McDade, 2003; McDade, 2005; Blackwell et al., 2010). By historical standards, people living in contemporary environments like the US enjoy unprecedented access to calorie-dense foods, low demands for physical activity, and regimes of sanitation and hygiene that have reduced—by orders of magnitude—the frequency and diversity of microbial exposures and burdens of infectious disease (Barrett et al., 1998; Popkin et al., 2004; Rook et al., 2004). From the perspective of ecology and evolutionary biology, it is reasonable to ask whether research conducted exclusively among over-nourished, “under-infected” populations can adequately capture the full range of variation that is necessary to understand the determinants of inflammatory phenotypes and their implications for disease (McDade, 2003; Gurven et al., 2008).
The implications of this perspective for research on social environments, inflammation, and disease have yet to be explored. The first objective of this paper is to investigate whether psychosocial stressors (perceived stress in adulthood, adversity during childhood), predict elevated CRP among young adults in the Philippines. The Philippines is a lower-middle income nation that exemplifies current global trends toward increasing rates of overweight/obesity, cardiovascular diseases, and the metabolic syndrome as a result of recent economic, dietary, and lifestyle transitions (Tanchoco et al., 2003; Adair, 2004; Pedro et al., 2007). However, infectious diseases still account for significant burdens of morbidity and mortality, and respiratory infections rank beside ischemic heart disease as the top causes of mortality (WHO, 2006). Previously, we have shown that lower birth weight is associated with higher CRP in young adulthood in the Philippines, and that elevated levels of microbial exposure in infancy predict lower CRP (McDade et al., 2010). We have interpreted these findings in light of prior research pointing toward microbial exposures in infancy as normative ecological inputs that are important to guiding the development of the immune system. In the absence of such inputs, poorly regulated, or self-directed inflammatory activity may be more likely to occur.
The second objective of this paper is to build on this prior research and consider whether associations between psychosocial stressors and inflammation are moderated by early environmental exposures—exposures that influence inflammatory phenotypes in adulthood but that may be overlooked by studies conducted in affluent industrialized settings. Specifically, we hypothesize that psychosocial stressors will be positively associated with CRP for individuals born at lower birth weight, and for individuals with lower levels of microbial exposure in infancy. For individuals born at higher birth weights, and with higher levels of microbial exposure, we hypothesize that associations between stressors and CRP will be attenuated or absent.
2. Methods
2.1. Participants and data collection
The Cebu Longitudinal Health and Nutrition Survey (CLHNS) is an ongoing cohort study in Metro Cebu, Philippines. Cebu is the second largest metropolitan area in the Philippines, and when the CLHNS began collecting data in 1983 Filipino families lived in a wide range of settlements, including rural towns and remote outlying areas, as well as dense urban areas with affluent neighborhoods and poorly constructed squatter camps (McDade et al., 2001; Adair et al., 2011). Approximately half the homes in the study had electricity, more than three quarters collected water from an open source, less than half used a flush toilet, and more than half had animals (e.g., dogs, chickens, goats, pigs) roaming under, around, or in the house. Since the CLHNS included households across the full range of socioeconomic conditions and settlement types in Cebu the sample includes substantial variation in environmental conditions.
The study began with the recruitment of 3,327 pregnant women representative of the childbearing population in Cebu (Adair et al., 2011). A single stage cluster sampling procedure was used to randomly select 17 urban and 16 rural neighborhoods, and households were surveyed to locate all pregnant women. Home visits were made prior to birth, immediately following birth, and every two months for two years. Follow-up surveys were conducted in 1991-92, 1994-95, 1998-99, and 2002, with the most recent comprehensive survey conducted in 2005 when the offspring were 20-22 years of age. Complete anthropometric, environmental, socio-demographic, psychosocial, and CRP data were available for 1,651 participants. An additional 29 women pregnant at the time of the survey were not included in the analyses due to the effect of pregnancy on inflammation, yielding a final sample size of 1,622. All data were collected under conditions of informed consent with institutional review board approval from the University of North Carolina, Chapel Hill.
Participants provided information on household demographics, economic activities and resources, environmental quality, psychosocial factors, and health behaviors in face-to-face interviews conducted in their homes. Interviewers also provided assessments of household and neighborhood attributes. Standard procedures (Lohman et al., 1988) were implemented in the home to collect anthropometric measures of standing height (without footwear), weight (in light clothing), skinfold thicknesses, and waist circumference.
The original sample is representative of live singleton births to women in Cebu in 1983-84, but a substantial number of participants have been lost to follow up, mainly due to migration out of the Metro Cebu area. We evaluated how the sample for this analysis differed from the original cohort as assessed when the study started in 1983. Compared to those with incomplete data, participants remaining in this study had higher mean birth weights (mean (SE) difference = 58.2 (15.9) grams, p<0.001), they were more likely to come from families that owned their homes in 1983 (73.2 vs. 57.6%, p<0.001), and that lived outside of urban communities (25.2 vs. 21.8%, p<0.05). Participants did not differ with respect to household income or assets at baseline.
2.2. CRP analysis
Blood samples were collected in 2005 into EDTA-coated vacutainer tubes in the participants’ homes in the morning after an overnight fast. Blood samples were kept in coolers on ice packs during transport for no more than 2 hours and were then centrifuged at a central facility at the University of San Carlos to separate plasma prior to freezing at −70°C. Samples were express shipped to Northwestern University on dry ice and stored frozen at −80°C until analysis. CRP concentrations were determined using a high sensitivity immunoturbidimetric method (Synchron LX20, lower detection limit: 0.1 mg/L).
2.3. Measures of childhood adversity and perceived stress in adulthood
As a lower-middle income nation the overall level of material deprivation is higher in the Philippines than in the US and other western nations, and measures of socioeconomic resources may have different meanings across these ecological settings. But since socioeconomic disadvantage has been a focus of prior life course research on inflammation (Nazmi et al., 2007; Miller et al., 2009), we considered two measures of socioeconomic resources in childhood: household income and household assets. Household income was assessed at delivery in 1983-4, in 1985-86 at the end of the two-year period of infancy follow up visits, and again in 1991-92 and 1994-95 when participants were approximately 8 and 11 years old, respectively. Preliminary analyses indicated similar patterns of association with CRP across all four income measures, therefore we generated a measure of average household income in childhood across the four measurement time points. We then divided the income distribution into quintiles because of the high degree of right skew, and to facilitate consideration of non-linear associations with CRP.
Household assets provide a more stable measure of socioeconomic status in low income settings due to volatility and/or inaccuracy in reporting of household income (Vyas et al., 2006). Assets included the following: home ownership, electricity in the home, type of housing material, and ownership of items such as air conditioner, television, refrigerator, or car. Assets were assessed in 1983-4, 1985-6, 1991-92, and 1994-5, and a simple sum of items was generated at each time point and averaged across all four surveys for a summary measure of household assets in childhood.
Childhood adversity was also defined as instability in caregiving relationships. A dichotomous variable was constructed to indicate whether a participant’s mother was absent for an extended period at any point in time prior to the 1994-95 survey, when participants were approximately 11 years old. Maternal absences were due primarily to divorce/separation with residence in a separate household (~46%), extended separations for employment outside of Cebu (~41%), or maternal death (~13%). A similar dichotomous variable indicating extended paternal absence prior to 1994-95 was also created, although questions regarding the reasons for paternal absence were not included in the survey.
The level of perceived stress in young adulthood was assessed during the 2005 survey using a modified version of the Perceived Stress Scale (Cohen et al., 1983). The PSS measures the degree to which respondents appraise situations in their lives as stressful, with an emphasis on the levels of stress experienced during the preceding month. The 10-item version of the PSS was implemented in face-to-face interviews, and participants were asked to rate on a five point scale (0=never, 4=very often) how often they felt a certain way. The PSS was administered in Cebuano, after being translated from English and back-translated to confirm accuracy. As a result of preliminary research with the PSS in Cebu, prior to implementation in the 2005 survey, two questions (item 9, “how often have you been angered”; item 10, “how often have you felt difficulties were piling up”) were replaced with two new questions: “In the last 4 weeks, how often have you dealt successfully with irritating life hassles?” and “In the last 4 weeks, how often have you felt that you were effectively coping with important changes that were occurring in your life?” Scores were summed across all 10 questions, following reverse scoring of six positively stated items.
2.4. Measures of prenatal nutrition and microbial exposure in infancy
In a prior analysis of this dataset we evaluated multiple measures of the prenatal and postnatal nutritional environment as predictors of CRP in young adulthood, including birth weight, gestational age, parity, breastfeeding, and growth in length and weight (McDade et al., 2010). Birth weight was the only significant predictor of CRP, and we therefore consider it in this analysis as an indicator of the quality of the prenatal nutritional environment following an extensive body of previous research (Barker et al., 1989; Kuzawa et al., 2003). As in many lower income nations, intra-uterine growth restriction (IUGR) is common in the Philippines, most likely due to high rates of maternal undernutrition during pregnancy. In the CLHNS, the prevalence of IUGR is 20.9 percent (Adair, 1989). Birth weight was measured at delivery, when newborns were weighed by hospital nurses or birth attendants trained in the use of Salter scales.
Similarly, following prior research in the Philippines and elsewhere (VanDerslice et al., 1994; Nurgalieva et al., 2002; Prado et al., 2003), we have previously considered several household-based measures of exposure to infectious microbes in infancy as predictors of CRP in young adulthood (McDade et al., 2010). Three measures emerged as significant predictors of CRP in this sample, and are therefore the focus of this analysis: frequency of infectious diarrhea, exposure to animal feces, and season of birth.
Information on infectious morbidity was collected during the first two years as part of in-home interviews conducted at bimonthly intervals following birth. Mothers were asked whether their infants had shown symptoms of diarrhea during the week preceding the interview, and the number of intervals when symptoms were present was summed. Since we do not have a complete accounting of diarrheal frequency in infancy it is not possible to determine whether this variable represents the level of infectious diarrhea experienced by a participant, or if it functions as a more general measure of the likelihood exposure to infectious microbes in the home (McDade et al., 2010). Exposure to animal feces in the home was assessed by summing the number of bimonthly intervals that the interviewer observed that the infant was crawling, and that animals were present in the home.
Lastly, a dichotomous variable was constructed to indicate birth in the dry season. The Philippines is a seasonal tropical environment, and prior analyses have shown that rainfall, poor drainage, and water supplies contaminated by heavy rains are associated with spread of pathogens and infectious morbidity in Cebu (Moe et al., 1991; VanDerslice et al., 1994). The dry season was defined as February through April. The frequency of infectious disease in the 12 months after delivery was significantly higher for individuals born in the dry season, suggesting higher levels of microbial exposure in early infancy for dry season births (McDade et al., 2010).
2.5. Additional covariates
In order to assess infectious morbidity at the time of blood collection, we asked participants if they were currently experiencing any symptoms of infection. Symptoms included runny nose, cough, fever, diarrhea, sore throat, as well as the more general categories of “flu,” “cold,” and “sinusitis”. Responses were used to construct a single variable indicating the presence of any infectious symptoms at the time of blood collection.
Other adult characteristics included anthropometric measures of adiposity (waist circumference, sum of triceps, subscapular, and supra-iliac skinfold thicknesses), individual health behaviors shown previously to be related to CRP in this sample (oral contraceptive use), and two measures of SES: household income and household assets. Following prior cross-sectional analyses of the predictors of CRP in this sample (McDade et al., 2009) we also constructed a household pathogen exposure variable based on five measures, each scored on a three point scale (0=low exposure, 1=moderate, 2=high) and assessed at the time of CRP measurement: cleanliness of the food preparation area, means of garbage disposal, presence of excrement near the house, level of garbage and excrement present in the neighborhood surrounding the household.
2.6. Data analysis
We evaluated a series of regression models investigating log-transformed (base 10) CRP as a continuous dependent variable. We applied Tobit regression models for censored data to account for non-normality in the distribution of log-CRP values. Left censoring of the distribution is due to the large number of observations with values below the lower detection limit of the CRP assay (N=612), which follows from the low median CRP concentration in this sample. The application of ordinary least squares regression procedures would likely result in biased and unstable parameter estimates, whereas Tobit regression takes into account the censored nature of the distribution to provide more reliable parameter estimates (Greene, 2000). All statistical analyses were conducted with Stata for Windows, version 11.1 (StataCorp, College Station, TX).
Studies measuring CRP as a biomarker of chronic, low-grade inflammation typically remove from the analysis individuals with acute elevations in CRP due to infection or injury since these values do not represent baseline levels of inflammatory activity that are predictive of subsequent risk for chronic disease. A recent joint scientific statement from the American Heart Association and the Centers for Disease Control and Prevention (Pearson et al., 2003) recommends using CRP > 10 mg/L as the cut point for identifying acute inflammation, although recent research has suggested that CRP concentrations above 10 mg/L are also predictive of CVD risk (Mueller et al., 2002). The 10 mg/L cut point is derived from research in the US, and it is not clear if it is appropriate in settings like the Philippines, particularly given the higher overall level of infectious disease, and the lower median CRP concentration in this population (McDade et al., 2009). Therefore, for this study we used the presence of infectious symptoms at the time of blood collection to identify individuals that were likely to have elevated CRP due to an active acute phase response.
Analyses proceeded in four stages. First, we considered the bivariate association between perceived stress and CRP concentration. We then added variables representing aspects of the environment in adulthood that may confound the association between stress and inflammation, and that our prior analyses have reported as predictors of CRP in this sample (McDade et al., 2009). These variables included gender, waist circumference, skinfold thickness, household hygiene, oral contraceptive use, and household assets and income in 2005. Third, we added variables representing prenatal undernutrition and postnatal infectious exposures, including birth weight, diarrhea episodes, exposure to animal feces, and season of birth. Fourth, we added interaction terms to test the hypothesis that the association between perceived stress and CRP was moderated by each of the early life variables. We then repeated this series of models using parental absence as the primary independent variable.
Alpha <0.05 was used as the criterion for statistical significance, and interaction terms with p<0.15 were interpreted due to the exploratory nature of the analyses, and because of reduced power in testing interaction terms (Selvin, 1996).
3. Results
Median CRP concentration for the entire sample was 0.2 mg/L, and was not significantly different by gender (Table 1). At the time of blood collection 226 participants (13.9%) reported symptoms of infectious disease. Median CRP was 0.65 mg/L for individuals with infectious symptoms, compared to 0.2 mg/L for those without symptoms. In a Tobit regression model predicting logCRP, infectious symptoms were significantly associated with elevated CRP (β =0.53, SE=0.07, p<0.001). Since elevations in CRP resulting from acute responses to infection are not indicative to chronic inflammation (Pearson et al., 2003), we excluded individuals with infectious symptoms at the time of blood collection from subsequent analyses.
Table 1.
Descriptive statistics for study participants. Mean (SD) values are presented for continuous variables (N=1,622).
| Assessed in infancy/childhood | |
| Birth weight (kg) | 3.016 (0.423) |
| Diarrheal episodes, 0-24 months (# intervals) | 2.3 (1.7) |
| Exposure to animal feces, 6-12 months (# intervals) | 1.2 (1.3) |
| Born in dry season (%) | 19.8 |
| Household assets, birth to ~11 years | 3.3 (1.7) |
| Household income, birth to ~11 years (pesos) | 352 (305) |
| Mother absence, birth to ~11 years (%) | 9.0 |
| Father absence, birth to ~11 years (%) | 8.2 |
| Assessed in adulthood | |
| Age (yrs) | 20.9 (0.3) |
| Waist circumference (cm) | 70.4 (7.9) |
| Sum of three skinfold measures (mm) | 48.7 (22.4) |
| Symptoms of infection (%) | 13.9 |
| Oral contraceptive use (%) | 1.5 |
| Household assets | 5.3 (2.0) |
| Household income (pesos) | 614 (1,314) |
| Perceived stress score | 17.2 (4.5) |
Parental absence in childhood was marginally associated with lower levels of perceived stress in adulthood (16.7 vs. 17.2, p=0.09). Measures of prenatal nutrition and microbial exposure in infancy were not significantly associated with parental absence or perceived stress, with the following exceptions: diarrhea episodes were more frequent for individuals with an absent parent (2.5 vs. 2.2, p=0.02), and perceived stress was lower for individuals born in the dry season (16.7 vs. 17.3, p=0.03). Consistent with our prior research in this population (McDade et al., 2010), birth weight was negatively associated with CRP in adulthood (β=−0.17, SE=0.064, p<0.01), while higher levels of exposure to animal feces in infancy were significantly associated with lower CRP (β=−0.054, SE=0.022, p<0.05). Birth in the dry season (β=−0.083, SE=0.070, p=0.23), and higher levels of infectious diarrhea in infancy (β=−0.011, SE=0.016, p=0.5) were associated with lower CRP as expected, although these associations were not statistically significant in this sample.
3.1. Association between CRP and perceived stress in adulthood
In a simple bivariate model, perceived stress was positively, but not significantly, associated with elevated CRP (Table 2, model 1). The association strengthened with the inclusion of measures of body mass, health behaviors, and socioeconomic status in adulthood, but remained statistically insignificant (model 2). The association with perceived stress remained similar with the addition of variables representing early life nutritional and microbial exposures (model 3).
Table 2.
Results of Tobit regression models evaluating the association between perceived stress and log-transformed CRP, excluding individuals with symptoms of infection (N=1,396).a
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | B | SE | B | SE | |
| Perceived stress score | 0.005 | 0.006 | 0.007 | 0.006 | 0.006 | 0.006 | 0.015+ | 0.008 | 0.011+ | 0.007 | 0.066§ | 0.042 |
| Exposure to animal feces, 6-12 mos | −0.052* | 0.021 | 0.074 | 0.085 | −0.054* | 0.021 | −0.051* | 0.021 | ||||
| Born in dry season | −0.080 | 0.067 | −0.085 | 0.067 | 0.340 | 0.265 | −0.081 | 0.067 | ||||
| Birth weight | −0.157* | 0.064 | −0.155* | 0.064 | −0.158* | 0.064 | 0.183 | 0.243 | ||||
| Perceived stress x fecal exposure | − 0.007 § | 0.005 | ||||||||||
| Perceived stress x dry season | − 0.025 + | 0.015 | ||||||||||
| Perceived stress x birth weight | − 0.020 § | 0.014 | ||||||||||
p<0.15,
p<0.10,
p<0.05
Model 1 includes the bivariate association between perceived stress score and CRP. Models 2-6 include the variables listed in the table, as well as covariates for gender, waist circumference, skinfold thickness, level of hygiene in the house in 2005, oral contraceptive use, household assets and income in 2005, and diarrhea episodes in infancy.
We next tested the hypothesis that early life exposures moderate the association between perceived stress and CRP in adulthood. We found trends toward significant interactions between perceived stress and environmental factors in infancy, including exposure to animal feces, season of birth, and birth weight (Table 2, models 4-6). For season of birth and fecal exposure, perceived stress was positively associated with CRP only for individuals with lower levels of microbial exposure in infancy (Figure 1A, B). For participants with high levels of exposure to animal feces in infancy, and for those born in the dry season (for whom post-natal infectious exposures were higher), perceived stress in adulthood was not associated with elevated CRP.
Figure 1.
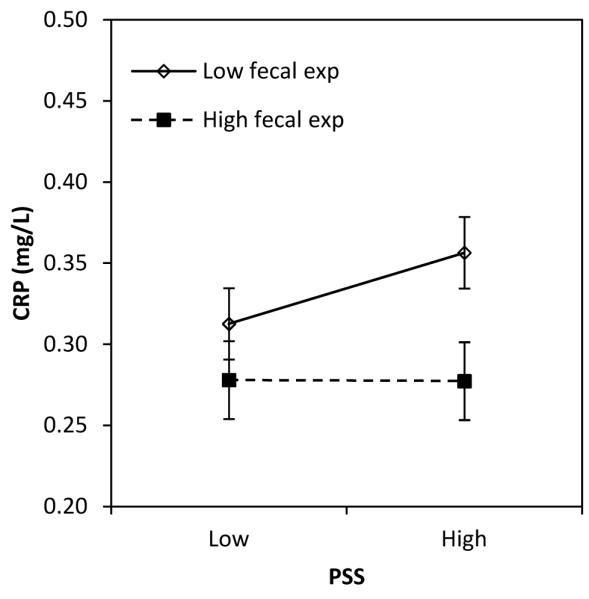
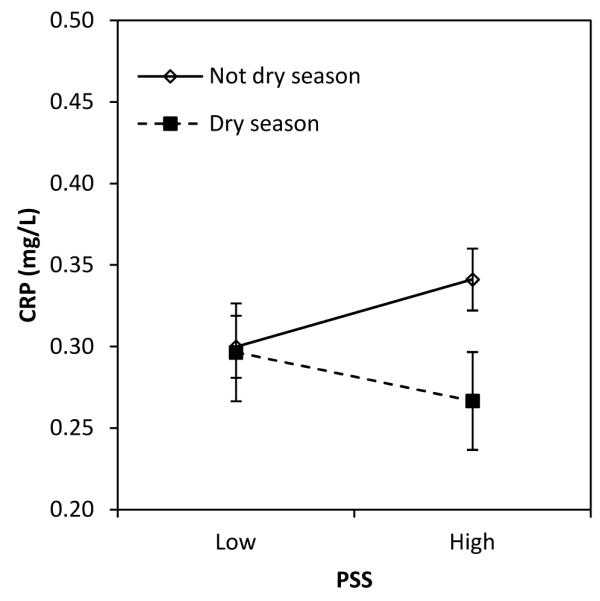
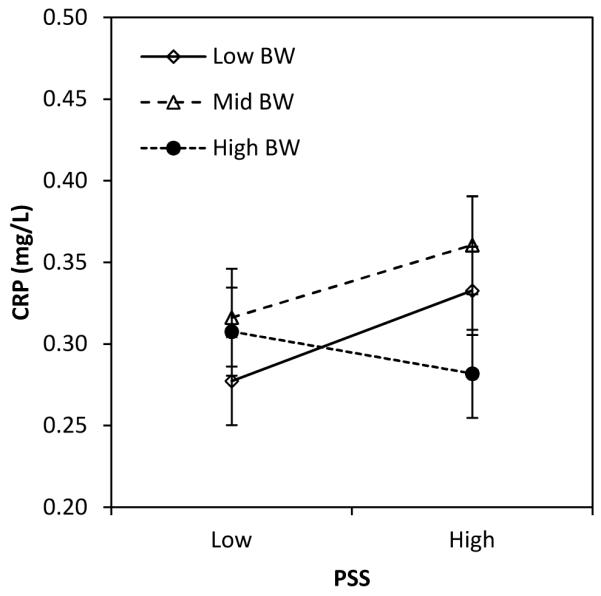
Association between perceived stress and CRP in adulthood, moderated by the level of fecal exposure in infancy (A), season of birth (B), and birth weight (C).
Note: Values are predicted concentration of CRP (±SE) based on coefficients from Table 2, models 4-6. Low and high values for predictors were set as follows: PSS (mean − 1 SD vs. mean + 1 SD); animal feces exposure (≤1 vs. 2 or more intervals); born in dry season (no, yes); birth weight (low: ≤2.8 kg; mid: >2.8 and ≤ 3.175 kg; high >3.175 kg). Original values were retained for other covariates.
Similarly, the pattern of association between perceived stress and CRP was distinct for individuals with higher versus lower birth weights. Perceived stress predicted elevated CRP for individuals born at lower birth weights, while perceived stress was negatively associated with CRP for individuals in the top third of the birth weight distribution (Figure 1C).
3.2. Association between CRP and childhood adversity
In the entire sample, 9.0% of participants experienced a prolonged maternal absence prior to age 11. Fathers were absent for 8.2% of participants. When combined, 15.7% of participants had an absent mother or father for extended periods of childhood. Father absence (β=0.169, SE=0.097, p=0.08) and mother absence (β=0.134, SE=0.095, p=0.16) both predicted elevated CRP in young adulthood in bivariate analyses, although the associations were not statistically significant. Since the number of participants with an absent mother or absent father was relatively low, and since the pattern of association was similar across both variables, we used a combined parental absence variable for subsequent analyses.
Parental absence in childhood predicted elevated CRP in young adulthood (Table 3, model 1). The association was attenuated when measures of body mass, health behaviors, socioeconomic status, and early life exposures were included in the model (Table 3, models 2 and 3). The patterns of association with CRP for all variables were virtually identical when measures of household income and assets in childhood were included in the model (results not shown).
Table 3.
Results of Tobit regression models evaluating the association between parental absence and log-transformed CRP, excluding individuals with symptoms of infection (N=1,396).a
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | B | SE | B | SE | |
| Parental absence (0, 1) | 0.149* | 0.075 | 0.132+ | 0.072 | 0.129+ | 0.072 | 0.260** | 0.081 | 0.108 | 0.080 | 0.717§ | 0.502 |
| Exposure to animal feces, 6-12 mos | −0.052* | 0.021 | −0.034§ | 0.023 | −0.052* | 0.021 | −0.052* | 0.021 | ||||
| Born in dry season | −0.082 | 0.067 | −0.083 | 0.067 | −0.100 | 0.073 | −0.081 | 0.067 | ||||
| Birth weight | −0.154* | 0.064 | −0.155* | 0.064 | −0.154* | 0.064 | −0.120+ | 0.070 | ||||
| Parental absence x fecal exposure | − 0.109 * | 0.057 | ||||||||||
| Parental absence x dry season | − 0.111 | 0.183 | ||||||||||
| Parental absence x birth weight | − 0.200 | 0.167 | ||||||||||
p<0.15,
p<0.10,
p<0.05,
p<0.01
Model 1 includes the bivariate association between perceived stress score and CRP. Models 2-6 include the variables listed in the table, as well as covariates for gender, waist circumference, skinfold thickness, level of hygiene in the house in 2005, oral contraceptive use, household assets and income in 2005, and diarrhea episodes in infancy.
We next evaluated whether the impact of parental absence on CRP was moderated by early life nutritional and infectious exposures (Table 3, model 4). Among individuals with low levels of exposure to animal feces in infancy, parental absence in childhood predicted a 47% increase in CRP concentration in young adulthood (Figure 2). In contrast, parental absence was associated with 17% lower CRP among individuals with high levels of fecal exposure during infancy. Interactions terms for parental absence and birth weight, season of birth, and frequency of diarrhea in infancy did not approach statistical significance, although associations were in the expected direction.
Figure 2.
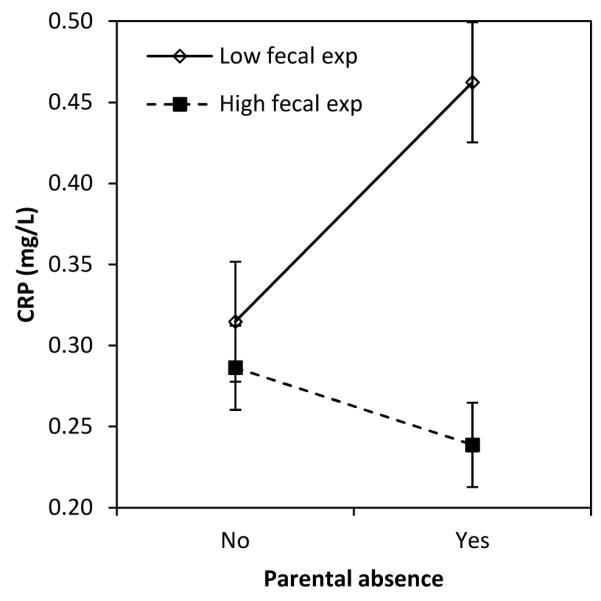
Association between parental absence in childhood and CRP in adulthood, moderated by the level of fecal exposure in infancy.
Note: Values are predicted concentration of CRP (±SE) based on coefficients from Table 3, model 4. Low and high values for predictors were set as follows: PSS (mean − 1 SD vs. mean + 1 SD); animal feces exposure (≤1 vs. 2 or more intervals). Original values were retained for other covariates.
We next considered measures of childhood socioeconomic adversity as predictors of CRP in young adulthood. We found no evidence for direct associations between adult CRP and childhood household income (β=0.003, SE=0.018, p=0.9) or assets (β=0.005, SE=0.026, p=0.7) in bivariate models. Coefficients did not change substantially, or approach statistical significance, with the addition of variables representing measures of body mass, health behaviors, adult socioeconomic status, and early life exposures. We also found no evidence for interactions between household income or assets in childhood and measures of nutritional and infectious exposures in infancy (all p-values > 0.4).
4. Discussion
Comparative, international research expands the range of ecological variation beyond what is typically considered in studies based in affluent industrialized settings, and it has the potential to generate unique insights into the determinants of human physiological function and health. In this study of young adults in the Philippines, where levels of prenatal undernutrition and postnatal microbial exposures were relatively high, we find initial evidence in support of the hypothesis that the impacts of psychosocial stressors on inflammation are moderated by environmental exposures in infancy. These findings underscore the value of a life course, ecological approach for future research on the social environment, inflammation, and disease.
Significant limitations to our study should be acknowledged up front. First, we rely on a single CRP measure to assess baseline levels of inflammation, which hampers our ability to differentiate acute from chronic, low-grade activation of inflammatory processes. Multiple CRP values would be preferable, but we use infectious symptoms to control for a major source of acute inflammation. In addition, a substantial proportion of samples were below the lower limit of detection for our CRP assay. While we implemented a relatively standard, widely used high sensitivity CRP method, it is possible that issues related to laboratory measurement or sample quality contributed to these low concentrations of CRP. We also rely on proxy measures of microbial exposure in infancy, which do not allow us to determine the type, or diversity, of microbes in the household. And lastly, since the study was originally designed to focus on issues related to maternal and child health, our measures of psychosocial stress are limited. Nonetheless, the large sample size, prospective cohort design, and wide range of environmental variation captured within the sampling frame provide exceptional opportunities for analyses of the life course determinants of health.
Positive associations in the Philippines between CRP and perceived stress in adulthood, and between CRP and adversity in childhood, are broadly consistent with prior findings from studies in the United States and New Zealand (Taylor et al., 2006; Danese et al., 2007). Similarly, analyses of gene expression have suggested that childhood adversity contributes to chronic inflammation by enhancing activation of pro-inflammatory signaling pathways, and reducing responsiveness to anti-inflammatory signaling (Miller et al., 2009; Champagne, 2010; Miller et al., 2010). Experimental animal models point toward epigenetic modifications as a potential mechanism through which early environments have lasting effects on inflammation in adulthood (Champagne, 2010; Meaney, 2010), perhaps by downregulating glucocorticoid receptor gene expression and reducing sensitivity to the anti-inflammatory effects of circulating glucocorticoids (Miller et al., 2002).
There is, however, a major difference between our study and prior research: We find no positive associations between stress and inflammation among individuals with high levels of microbial exposure in infancy. Phrased another way, psychosocial stressors predicted higher CRP only for individuals born into more hygienic environments—environments that in this respect are probably more similar to the microbial environments of affluent industrialized populations that have been the focus of prior studies. These findings should be regarded as tentative and suggestive for future research, but they are based on tests of a priori hypotheses emerging from a developmental, ecological model of inflammation that highlights the importance of microbial exposures in infancy as determinants of the regulation of inflammation in adulthood (McDade et al., 2010). They are also consistent with experimental animal model research, in which postnatal exposure to endotoxin results in larger and more rapid glucocorticoid responses to stressors later in life, and lower levels of inflammatory disease (Shanks et al., 2000).
Infectious microbes have been part of the human ecology for millennia, and it is only recently that more hygienic environments in affluent industrialized settings have substantially reduced the level and diversity of exposure (Armelagos et al., 2005; Rook, 2009). To the extent that microbial exposures are important in establishing effective regulatory networks during sensitive periods of immune development in infancy, the absence of these exposures may bias inflammatory phenotypes toward poorly regulated or self-directed activity in adulthood (Yazdanbakhsh et al., 2002; Rook et al., 2004). This may describe the situation of individuals born into more hygienic environments in the Philippines, for whom psychosocial stressors contribute to elevated concentrations of CRP. In contrast, higher levels of microbial exposure promote the development of effective anti-inflammatory regulatory networks that reduce sensitivity to the potentially pro-inflammatory effects of psychosocial stressors. Similarly, prenatal undernutrition—indicated in this study by lower birth weight—may impede the development of these regulatory networks regardless of the level of microbial exposure, and therefore enhance vulnerability to stress-induced increases in inflammation.
Consistent with the idea that microbial exposures during development shape the regulatory dynamics of inflammation in adulthood, we recently reported that chronic low-grade inflammation was absent among adults in lowland Ecuador, in a setting where infectious diseases continue to be major sources of morbidity and mortality (McDade et al., 2012). Analysis of weekly blood samples for CRP revealed frequent bouts of acute inflammation, but not a single case of elevated CRP across two or more sampling intervals. This pattern provides a striking contrast to prior studies of CRP variability in the US, where a substantial fraction of adults have consistently elevated concentrations of CRP indicative of chronic inflammatory activity (Macy et al., 1997; Ockene et al., 2001). Results from Ecuador provide evidence for the possibility of a distinct inflammatory phenotype characterized by efficient activation of inflammatory pathways, followed by downregulation of inflammation to very low levels of activity. We speculate that infectious exposures during sensitive periods of immune development may be particularly important for establishing these anti-inflammatory pathways, thereby reducing sensitivity to potentially pro-inflammatory stimuli such as psychosocial stress.
A wide range of ecological diversity is a defining characteristic of the human species, and recent attention to the early life origins of health and disease has underscored the critical role that aspects of the environment in infancy play in shaping the function of multiple physiological systems (Barker, 1994; Bateson et al., 2004; Kuzawa, 2007). A renewed focus on the life course has generated important insights into the etiology of cardiovascular and metabolic diseases in particular, and the idea that early environments influence inflammatory phenotypes may have substantial implications for future research in psychoneuroimmunology. In particular, additional explanatory power may be achieved by explicitly modeling interactions between psychosocial or cognitive/emotional factors in adulthood, and environmental exposures early in life. Interaction terms or stratified analyses may identify subgroups of individuals uniquely sensitive to the pro-inflammatory effects of, for example, perceived stress or depression, and point toward measurable factors that account for previously unexplained variance while enriching scientific understanding of the complex determinants of inflammation over the life course. It is our hope that the results presented above will serve as a catalyst for additional research along these lines.
Highlight.
The association between psychosocial stressors and C-reactive protein among adults in the Philippines is moderated by microbial and nutritional environments in infancy.
Acknowledgments
Funding for this study was provided by grants from the National Institutes of Health (RO1HL085144; 5RO1TW05596), including the Fogarty International Center (5RO3TW008133); biomarker data collection was supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Adair LS. Low birth weight and intra-uterine growth retardation in Filipino infants. Pediatrics. 1989;84:613–612. [PubMed] [Google Scholar]
- Adair LS. Dramatic rise in overweight and obesity in adult Filipino women and risk of hypertension. Obesity Research. 2004;12:1335–1341. doi: 10.1038/oby.2004.168. [DOI] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, et al. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40:619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Glynn RJ, et al. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) The American Journal of Cardiology. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- Araújo F, Pereira AC, et al. High-sensitivity C-reactive protein concentration in a healthy Brazilian population. International Journal of Cardiology. 2004;97:433–438. doi: 10.1016/j.ijcard.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Armelagos GJ, Brown PJ, et al. Evolutionary, historical and political economic perspectives on health and disease. Soc Sci Med. 2005;61:755–765. doi: 10.1016/j.socscimed.2004.08.066. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, Babies and Diseases in Later Life. BMJ Publishing Group; London: 1994. [Google Scholar]
- Barker DJ, Osmond C, et al. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43:237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RL, Kuzawa CW, et al. Emerging and re-emerging infectious diseases: The third epidemiological transition. Annual Review of Anthropology. 1998;27:247–271. [Google Scholar]
- Bateson P, Barker D, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain, Behavior, and Immunity. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Snodgrass JJ, et al. Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am J Hum Biol. 2010;22:836–848. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental Psychobiology. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, et al. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Danese A, Pariante CM, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano TA, Bitton A, et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WH. Econometric Analysis. 4th edition Prentice Hall; New Jersey: 2000. [Google Scholar]
- Gurven M, Kaplan H, et al. Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci. 2008;63:196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TBF, Tracy RP, et al. Associations of elevated Interleukin-6 and C-Reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Jenny NS, Yanez ND, et al. Inflammation biomarkers and near-term death in older men. American Journal of Epidemiology. 2007;165:684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, et al. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113–121. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Clermont G, et al. The dynamics of acute inflammation. Journal of Theoretical Biology. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Kuo H-K, Bean JF, et al. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Developmental origins of life history: growth, productivity, and reproduction. Am J Hum Biol. 2007;19:654–661. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. Am J Clin Nutr. 2003;77:960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, et al. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, et al. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- Lutgendorf SK, Garand L, et al. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. Journal of Gerontology: Medical Sciences. 1999;54A:M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy EM, Hayes TE, et al. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clinical Chemistry. 1997;43:52–58. [PubMed] [Google Scholar]
- Maes M, Lin A, et al. Elevated Serum Interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biological Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history theory and the immune system: Steps toward a human ecological immunology. American Journal of Physical Anthropology Suppl. 2003;4:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. Am J Hum Biol. 2005;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McDade TW, Adair LS. Defining the “urban” in urbanization and health: a factor analysis approach. Social Science & Medicine. 2001;53:55–70. doi: 10.1016/s0277-9536(00)00313-0. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, et al. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, et al. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of the Royal Society B-Biological Sciences. 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford JN, et al. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Tallman PS, et al. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. American Journal of Human Biology early view. 2012 doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, et al. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, et al. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe CL, Sobsey MD, et al. Bacterial indicators of risk of diarrhoeal disease from drinking water in the Philippines. Bulletin of the World Health Organization. 1991;9:305–317. [PMC free article] [PubMed] [Google Scholar]
- Mueller C, Buettner HJ, et al. Inflammation and long-term mortality after non-ST elevation acute coronary syndrome treated with a very early invasive strategy in 1042 consecutive patients. Circulation. 2002;105:1412–1415. doi: 10.1161/01.cir.0000012625.02748.62. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007:7. doi: 10.1186/1471-2458-7-212. doi:10.1186/1471-2458-1187-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgalieva ZZ, Malaty HM, et al. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. American Journal of Tropical Medicine and Hygiene. 2002;67:201–206. doi: 10.4269/ajtmh.2002.67.201. [DOI] [PubMed] [Google Scholar]
- Ockene IS, Matthews CE, et al. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clinical Chemistry. 2001;47:444–450. [PubMed] [Google Scholar]
- Owen N, Poulton T, et al. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain, Behavior, and Immunity. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pedro MR, Barba CVC, et al. Nutrition transition in the Philippines. Philippine Population Review. 2007;6:1–19. [Google Scholar]
- Popkin BM, Gordon-Larsen P. The nutrition transition: Worldwide obesity dynamics and their determinants. International Journal of Obesity. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prado MS, Strina A, et al. Risk factors for infection with Giardia duodenalis in pre-scholl children in the city of Salvador, Brazil. Epidemiology and Infection. 2003;131:899–906. doi: 10.1017/s0950268803001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Buring, Julie E, Shih, Jessie, Matias, Mathew, Hennekens, Charles H. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Rook GA, Adams V, et al. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- Rook GAW. Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press; New York: 1996. [Google Scholar]
- Shanks N, Windle RJ, et al. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, et al. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Tanchoco CC, Cruz AJ, et al. Prevalence of metabolic syndrome among Filipino adults aged 20 years and over. Asia Pacific J Clin Nutr. 2003;12:271–276. [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, et al. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- VanDerslice J, Popkin B, et al. Drinking-water quality, sanitation, and breast-feeding: their interactive effects on infant health. Bulletin of the World Health Organization. 1994;72:589–601. [PMC free article] [PubMed] [Google Scholar]
- Vikram NK, Misra A, et al. Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis. 2003;168:305–313. doi: 10.1016/s0021-9150(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- WHO . Mortality Country Fact Sheet 2006. World Health Organization; 2006. [Google Scholar]
- Yazdanbakhsh M, Dremsner PG, et al. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Ye X, Yu Z, et al. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. Journal of the American College of Cardiology. 2007;49:1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]


