Abstract
Problem
Vaginal microbicides represent a promising approach for preventing heterosexual HIV transmission. However, preclinical evaluation should be conducted to ensure that microbicides will be safe for human cells and healthy microflora of the female reproductive tract. One microbicide candidate, RC-101, has been effective and well-tolerated in preliminary cell culture and macaque models. However, the effect of RC-101 on primary vaginal tissues and resident vaginal microflora requires further evaluation.
Method of Study
We treated primary vaginal tissues and vaginal bacteria, both pathogenic and commensal, with RC-101 to investigate effects of this microbicide.
Results
RC-101 was well-tolerated by host tissues, and also by commensal vaginal bacteria. Simultaneously, pathogenic vaginal bacteria, which are known to increase susceptibility to HIV acquisition, were inhibited by RC-101.
Conclusions
By establishing vaginal microflora, the specific antibacterial activity of RC-101 may provide a dual mechanism of HIV protection. These findings support advancement of RC-101 to clinical trials.
Keywords: Bacterial Vaginosis, Lactobacilli, RC-101, Theta-Defensin, Vaginal Microbicide
INTRODUCTION
More than 30 million people worldwide are infected with HIV, and every year another two million individuals will become newly infected. In sub-Saharan Africa, the region most affected by HIV, women make up 60% of the infected population, with sexual transmission being a main mode of transmission.1 Therefore, prophylactic approaches designed to halt the transmission of HIV in the female reproductive tract (FRT) are being actively pursued. These methods include the development of vaginal microbicides, antimicrobial agents that prevent the transmission of HIV and reduce the user’s susceptibility to viral acquisition.1,2
The search for an anti-HIV microbicide has been complicated by unforeseen inconsistencies between the bench and the clinic. These inconsistencies result from poor recapitulation of in vivo environments during initial characterization of the candidate microbicide. The FRT is a complex environment, lined by host epithelia and colonized by commensal lactobacilli that contribute to mucosal integrity, and the disruption of either host tissues or bacterial inhabitants can actually increase susceptibility to HIV. The former was exemplified by the well-publicized nonoxonyl-9 clinical trials, wherein antiviral effects observed in cell culture were reversed in clinical trials due to the uncharacterized damaging effects of the surfactant microbicide on FRT tissues.3 Similarly, disruption of healthy FRT microflora can also render women more susceptible to HIV acquisition, as is demonstrated in the common gynecological condition bacterial vaginosis (BV).4 In BV, commensal vaginal lactobacilli are displaced by mixed populations of pathogenic bacteria, including Gardnerella vaginalis, Atopobium vaginae, Mobiluncus curtisii and Prevotella bivia.5,6 The displacement of lactobacilli, which otherwise prevent infections by secreting lactic acid and hydrogen peroxide, weakens inherent mucosal defenses to reproductive pathogens. At the same time, intruding pathogenic bacteria induce an inflammatory host response that recruits immune cells, including CD4+ target cells for HIV infection (our unpublished data), and simultaneously activates NF-κB, which promotes HIV viral transcription.7 Combined, these effects of BV result in a 60% increased susceptibility to HIV acquisition.8 Therefore, in order to prevent side effects that counter antiviral activity, anti-HIV vaginal microbicide candidates must demonstrate compatibility with FRT tissues and microflora prior to clinical trials.
One such microbicide candidate, the cyclic peptide RC-101, has thus far demonstrated a promising safety and therapeutic profile. RC-101 is an analogue of the retrocyclin RC-100, a cyclic theta-defensin whose expression was lost over the course of primate evolution; retrocyclins are encoded in the human genome, but not expressed due to a premature stop codon in the peptide proregion.9 Retrocyclins have been synthetically recreated, and the resulting peptides exhibit broad-spectrum antimicrobial activity, including anti-HIV and antibacterial activity.10,11 RC-100, the peptide encoded by the human retrocyclin pseudogene, inhibits HIV at IC50 as low as 1.0 μg/mL, while its analogue RC-101 exhibits slightly better activity, with reported IC50 as low as 0.19 μg/mL.2,12,13 Ongoing research seeks to restore translation of endogenous RC-100 to the FRT using premature termination codon readthrough agents,14 while RC-101 has become the primary focus of microbicide development.
It stands to reason that since retrocyclins are derivatives of endogenously encoded primate peptides, their reintroduction to the human FRT would be well-tolerated by both human tissue and healthy bacterial inhabitants that evolved in the presence of host theta-defensins. In agreement with this hypothesis, safety studies in an ex vivo human cervical organ model15 and in vivo pigtailed macaque studies showed that application of RC-101 was well-tolerated by vaginal and cervical tissue, inducing no inflammation or adverse side effects upon gynecological examination.16 At the same time, recovered RC-101 peptide remained bioactive16 and is stable in the macaque vaginal environment for up to 14 days after application (our unpublished data). Furthermore, RC-101 application did not disrupt endogenous populations of commensal lactobacilli.16 This is an important consideration, since the tissues and microbes of the macaque vaginal canal are highly similar to those of the human FRT.17 These studies suggest that RC-101 will remain safe, stable and active among the complex environment of the human FRT.
To expand the promising profile of RC-101 as a vaginal anti-HIV microbicide, we sought to confirm its compatibility with epithelia, primary tissues, and commensal bacteria of the human FRT. Furthermore, we examined the stability and activity of RC-101 amongst pathogenic BV-associated bacteria, to ensure that this common affliction would not disrupt treatment regimens of RC-101. These studies demonstrate the compatibility of RC-101 with host tissues and microflora, and additionally demonstrate that RC-101 can inhibit BV-associated bacteria, thereby promoting healthy vaginal flora and providing a dual mechanism of HIV prevention.
METHODS
Epithelial and Tissue Cultures
HeLa cells (CCL-2) were purchased from ATCC and maintained in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum. Primary vaginal epithelial cells (VEC-CRY-OV) and full-thickness EpiVaginal tissues (VLC-100-FT) are engineered specimens that were purchased from MatTek Corporation and maintained in provided media according to supplier’s instructions.
For epithelial experiments, treatments were prepared in maintenance media and applied to confluent monolayers. For tissues, treatments were prepared in 100 μL PBS and applied apically. After incubation, epithelial treatment media or tissue underlay media were collected, clarified, and stored at −80°C until analysis. Cells were rinsed with PBS before lysing for phosphoprotein analysis.
Bacterial Cultures
The following bacterial cultures were purchased from ATCC: Lactobacillus crispatus (33197); Lactobacillus acidophilus (4356); Lactobacillus johnsonii (11506); Lactobacillus jensenii (25258); Lactobacillus gasseri (9857); Lactobacillus vaginalis (49540); Gardnerella vaginalis (49145); Atopobium vaginae (BAA-55); Mobiluncus curtisii (35241); Prevotella bivia (29303). All lactobacilli were grown in de Man, Rogosa and Sharpe (MRS) broth or on MRS agar plates at 37°C, 5% CO2 atmosphere. G. vaginalis, A. vaginae, M. curtisii, and P. bivia maintenance cultures were all grown in tryptic soy broth (TSB) supplemented with 5% defibrinated rabbit blood (Becton, Dickinson and Company), or on agar plates of the same composition. G. vaginalis was grown at 37°C, 5% CO2, while the other three bacteria were grown in anaerobic GasPak chambers (Becton, Dickinson and Company) at 37°C.
Bacterial Inhibition Assays
For experiments, anaerobic BV-associated bacteria (A. vaginae, P. bivia and M. curtisii) were taken directly from snap-frozen vials, and were washed and resuspended in prereduced brain heart infusion (BHI) media. Bacterial suspensions were mixed with preparations of RC-100, RC-101, clindamycin, or vehicle diluted in the same media. 5 μL of the final culture were placed under 3 μL liquid wax on a Terasaki microtiter plate as previously described11 and incubated anaerobically for up to 24 h. Extended incubation times such as this are typical for these anaerobic species.18,19,20 Cultures were periodically diluted in prereduced BHI and plated on prereduced 5% blood TSA plates. Plates were incubated anaerobically, and colony forming units (CFUs) were subsequently quantified for each condition.
For lactobacilli, snap-frozen vials of each species were first grown for 2 h in MRS broth at 37°C, 5%CO2 to allow cultures to recover. Actively growing bacteria were then mixed with RC-101 or vehicle diluted in the same media, and plated in Terasaki wells as done for anaerobes. These cultures were incubated at 37°C, 5%CO2 for up to 6 h. This duration was chosen based on previous studies demonstrating that RC-101 inhibits susceptible aerobic species in less than three hours,11 and because with longer incubations the density of some cultures began to decline. Culture growth was monitored by diluting and plating on MRS, then incubating at 37°C, 5% CO2 for CFU determination.
RC-101 Recovery from Pathogenic Bacterial Cultures
For coincubation with RC-101, snap-frozen vials of G. vaginalis were grown for 2 h in TSB to achieve log-phase growth, while anaerobic bacteria were taken directly from snap-frozen vials. All bacteria were diluted in maintenance media, and combined with RC-101 diluted in the same preparation for a final culture volume of 100 μL. These cultures were incubated at the appropriate atmosphere for 24 h at 37°C, after which each culture was acid extracted as previously described.16 Soluble extracts were neutralized by sequential drying and dilution with water, and neutralized extracts were resolved by Tricine-SDS PAGE, followed by western blotting with an anti-RC-101 antibody. Recovered peptide was run alongside a peptide standard for semi-quantitative comparison.
Bio-plex Analysis of Lysates and Conditioned Media
For phosphoprotein quantification, cells were harvested with Bio-Rad Cell Lysis kit, and equal amounts of total protein were assayed by multiplex phosphoprotein array. For cytokine analysis, conditioned media were clarified and equal volumes were assayed by multiplex cytokine array. Experimental analysis was performed according to manufacturer’s instructions. In addition to cytokines appearing in our results, the following cytokines were assayed, but were not produced by our cells or tissues at measurable levels: PDGF-BB, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17, IL-2Rα, IL-18, Eotaxin, FGF-β, G-CSF, IFN-γ, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α, LIF, MCP-3, β-NGF, SCF, SCGF-β, SDF-1α, TFN-β, TRAIL, HGF, IFN-α2.
Statistical Analyses
For bacterial inhibition assays, culture densities were log-transformed and treatments were compared to vehicle at each time point by a two-tailed paired Student’s t-test.11 For RC-101 recovery analysis, densitometric quantification between bacteria condition or media alone was compared by a two-tailed paired Student’s t-test. For Bio-Plex analysis of conditioned media, cytokine concentrations of RC-101 treated cultures were compared to appropriate vehicle by a two-tailed paired Student’s t-test.
RESULTS
Retrocyclin Theta-Defensins are Active Against BV-Associated Bacteria
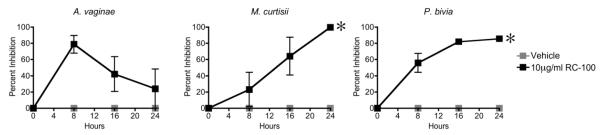
Retrocyclins RC-100 and RC-101 have been previously shown to inhibit a variety of microbes, including viruses, fungi, and Gram-positive and Gram-negative bacteria.10,11,21 As potential vaginal microbicides, the ability of retrocyclins to provide simultaneous protection against both HIV and pathogenic bacteria of the FRT is of immediate interest. To determine whether the restoration of retrocyclins to the FRT can inhibit bacterial pathogens, we first incubated RC-100, the endogenously encoded theta-defensin, with BV-associated bacteria Atopobium vaginae, Mobiluncus curtisii and Prevotella bivia. Treated cultures were incubated anaerobically for up to 24 h to determine the effects of the peptide on bacterial growth. Figure 1 shows that the peptide RC-100 significantly inhibited two of the three pathogenic anaerobes tested. M. curtisii was inhibited 95% by RC-100 after 24 h, while P. bivia inhibition was 85%. The third species, A. vaginae, was inhibited by RC-100 at 8 h, but the effects of RC-100 decreased by the completion of the 24 h experiment.
Figure 1. RC-100 Inhibits BV-Associated Bacteria.
RC-100 was incubated with BV-associated bacteria Atopobium vaginae, Mobiluncus curtisii, or Prevotella bivia (5×106 CFU/mL) anaerobically and cultures were plated at indicated timepoints to determine culture density. Percent inhibition was calculated relative to vehicle-treated bacteria. All inhibition values less than zero were plotted as zero. Asterisks indicated treatments for which one or more timepoints were significantly (p<0.05) different from vehicle. n = 3-4 for each condition.
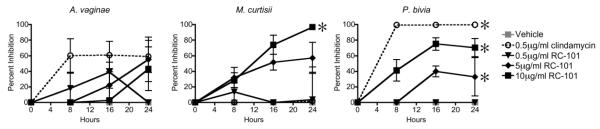
Having determined that RC-100 inhibited BV-associated bacteria, we next investigated whether the retrocyclin analogue RC-101, which is being actively developed as a topical microbicide, exhibits similar antibacterial activity. The same panel of pathogenic bacteria was incubated with RC-101 at concentrations ranging from 0.5 - 10 μg/mL, or with clindamycin, a standard antibiotic used to treat BV,22 for comparison. Similar to trends observed for RC-100, RC-101 exhibited significant, dose-dependent inhibition of both M. curtisii and P. bivia, while inhibition of A. vaginae was not significant (Figure 2). Interestingly, clindamycin was unable to inhibit the pathogen M. curtisii, in contrast to RC-101, which exerted >95% inhibition of M. curtisii by 24 h. P. bivia, on the other hand, was significantly inhibited by RC-101, and also by clindamycin.
Figure 2. BV-Associated Bacteria are Inhibited by RC-101.
RC-101 at 10 μg/mL, 5 μg/mL or 0.5 μg/mL, or clindamycin at 0.5 μg/mL was incubated with BV-associated bacteria anaerobically and cultures were plated at indicated timepoints to determine culture density. Percent inhibition was calculated relative to vehicle-treated bacteria. All inhibition values less than zero were plotted as zero. Asterisks indicate treatments for which one or more timepoints were significantly (p<0.05) different from vehicle. n = 3-5 for each condition.
Commensal Vaginal Lactobacilli are not Inhibited by RC-101
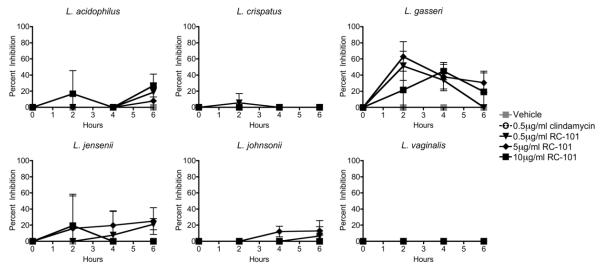
The ability of RC-101 to inhibit BV-associated bacteria is a favorable secondary effect that complements the peptide’s anti-HIV activity. However, vaginal microbicides must not exert antibacterial effects on the commensal bacteria that inhabit the FRT and promote reproductive health. Thus, we next examined the effect of RC-101 on the beneficial lactobacilli that comprise healthy vaginal flora. Six strains of lactobacilli that are common to the FRT,23,24 were subjected to microassay analysis to determine whether they were equally affected by RC-101. Figure 3 shows the effect of different concentrations of RC-101 on Lactobacillus acidophilus, crispatus, gasseri, jensenii, johnsonii and vaginalis over a 6 h time course of treatment. Unlike the pathogenic bacteria, none of the commensal lactobacilli were significantly inhibited by RC-101 at treatments as high as 10 μg/mL. Overall, the lack of significant antibacterial effects on lactobacilli suggests that RC-101 administered at anti-HIV concentrations would not disrupt the endogenous healthy bacterial flora of the FRT.
Figure 3. RC-101 Does Not Inhibit Commensal Vaginal Lactobacilli.
RC-101 at 10 μg/mL, 5 μg/mL or 0.5 μg/mL was incubated with six different species of vaginal lactobacilli, and cultures were plated at indicated timepoints. Percent inhibition was calculated relative to vehicle-treated bacteria and all inhibition values less than zero were plotted as zero. No treatments resulted in significant (p<0.05) inhibition of lactobacilli. n = 3 for each condition.
RC-101 is Recovered from BV-Associated Bacterial Cultures
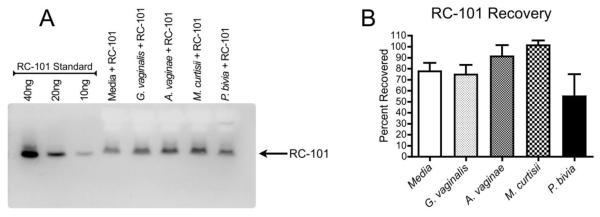
While the recovery and bioactivity of RC-101 has been characterized in the presence of commensal microflora, we sought to ensure that BV-associated bacteria would not affect the stability of this peptide microbicide. To do so, we incubated RC-101 in cultures of BV-associated pathogens, and analyzed peptide recovery and electrophoresis after 24 h. Figure 4A shows that RC-101 was recovered from all cultures, and that the peptide migrated at the appropriate size. Based on densitometric quantification, there were no significant differences in percent recovery between bacterial cultures and media alone, however there was a trend toward lower recovery from P. bivia cultures compared to the other three cultures (Figure 4B). We occasionally observed a slower migrating band in this sample extract, which was not included in densitometric quantification. Ongoing studies are investigating whether RC-101 is actively degraded by P. bivia, or whether the complexity of this bacterial culture alters peptide recovery or migration.
Figure 4. RC-101 is Recovered from BV-Associated Bacterial Cultures.
RC-101 (at 5 μg/mL) was incubated with BV-associated bacteria (5×106 CFU/mL) anaerobically for 24 h, then culture extracts were immunoblotted for RC-101 recovery determination. A) A representative immunoblot demonstrates the recovery of RC-101 from bacterial cultures or media alone, run alongside a standard of known RC-101 concentrations. B) Densitometry from three independent experiments. RC-101 recovery from bacterial cultures was not significantly different from media alone.
RC-101 is Well-Tolerated by Reproductive Cells and Tissues
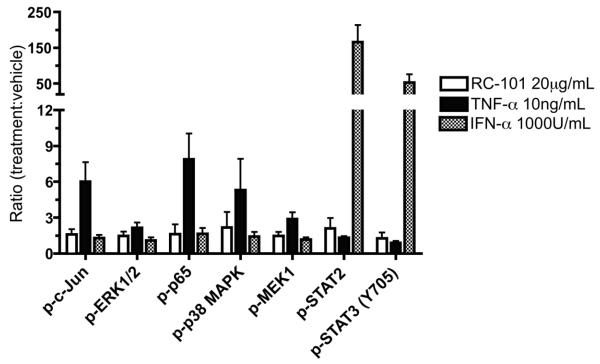
In addition to testing the compatibility of RC-101 with commensal vaginal bacteria, we also examined the effects of RC-101 on the epithelial cells that line the FRT to ensure that the peptide would be well-tolerated by host tissues. Recent studies have monitored select cytokine responses to RC-101 in a cervical organ model, but do not provide a comprehensive cytokine and signaling evaluation.15 To more broadly survey host response to RC-101, HeLa cells were treated with RC-101, and cellular response was gauged by monitoring intracellular signaling pathways. Figure 5 shows the phosphoprotein signaling response of reproductive epithelia to 30 min of RC-101 treatment. Importantly, none of seven monitored signal-transducing proteins was phosphorylated >2.1 fold in response to RC-101 compared to vehicle alone. This includes mediators of proinflammatory responses and regulators of cellular proliferation and turnover. The attenuated phosphorylation response to RC-101 is in contrast to control stimuli TNF-α and IFN-α, which elicit robust phosphorylation responses.
Figure 5. Reproductive Epithelial Cells Exhibit Minimal Phosphoprotein Response to RC-101.
HeLa cells were treated with vehicle, 20 μg/mL RC-101, or positive stimuli 10 ng/mL TNF-α or 1000 U/mL IFN-α for 30 min, then lysed for phosphoprotein quantification. Phosphoprotein ratios relative to vehicle-treated cells are shown for seven intracellular signaling proteins. Ratios are averaged from three or more independent experiments, except for quantification of p-MEK1 and p-STAT3 after RC-101 treatment, which are averaged from two independent experiments.
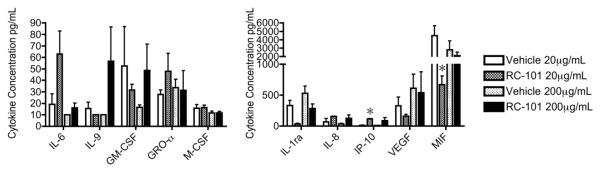
The lack of an intracellular phosphoprotein response was corroborated by an equivalent absence of cytokine response. Figure 6 shows the cytokine response of primary cultures of vaginal epithelial cells to 24 h of RC-101 exposure. In comparison to matched vehicle control, neither 20 μg/mL nor the excessive 200 μg/mL treatment stimulated significant increases in immune mediators such as IL-6, IL-8, Gro-α, M-CSF or GM-CSF. Of the ten analytes shown, only two (IP-10 and MIF) displayed significant differences from vehicle treatments, and for these two cytokines the significant difference observed at 20 μg/mL did not repeat at the higher dose of 200 μg/mL. These phosphoprotein and cytokine data suggest that RC-101 elicits a very minimal response from reproductive epithelial cells.
Figure 6. RC-101 Does Not Induce Proinflammatory Cytokines In Primary Vaginal Epithelia.
Primary vaginal epithelia were treated with either 20 or 200 μg/mL RC-101, or paired vehicles. After 24 h, conditioned media were collected and analyzed by multiplex cytokine array. The ten cytokines shown are expressed as raw cytokine concentrations and are grouped for graphing purposes. Asterisks indicated significant differences between RC-101 treatment and matched vehicle (p<0.05). n = 4-6.
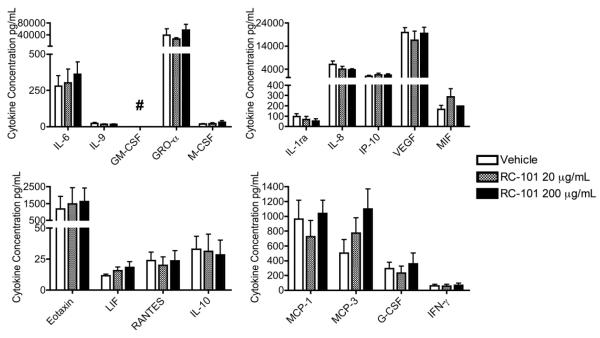
To expand this evaluation, we next utilized MatTek ex vivo vaginal tissues to examine the effects of RC-101 on intact tissues of the FRT. These full thickness tissues containing stratified epithelia, a basal lamina and submucosal dendritic cells were treated by apical application of RC-101 at the air-liquid interface. After 24 h, the basal media was analyzed for cytokine expression. Figure 7 shows the matched ten analytes from our epithelial analysis, with an additional eight characterized cytokines. One cytokine, GM-CSF, could not be accurately quantified, as it was found in tissue maintenance media alone. For the 17 cytokines that were measured, neither the 20 μg nor the 200 μg application per tissue resulted in significant differences from vehicle. This includes the two analytes IP-10 and MIF that showed inconsistent trends in our epithelial model (Figure 6). The absence of any significant cytokine changes in these primary organotypic tissues is in agreement with epithelial trends and suggests that RC-101 is well-tolerated by host tissues of the FRT.
Figure 7. RC-101 is Well Tolerated by Organotypic Vaginal Tissue Model.
Full-thickness organotypic tissues were treated with apical application of RC-101 at either 20 or 200 μg/mL RC-101 or vehicle. After 24 h, underlay media were collected and analyzed by multiplex cytokine array. In addition to the ten cytokines shown in Figure 6 for epithelia, another eight cytokines are shown here, all expressed as raw cytokine concentrations and grouped for graphing purposes. No RC-101 treatments resulted in significant differences from vehicle. ## = GM-CSF was detected in the maintenance media. n = 3-7.
DISCUSSION
Recent failures of anti-HIV microbicides3,25 have prompted more extensive preclinical characterization of candidate prophylactics. In this study, the microbicide candidate RC-101 was evaluated in order to determine its safety for host tissues and microflora prior to clinical trial. In agreement with other recent studies,15,16 we observed a desirable safety profile when RC-101 was applied to human FRT epithelia and tissues. Even at concentrations >40 times its antiviral IC50 range, RC-101 did not induce significant changes in cytokine release from primary FRT tissues. This evaluation included chemokines that mediate inflammation and chemotaxis of immune cells, such as IL-8, Gro-α, MCP-1 and MCP-3,26-29 and other important immune effectors such as IL-6.30
RC-101 did not elicit substantial increases in phosphorylation of the signaling transducers STATs 2 and 3,31 nor of the proinflammatory mediator, the p65 subunit of NF-κB.32 The lack of p65 phosphorylation is especially notable, as NF-κB activation is implicated in HIV proviral replication,7 and its unintended activation could counter the anti-HIV activity of applied microbicides. At the same time, the phosphorylation of mitogenic signaling intermediates such as MEK-1, ERK1/2, p38, and c-Jun was essentially unaffected by RC-101 application, indicating that this microbicide candidate is unlikely to induce unexpected effects on cellular proliferation, turnover or stress response.33
In addition to exhibiting compatibility with FRT epithelia and tissues, we also observed that RC-101 was well-tolerated by vaginal lactobacilli. Of our panel of six Lactobacillus spp., none was significantly inhibited by RC-101. This validates previous in vivo observations of macaque vaginal flora, in which lactobacilli remained unaffected by vaginal film formulations of RC-101.16 While lactobacillus growth is not inhibited by RC-101, we observed that BV-associated bacteria were significantly inhibited by retrocyclins. For the two strains that were susceptible, bacterial inhibition occurred at 10 μg/mL, well within expected therapeutic concentrations, and for one species, M. curtisii, RC-101 treatment at all concentrations (0.26 - 5.3 μM) exerted significantly greater inhibition compared to clindamycin given at 0.5 μg/mL (1.2 μM), above its reported MIC90 of 0.125 μg/mL.18
Furthermore, RC-101 was recovered from coincubation with these bacterial pathogens, though recovery was decreased when the peptide was coincubated with P. bivia. Of note, we occasionally observed a slower migrating immunoreactive band in this sample. As many positively charged antimicrobial peptides exert their antibacterial effect by binding and oligomerizing on bacterial surfaces to permeabilize cells,34 we expect that this band might be either bound or oligomerized RC-101. Interestingly, P. bivia is distinct in the panel of bacteria we evaluated, in that it is gram negative. The presence of negatively charged outer cell membrane components in this culture in particular could bind positively charged RC-101, slowing its electrophoretic mobility.35 While this hypothesis might explain our slightly lower RC-101 recovered from P. bivia culture, overall our results demonstrated good recovery of the peptide from the panel of bacteria, suggesting that RC-101 stability in the FRT would withstand transient fluctuations in microflora.
Though the molecular determinants of susceptibility remain unknown, the specificity of RC-101’s antibacterial activity against pathogenic bacteria but not against commensal lactobacilli supports the notion that the dynamic and complex primate vaginal microflora evolved in the presence of similar theta-defensins. Consequently, endogenous lactobacilli are uninhibited by the reintroduction of a theta-defensin analogue, while pathogenic species remain susceptible to this class of antimicrobial host defense peptides.
These data suggest that RC-101 would be an ideal anti-HIV microbicide. In addition to being well-tolerated by human vaginal epithelia and tissues, by restoring a lost host defense mechanism, RC-101 provides not only potent antiviral activity, but also specific antibacterial activity that stabilizes the mucosal microflora. These desirable attributes make RC-101 a promising candidate for vaginal anti-HIV microbicide development.
Acknowledgements
Funding: This work was supported by the National Institutes of Health (grant numbers AI052017 and AI082693 to AMC, grant number AI082623 to PG, AMC, LCR, MAP, PM, and PMT, and grant number P51 RR00164-50 to PM).
Footnotes
Competing Interests: None to declare.
References
- 1.WHO, UNICEF, UNAIDS [23 May 2012, date last accessed];Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. http://www.who.int/hiv/pub/progress_report2011/en/index.html.
- 2.Eade CR, Wood MP, Cole AM. Mechanisms and modifications of naturally occurring host defense peptides for anti-HIV microbicide development. Curr HIV Res. 2012;10:61–72. doi: 10.2174/157016212799304580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiègne-Traoré V, Uaheowitchai C, Karim SS, Mâsse B, Perriëns J, Laga M. COL-1492 Study Group: Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 4.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 5.Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics. 2010;11:488. doi: 10.1186/1471-2164-11-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer MC, Desmond RA, Schwebke JR. Association of Mobiluncus curtisii with recurrence of bacterial vaginosis. Sex Transm Dis. 2008;35:611–3. doi: 10.1097/OLQ.0b013e318167b105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–8. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–54. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Cole AM, Hong T, Boo LM. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002;99:1813–8. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers RP, Eade CR, Waring AJ, Cole AL, Cole AM. Characterization of the retrocyclin analogue RC-101 as a preventative of Staphylococcus aureus nasal colonization. Antimicrob Agents Chemother. 2011;55:5338–46. doi: 10.1128/AAC.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Münk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR, Cole AM. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res Hum Retroviruses. 2003;19:875–81. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- 13.Gupta P, Lackman-Smith C, Snyder B, Ratner D, Rohan L, Patton D, Ramratnam B, Cole A. Antiviral Activity of Retrocyclin RC-101, a Candidate Microbicide Against Cell-Associated HIV-1. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0135. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkataraman N, Cole AL, Ruchala P, Waring AJ, Lehrer RI, Stuchlik O, Pohl J, Cole AM. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 2009;7:e95. doi: 10.1371/journal.pbio.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta P, Ratner D, Ding M, Patterson B, Rohan LC, Reinhart TA, Ayyavoo V, Huang X, Patton DL, Ramratnam B, Cole AM. Retrocyclin RC-101 Blocks HIV-1 Transmission Across Cervical Mucosa in an Organ Culture. J Aquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e318258b420. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole AM, Patton DL, Rohan LC, Cole AL, Cosgrove-Sweeney Y, Rogers NA, Ratner D, Sassi AB, Lackman-Smith C, Tarwater P, Ramratnam B, Ruchala P, Lehrer RI, Waring AJ, Gupta P. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS ONE. 2010;5:e15111. doi: 10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patton DL, Sweeney YC, Tsai C-C, Hillier SL. Macaca fascicularis vs. Macaca nemestrina as a model for topical microbicide safety studies. J Med Primatol. 2004;33:105–8. doi: 10.1111/j.1600-0684.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel CA. Susceptibility of Mobiluncus species to 23 antimicrobial agents and 15 other compounds. Antimicrob Agents Chemother. 1987;31:249–52. doi: 10.1128/aac.31.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka K, Kato N, Watanabe K. In vitro activity of an evernimicin derivative, SCH27899, against anaerobic bacteria and Propionibacterium acnes. J Antimicrob Chemother. 2000;46:465–9. doi: 10.1093/jac/46.3.465. [DOI] [PubMed] [Google Scholar]
- 20.Lopes Dos Santos Santiago G, Grob P, Verstraelen H, Waser F, Vaneechoutte M. Susceptibility testing of Atopobium vaginae for dequalinium chloride. BMC Res Notes. 2012;5:151. doi: 10.1186/1756-0500-5-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran D, Tran PA, Tang Y, Yuan J, Cole T, Selsted ME. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem. 2001;277:3079–84. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 22.Hillier S, Krohn MA, Watts DH, Wolner-Hanssen P, Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol. 1990;76:407–13. [PubMed] [Google Scholar]
- 23.Lopes Dos Santos Santiago G, Cools P, Verstraelen H, Trog M, Missine G, Aila N, Verhelst R, Tency I, Claeys G, Temmerman M, Vaneechoutte M. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS ONE. 2011;6:e28180. doi: 10.1371/journal.pone.0028180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Daroczy K, Xiao B, Yu L, Chen R, Liao Q. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J Med Microbiol. 2012;61:729–39. doi: 10.1099/jmm.0.038687-0. [DOI] [PubMed] [Google Scholar]
- 25.Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D, CS Study Group Lack of Effectiveness of Cellulose Sulfate Gel for the Prevention of Vaginal HIV Transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–93. [PubMed] [Google Scholar]
- 27.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–24. [PubMed] [Google Scholar]
- 28.Yadav A, Saini V, Arora S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clinica Chimica Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J Leukoc Biol. 1996;59:67–74. doi: 10.1002/jlb.59.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–8. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Shared Principles in NF-kappaB Signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 34.Huang HW. Molecular mechanism of antimicrobial peptides: The origin of cooperativity. Biochim Biophys Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Aroutcheva A, Ling Z, Faro S. Prevotella bivia as a Source of Lipopolysaccharide in the Vagina. Anaerobe. 2008;14:256–60. doi: 10.1016/j.anaerobe.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]