Abstract
An emerging body of research suggests that mindfulness-based interventions may be beneficial for smoking cessation and the treatment of other addictive disorders. One way that mindfulness may facilitate smoking cessation is through the reduction of craving to smoking cues. The present work considers whether mindful attention can reduce self-reported and neural markers of cue-induced craving in treatment seeking smokers. Forty-seven (n = 47) meditation-naïve treatment-seeking smokers (12-h abstinent from smoking) viewed and made ratings of smoking and neutral images while undergoing functional magnetic resonance imaging (fMRI). Participants were trained and instructed to view these images passively or with mindful attention. Results indicated that mindful attention reduced self-reported craving to smoking images, and reduced neural activity in a craving-related region of subgenual anterior cingulate cortex (sgACC). Moreover, a psychophysiological interaction analysis revealed that mindful attention reduced functional connectivity between sgACC and other craving-related regions compared to passively viewing smoking images, suggesting that mindfulness may decouple craving neurocircuitry when viewing smoking cues. These results provide an initial indication that mindful attention may describe a ‘bottom-up’ attention to one’s present moment experience in ways that can help reduce subjective and neural reactivity to smoking cues in smokers.
Keywords: mindfulness, craving, fMRI
INTRODUCTION
Nearly, half of the adult smokers attempt to quit each year, but the majority of quit attempts are unsuccessful, even with clinical intervention (Hughes et al., 2004; Center for Disease Control and Prevention, 2008; United States Public Health Service, 2008). Whether successful or not, smoking cessation is a significant stressor, causing disturbances of mood, cognition, sleep and cigarette craving, all of which may persist long term (Piasecki et al., 1998; Gilbert et al., 1999, 2002). Therefore, identifying novel behavioral treatments to reduce these consequences is of the utmost importance.
Recently, interest has grown in the use of mindfulness-based treatments for addictive disorders, including smoking (Witkiewitz et al., 2005; Brewer et al., 2010). Mindfulness is often defined as attention to moment-to-moment experience, coupled with a nonjudgmental, accepting attitude toward that experience (Bishop et al., 2004). Mindfulness-based approaches have demonstrated efficacy for a variety of psychiatric concerns (Brown et al., 2007; Chiesa and Serretti, 2011; Fjorback et al., 2011). To date, mindfulness-based addiction treatments have shown promise in nonrandomized pilot studies for smoking cessation (Altner, 2002; Witkiewitz et al., 2005, 2010; Davis et al., 2007; Bowen and Marlatt 2009). In the first randomized, controlled trial of a mindfulness-based intervention for smoking cessation, Brewer et al. (in press) found that mindfulness training was associated with reductions in smoking and improvements in biochemically validated abstinence, both immediately after treatment and at 17-week follow-up, in comparison to a standard behavioral cessation paradigm.
One way mindful attention might help smokers is through reductions in craving (Brewer et al., in press). Cigarette craving has been identified as an important factor in cessation attempts, as individuals with high levels of craving are more likely to relapse (e.g. Killen and Fortmann, 1997), and craving often directly precedes relapse (Allen et al., 2008). In the Buddhist tradition, craving is considered one of the five hindrances, whose influence could be lessened by the deployment of mindful attention (Fronsdal, 2005). Mindful attention has been shown to help alleviate negative emotional states, such as distress (e.g. Jain et al., 2007) and may therefore have benefits against craving as well. (For a theoretical and empirical review of mindfulness, see Brown et al., 2007.)
Several studies have examined the relationship between mindfulness, cigarette craving and smoking. Vidrine et al. (2009) found that among smokers enrolled in a cessation study, dispositional mindfulness was linked to lower baseline nicotine dependence, greater sense of agency regarding cessation and other factors known to predict success in quitting. Bowen and Marlatt (2009) randomly assigned smokers to receive mindfulness instructions or no instructions during cue-induced craving. Mindfulness instructions were associated with significant decreases in smoking over the subsequent 7 days. Similarly, Rogojanski et al. (2011) randomly assigned smokers to apply either mindfulness-based or suppression-based coping skills in response to experimentally induced craving, and found that smokers who applied mindfulness-based strategies reported reductions in negative affect, depressive symptoms and nicotine dependence 1 week later.
Experimental methods for studying craving
Cue-induction methods have been developed to explore the subjective experience of craving in drug-dependent populations (Carter and Tiffany, 1999). In these paradigms, individuals view drug-related stimuli and report on their craving (which can increase during cue exposure in abstinent populations). Neuroimaging studies of cue-induced craving have revealed increases in activity in anterior cingulate cortex (ACC), ventromedial prefrontal cortex (VMPFC) and orbitofrontal cortex (OFC), ventral striatum (VS), precuneus and cuneus, motor control areas in the basal ganglia and supplementary motor areas (e.g. Due et al., 2002; David et al., 2005; Lee et al., 2005; McBride et al., 2006; Smolka et al., 2006; Brody et al., 2007; McClernon et al., 2008). A recent meta-analysis highlights a central role for craving-related activity in VS, amygdala, temporo-parietal junction and ACC, including the subgenual region (sgACC), in nicotine dependence (Kühn and Gallinat, 2011). Moreover, Sinha and Li (2007) suggest that cue-induced activity in medial prefrontal cortex (PFC), ACC (and posterior cingulate cortex), striatum and posterior insula predict relapse after a cessation attempt. Further evidence of the role of cue-induced craving networks for cigarette smoking comes from pharmacalogical studies. Bupropion (a prescription medication used to aid in smoking cessation) was found to attenuate cigarette cue-induced sgACC activity (Brody et al., 2004), and extinction-based treatment with nicotine replacement therapy attenuates cue-induced activity in amygdala (McClernon et al., 2007).
Cue-induced craving approaches have recently attracted criticism due to the limited predictive ability of self-reported craving in such paradigms (Perkins, 2009; see also Shiffman, 2009; Tiffany, 2009). Nevertheless, much evidence directly links craving to drug taking (Shiffman et al., 1996, 1997; Killen and Fortmann, 1997; Catley et al., 2000; O'Connell et al., 2004; Allen et al., 2008; Epstein 2009, 2010; Preston et al., 2009). Further, exposure to drug cues has been linked to relapse following treatment (Shiffman et al., 1986; Bliss et al., 1989). Finally, as patterns of cue-induced neural activity and concomitant self-reported craving have been increasingly studied in relation to both applied cognitive strategies and smoking cessation treatments, cue-reactivity paradigms have greater potential to inform a mechanistic understanding of nicotine addiction (Brody et al., 2007; McClernon et al., 2007; Sinha and Li, 2007; Janes et al., 2010; Chua et al., 2011).
Interestingly, of two prior studies that have examined mindful attention during a cue-induction paradigm, neither found significant reductions in self-reported craving (Bowen and Marlatt, 2009; Rogojanski et al., 2011). However, to our knowledge, no cue exposure studies have examined how mindful attention affects craving in a neuroimaging environment.
Neural pathways linking mindful attention to reduced craving: regulation vs reduced reactivity
There are two candidate neural pathways that link mindful attention to reduced craving. First, mindful attention may recruit ‘regulatory’ regions in a top-down manner. A large body of work has identified the neural circuitry underlying cognitive regulation of emotions (Ochsner et al., 2002; Lieberman, 2007), comprising upregulation of prefrontal regions such as dorsolateral PFC (DLPFC), and down-regulation of subcortical limbic regions such as amygdala and VS (Garavan et al., 2000; Heatherton, 2011; Heatherton and Wagner, 2011). There is some indication that craving can be regulated in this manner as well (Kober et al., 2010). In support of this regulatory pathway, we have previously found that individuals high in dispositional mindfulness show increased lateral PFC and decreased amygdala activity when explicitly instructed to label affective stimuli (Creswell et al., 2007). Likewise, Farb et al., (2007) trained participants in an experiential attention (similar to mindful attention), and found increased activity in executive regions including DLPFC and ventral lateral PFC (VLPFC). More recent work by this group likewise found increased activity in attention-related regions such as dorsal anterior cingulate and lateral PFC during negative emotion induction in participants who received 8 weeks of mindfulness training compared to controls (Farb et al., 2010). Supporting this view, a recent review of neuroimaging studies of mindfulness meditation concluded that alterations to top-down processing underlie the beneficial effects of mindfulness for psychiatric concerns (Chiesa et al., 2010).
Alternatively, however, mindful attention may reduce craving by directly decreasing craving-related neural ‘reactivity’. Specifically, this perspective posits that mindful attention operates in a more ‘bottom-up’ manner, through a nonjudgmental stance toward one's experience. Indeed, several reviews have pointed out the difference between mindful awareness and explicit emotion regulation (Shapiro et al., 2006; Chambers et al., 2009). Support for this hypothesis comes from recent work demonstrating that mindfulness practice improves bottom-up attentional processes (Jha et al., 2007; Slagter et al., 2007). Recently, van den Hurk et al. (2010) found that experienced meditators showed decreased intersensory facilitation, directly supporting the reduced reactivity account. Moreover, several studies suggest that mindfulness can reduce activity in craving related limbic and paralimbic regions without any recruitment of PFC regulatory regions. For example, there is reduced resting state activity in bilateral amygdala in mindful individuals (Way et al., 2010), and mindful attention has been found to reduce reactivity in pain-related regions in experimentally induced pain (Kober et al., 2011; Zeidan et al., 2011).
The purpose of the present study is to test the ‘regulation’ vs ‘reduced reactivity’ account for how mindful attention may reduce craving among adult smokers. This study focuses on tasks conducted prior to treatment delivery in a broader smoking cessation trial. Specifically, we used a cue-induction paradigm in meditation-naïve smokers who had been abstinent for 12 h (biochemically verified). An active ‘regulation’ account predicts that mindful attention acts in a top-down manner and will recruit lateral PFC regions (previously associated with regulation), which will modulate activity in craving-related neural regions (e.g. ACC, VS). This ‘regulation’ account would further predict that regions modulated by mindful attention will be more strongly functionally connected to lateral PFC regions during mindfulness. In contrast, a ‘reduced reactivity’ account posits that mindful attention acts in bottom-up manner in ways where one can nonjudgmentally experience craving-related stimuli without reacting to it, thus directly reducing neural reactivity in craving-related regions (e.g. sgACC, VS) without concomitant activation of PFC. This account further predicts that regions modulated by mindful attention will be less strongly connected to other craving-related regions, and will not be more strongly connected to lateral PFC regions during mindfulness.
METHODS
Participants
Participants were 54 right-handed smokers recruited as part of the Healthier Brains in Treating Smoking (HaBITS) study (P.I. Tindle), conducted at the University of Pittsburgh. All participants were adults ≥18 years who smoked at least 10 cigarettes per day at baseline and expressed a strong desire to quit smoking within the following month. Exclusion criteria included medication that could affect the nervous system during functional magnetic resonance imaging (fMRI) scanning (such as β-blocker, analgesic or any psychotropic medication), pregnancy, history of brain injury, cognitive impairment such as dementia, untreated psychiatric illness such as hallucinations or active depression, and concomitant substance use. Participants first completed a telephone screen to determine eligibility, and within 2 weeks conducted an initial visit to deliver informed consent and administer a baseline questionnaire. During the phone screen and the baseline visit, participants were screened verbally for psychiatric or substance abuse disorders and informed that they would be screened for drugs at both fMRI visits. They also completed the Beck Depression Inventory II (Beck et al., 1996) as a means of assessing depressive symptomatology. This study was approved by both University of Pittsburgh and Carnegie Mellon Internal Review Boards. All participants provided informed consent and were reimbursed for their participation.
Stimuli
Participants viewed different types of pictures and were instructed to think about them in different ways depending on the instruction they were given. There were three types of pictures (smoking, e.g. a lit cigarette; neutral, e.g. bookcases; and aversive, e.g. injured people), preceded by one of three instructions (Look, Mindfully Attend or Reappraise) for a total of seven conditions (LookSmoking, LookNeutral, LookDistressing, MindfulSmoking, MindfulDistressing, ReappraiseSmoking and ReappraiseDistressing). Findings associated with the reappraisal instruction and aversive stimuli will be described in a separate paper and are not reported here.
Neutral and aversive stimuli were selected from the International Affective Picture System (IAPS; Lang et al., 1997). Smoking stimuli came from two sources: the ‘International Smoking Image Series’ (ISIS; Gilbert and Rabinovich, 1999) and images purchased from istockphoto.com. For the latter, a separate sample of cigarette smokers viewed the pictures and provided ratings of craving on a 7-point scale; these were averaged and matched to the standardized ratings provided with the ISIS stimuli. The final sample consisted of 12 pictures, all of which were >3 on this scale; mean craving rating was 4.6 out of 7 in the pilot. Smoking-related images were balanced between the LookSmoking and MindfulSmoking conditions, such that average craving score did not differ between the two conditions. Stimuli for smoking and neutral conditions contained roughly equal numbers of pictures of faces, balanced for gender and were counterbalanced across the three types of instruction.
Procedure and training
Prior to scan day, participants were asked to abstain from smoking for at least 12 h. Abstinence (determined as <13 ppm) was biochemically validated upon arrival at the scan facility using a carbon monoxide monitor (Bedfont, Rochester, UK). Additionally, all participants were required to provide a negative a urine screen for cocaine, THC, methamphetamine, and opiates at the scan site.
Before entering the scanner, a researcher conducted a brief training session. After explaining the task instructions, the researcher walked participants through a set of practice pictures. Participants practiced each instruction [Look, Mindfully Attend and Reappraise (not reported here)], verbalizing their thought processes, and the researcher gave corrective feedback. For the LOOK instruction, participants were asked simply to relax and view the picture as naturally as possible. For the MINDFULLY ATTEND condition, participants were instructed to actively focus on their responses to the picture, including thoughts, feelings, memories and bodily sensations, while maintaining a nonjudgmental attitude toward those responses. MINDFULLY ATTEND was not described as a strategy to reduce craving; rather, researchers emphasized that whatever sensations the participant experienced—including craving—were to be noticed as open-mindedly as possible. Instructions explicitly asked participants to ‘notice and accept’ their internal experience. Finally, participants practiced rating their craving and negative affect.
Scan session
The task consisted of four 7-min runs in an event-related design. Within each run, each condition (e.g. MindfulSmoking) was repeated three times for a total of 21 pictures. The order of conditions was pseudo-randomized with the constraint that no two consecutive pictures would be of the same type or instruction.
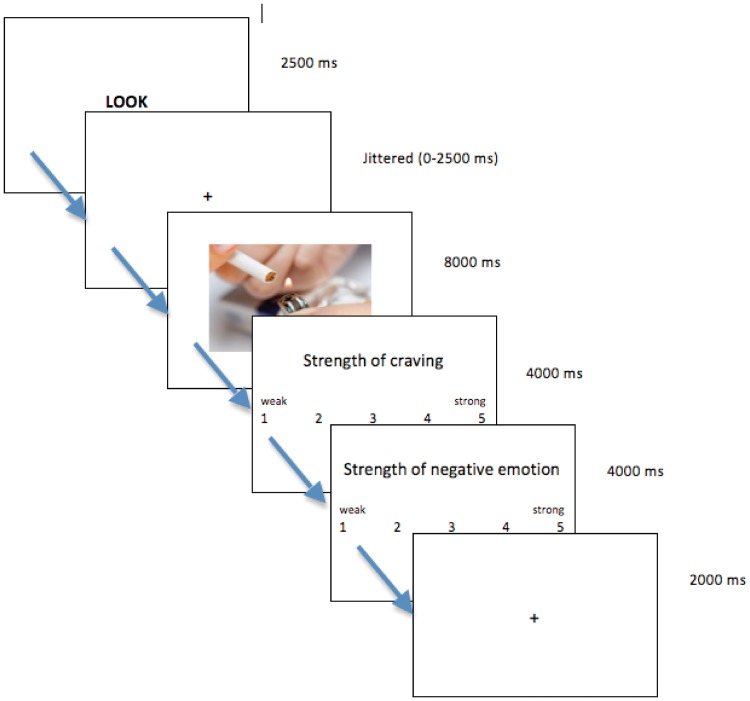
Each trial was constructed as follows: participants saw a 2-s instruction slide (LOOK or MINDFULLY ATTEND), a fixation cross of jittered duration (∼1.5 s), the stimulus picture for 8 s, two 4-s rating slides (the first for craving and the second for negative emotion) and finally a 2-s rest before the next trial began (Figure 1). Participants made ratings using a data glove with a button for each finger (Psychology Software Tools, Pittsburgh, PA, USA). Ratings ranged from 1 (weak) to 5 (strong). Inter-stimulus intervals were jittered from 0 to 2500 ms, distributed exponentially; the order of jitters was randomized and was the same for all participants. The task was constructed using E-Prime 2.0 Professional (Psychology Software Tools). Stimuli were viewed on a 6 × 9″ screen projected to a mirror mounted on the head coil (approximating 18″ distance from head).
Fig. 1.
Scanning task design. Schematic illustration of a single trial. Each trial began with a 2500-ms long instruction (LOOK or MINDFULLY ATTEND or REAPPRAISE), followed by a jittered interval. A photo was then presented onscreen for 8000 ms (neutral, negative or smoking). Subsequently, participants were asked to rate the strength of their craving and negative affect. Trials were separated by a 2000-ms intertrial interval.
Data acquisition and analysis
All scans were performed at the Brain Imaging Research Center in Pittsburgh. Data were collected on a Siemens Allegra 3.0T scanner using a one-channel birdcage head coil. Participants’ heads were restrained using foam padding and surgical tape across the forehead. For each participant, a high-resolution 3-dimensional T1-weighted gradient echo image was acquired with TI = 800 ms, TR = 1630 ms, TE = 2.48 ms and flip angle = 8°. This scan recorded 224 slices with acquisition matrix 256 × 256, field of view = 205 mm and voxel size of 0.8 × 0.8 × 0.8 mm3. Functional scans were acquired using an echo-planar pulse sequence with TR = 2 s, TE = 28 ms and flip angle = 79°. Each pulse recorded 34 oblique axial slices with slice thickness 3.2 mm (no gap); field of view was 205 mm and matrix size was 64 × 64 generating 3.2 × 3.2 × 3.2 mm3 voxels. Four runs were acquired, each comprising 218 volumes.
The imaging data were analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). The data were field-map corrected, slice-timing corrected, realigned to the mean image of the first functional run and smoothed with a 4-mm Gaussian kernel (FWHM) to be in the preferred format for the motion correction program, ArtRepair (Mazaika et al., 2009). ArtRepair applied an algorithm to each run to suppress interpolation errors due to large motion. Then, TRs with excessive fast motion (>1.5 mm/TR) or large global signal variation were repaired using linear interpolation. This motion corrected data was then co-registered to the T1 structural image, which was normalized to a standard stereotactic space as defined by the Montreal Neurological Institute (MNI). Finally, the normalization parameters were applied to the co-registered functional images, and smoothed with a 7-mm Gaussian kernel (FWHM).
For each participant, each condition (e.g. MindfulSmoking) was modeled as an event convolved with the canonical hemodynamic response function. The rest period after instruction was modeled as an explicit baseline and rests between trials were left unmodeled. Planned comparisons between conditions of interest were computed in SPM8 as linear contrasts. The single subject results were then submitted to a second-level random-effects group analysis. A Monte Carlo Simulation using AlphaSim implemented in AFNI (Cox, 1996) of our whole-brain volume demonstrated that a cluster extent cutoff of at least 54 contiguous voxels exceeding a voxel-wise threshold of P < 0.001 provided a multiple-comparison correction at P < 0.05.
To further test our alternative hypotheses regarding the mechanism by which mindful attention may modulate craving, additional psychophysiological interaction (PPI) analyses (Friston et al., 1997) were conducted using the SPM PPI toolbox. For each subject, volumes of interest were extracted and used as seeds in single-subject whole-brain PPI analyses. These were combined into a group-level t-test to identify regions exhibiting connectivity with the seed region.
RESULTS
Sample characteristics
Several participants (n = 7) were excluded in the imaging analyses due to excessive head motion, errors with the response glove, or failure to perform the task correctly, and thus we present the self-reported craving results on the full sample (n = 54) and on the neuroimaging subsample (n = 47). The neuroimaging sample did not differ from the full sample on demographic or nicotine dependence variables. Self-reported craving was not associated with SES, ethnicity, sex, income or grade level (all P's > 0.05). Psychiatric comorbidity was not formally assessed, but scores on the Beck Depression Inventory II (Beck et al., 1996) were on average below the clinical cutoff for depression (13), suggestive of a low level of depressive mood comorbidity within our sample. Participant characteristics are reported in Table 1.
Table 1.
Participant characteristics
| Characteristic | |
|---|---|
| Age [M (s.d.)] (years) | 45 (11.35) |
| Sex (%), female | 31 |
| Race (%) | |
| African-American | 47 |
| Caucasian | 51 |
| Other | 2 |
| Completed high school (%) | 49.5 |
| Annual income (%) | |
| <$20 000 | 55.8 |
| $20 000–50 000 | 25.3 |
| $50 000–75 000 | 15.8 |
| >$75 000 | 3.2 |
| Beck depression inventory IIa score [M (s.d.)] | 7.41 (6.53) |
| Scored ≤13 (%) | 86.5 |
| M (s.d.) | |
| Nicotine dependence (FTND)b | 5.03 (2.25) |
| Cigarettes per day | 17.58 (8.10) |
| Years of smoking | 25.78 (11.11) |
| Baseline CO level | 13.94 (6.79) |
Behavioral analyses: self-reported craving
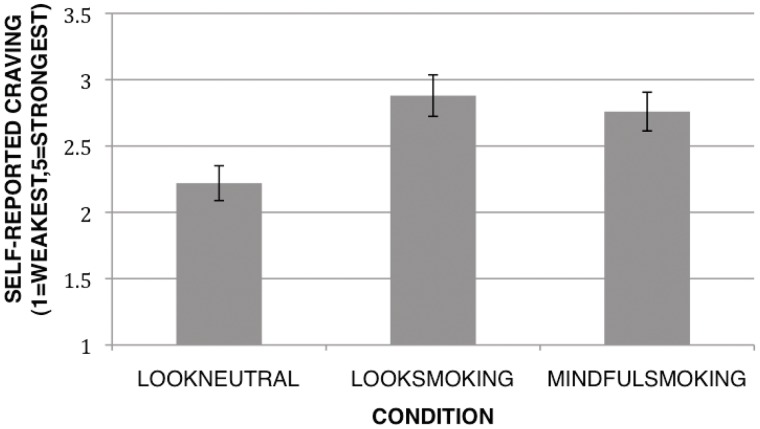
To determine whether mindful attention reduced self-reported craving to smoking and neutral images, a repeated measures analysis of variance (ANOVA) was conducted to test for significant differences between the three conditions: passively viewing neutral images (LookNeutral), passively viewing smoking images (LookSmoking) or mindfully attending to smoking images (MindfulSmoking). We conducted these tests first with the entire sample (n = 54). Consistent with predictions, the repeated measures ANOVA indicated a significant difference between conditions [F(2,53) = 14.57, P < 0.001, η2 = 0.36]. Specifically, looking at smoking images produced the highest self-reported craving (M = 2.82, s.d. = 1.07), followed by mindful attention (M = 2.70, s.d. = 1.01), and looking at neutral images (M = 2.20, s.d. = 0.90) (Figure 2). We then performed follow-up paired samples t-tests between conditions. Consistent with predictions, craving was greater for LookSmoking than for LookNeutral [t(46) = 5.25, P < 0.0001] or MindfulSmoking [t(46) = −2.09, P < 0.05], indicating that mindful attention helped to reduce self-reported craving. The same pattern of results was observed in the neuroimaging subsample (n = 47): the repeated measures ANOVA was significant [F(2,46) = 22.63, P < 0.001, η2 = 0.33], as was the paired t-test between LookSmoking and LookNeutral [t(46) = 4.93, P < 0.0001]. The paired t-test comparing MindfulSmoking to LookSmoking was marginally significant [t(46) = −1.76, P = 0.09] in this subsample.
Fig. 2.
Differences in self-reported craving. Mean cue-induced craving reported by condition for the fMRI sample.
A similar pattern emerged for ratings of distress to smoking and neutral images. The repeated measures ANOVA was significant [F(2,46) = 6.30, P < 0.01, η2 = 0.12]. Paired t-tests revealed that distress ratings were significantly lower in the LookNeutral condition (M = 1.99, s.d. = 0.85) than in the LookSmoking condition [M = 2.31, s.d. = 1.03, t(46) = 2.90, P < 0.01]. Distress was also decreased in the MindfulSmoking condition (M = 2.19, s.d. = 0.93) [t(46) = −2.30, P < 0.05) compared to LookSmoking. This pattern of behavioral results suggested that mindful attention can reduce both craving and concomitant distress that can accompany viewing smoking stimuli in 12-h abstinent smokers.
fMRI analyses
Smoking cue manipulation check: passively viewing smoking images increases activity in craving-related regions (LookSmoking > LookNeutral).
We first examined whether viewing smoking images activated craving-related neural regions in the LookSmoking > LookNeutral contrast. Consistent with expectations, craving-area activations were observed in right precuneus and left medial frontal gyrus/ventral ACC, as previously reported (Brody et al., 2004; Sinha and Li, 2007) (details displayed in Table 2).
Table 2.
Activations seen in the LookSmoking > LookNeutral contrast
| Anatomic region | BA | Side | Cluster size | Peak activation (MNI) |
t | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| Activations | |||||||
| Precuneus | 31 | R | 300 | 18 | −46 | 30 | 4.77 |
| Medial frontal gyrus | 10 | L | 125 | −4 | 50 | −6 | 4.22 |
| Deactivations | |||||||
| L | −22 | 2 | 34 | 3.58 | |||
| Fusiform gyrus | 37/19 | R | 111 | 30 | −64 | −12 | 4.90 |
Mindfully attending to smoking images reduces craving-related neural reactivity (MindfulSmoking > LookSmoking).
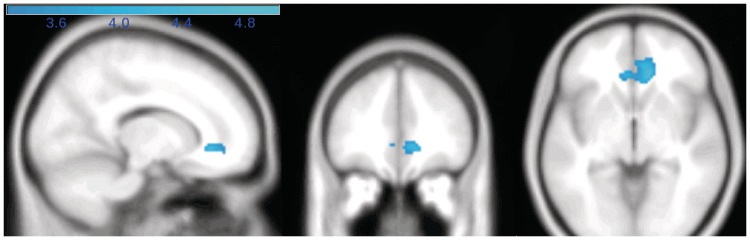
Activity during the MindfulSmoking condition was contrasted with the LookSmoking condition to determine which brain areas showed increased or decreased activity while mindfully attending to smoking images. In support of the reduced reactivity account, we found a large region of bilateral subgenual ACC/VMPFC to show reduced activity in the MindfulSmoking > LookSmoking contrast (BA 32/24/10, 12, 34, −4 Z = −4.77, k = 279) (Figure 3). To test the regulation account, namely that mindful attention would be associated with increased activity in lateral PFC regions, we looked for regions of relatively increased activity during mindful attention (in the MindfulSmoking > LookSmoking contrast), but no areas in a whole-brain analysis showed increased activity.
Fig. 3.
Activations during MindfulSmoking > LookSmoking. Regions modulated by mindful attention. Sagittal (x = 14), coronal (y = 38) and axial slices (z = −4) views of sgACC, which was significantly less activated during mindful attention to smoking pictures, compared to passive viewing.
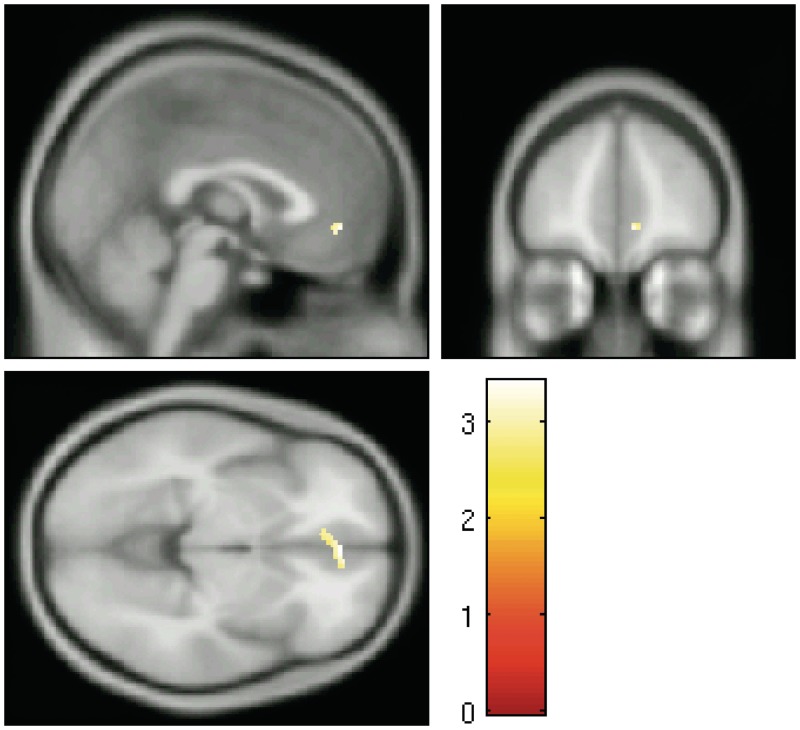
To determine whether the region of ACC that was modulated in MindfulSmoking overlapped with the sgACC activation observed in the craving contrast (LookSmoking > LookNeutral), the deactivated region in MindfulSmoking > LookSmoking was used to generate a functional mask, which was applied to the LookSmoking > LookNeutral contrast. Indeed, there was a significant cluster in left sgACC that was active while viewing smoking images and which was deactivated during mindful attention (BA 32/10, 8, 46, −8, k = 53). This region is shown in Figure 4.
Fig. 4.
Region of overlap between MindfulSmoking > LookSmoking contrast and LookSmoking > LookNeutral contrast (BA 32/10, 8, 46, −8, k = 53).
In the MindfulSmoking > LookNeutral contrast, however this region showed neither activation nor deactivation. Together these results suggest that this region was activated by passive looking at smoking images (LookSmoking) compared to neutral images (LookNeutral). Mindful attention to smoking images (MindfulSmoking), however, appeared to decrease activity back to the level of LookNeutral. This finding supports the reduced reactivity account for mindful attention, suggesting that mindful attention reduces neural activation in a known craving-related region (sgACC).
Functional connectivity of sgACC.
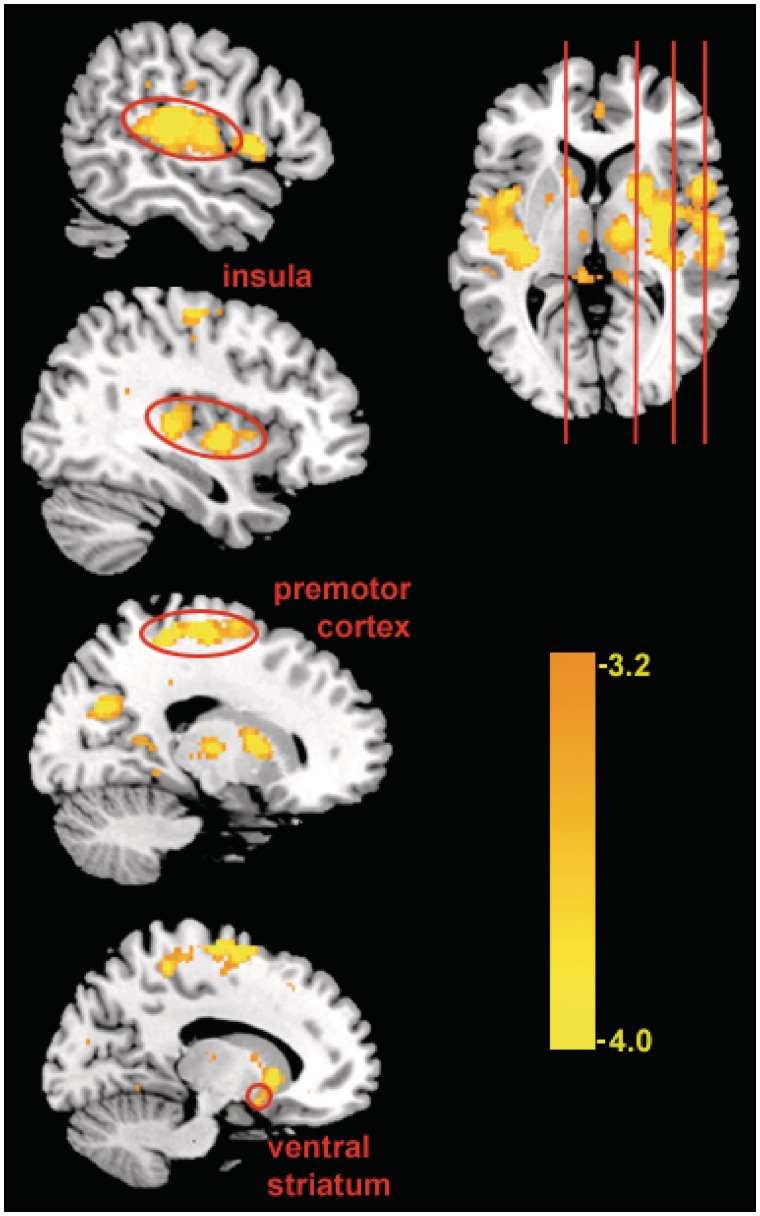
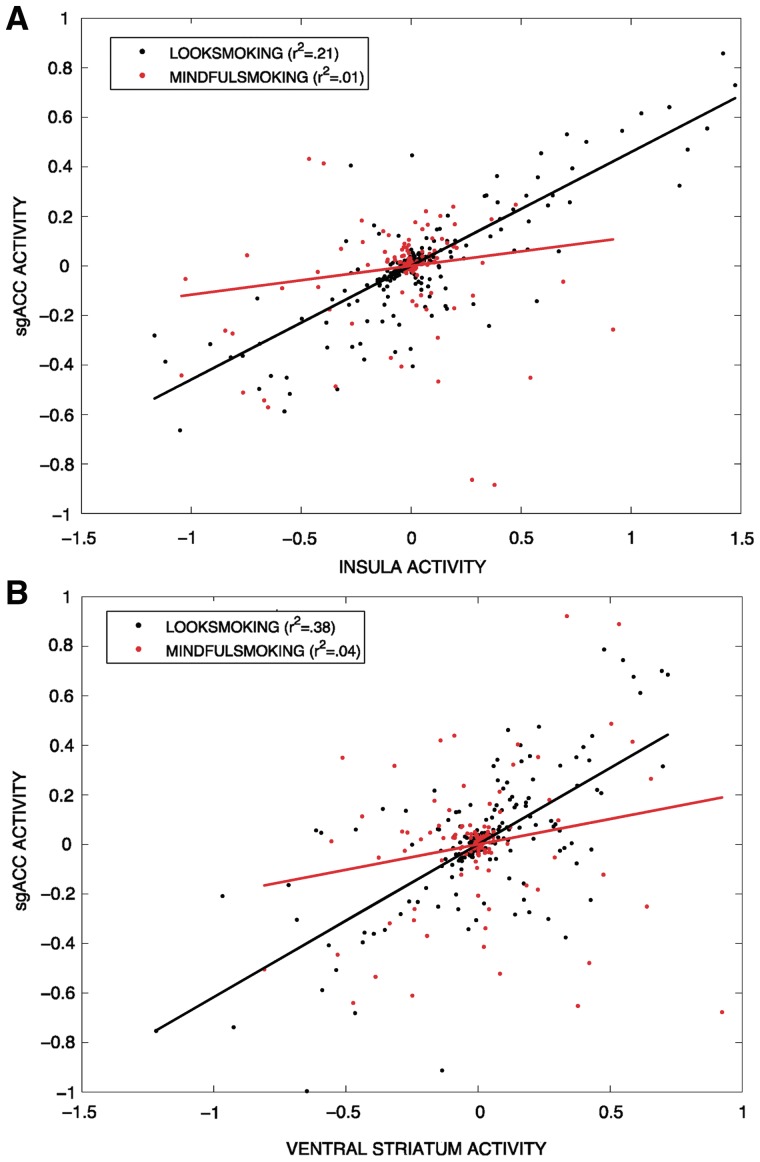
To further test our regulation vs reactivity hypotheses, PPI analysis was conducted to identify neural regions that were functionally connected with the sgACC cluster that was modulated during mindful attention (MindfulSmoking). An 8-mm cluster centered around the peak right sgACC cluster in the MindfulSmoking > LookSmoking group contrast was functionally defined as a seed region, and used in a group-level PPI connectivity analysis. Consistent with the reduced reactivity account, the sgACC cluster showed reduced functional connectivity with other craving-related regions, including bilateral insula and VS (Figure 5 and Table 3), during the MindfulSmoking condition compared to the LookSmoking condition. To help visualize this interaction, we provide representative single-subject plots comparing connectivity of sgACC with two regions (VS and insula) between the MindfulSmoking and LookSmoking conditions (Figure 6). This finding suggests that functional coupling in craving neurocircuitry during passive viewing of smoking images is reduced during mindful attention. This connectivity also did not support the regulation account, we found no lateral PFC regions that were more strongly connected with sgACC during mindfulness compared to passive viewing.
Fig. 5.
Functional connectivity in MindfulSmoking > LookSmoking. Regions that were functionally connected to sgACC seed during mindful attention to smoking stimuli compared to passive viewing. Highlighted regions are part of a network previously associated with craving, including the VS and insula.
Table 3.
Regions showing negative PPI with right vACC during MindfulSmoking > LookSmoking
| Anatomic region | BA | Side | Cluster size | Peak activation (MNI) |
t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Premotor | 6 | R | 3228 | 6 | −8 | 68 | −5.33 |
| Middle frontal gyrus | 9 | L | 83 | −22 | 30 | 40 | −4.36 |
| Precuneus | 18 | R | 230 | 16 | −70 | 26 | −4.15 |
| Inferior parietal lobule | 40 | L | 117 | 54 | 34 | 32 | −4.26 |
| Insula | 13/22 | L | 1412 | −46 | −18 | 8 | −5.32 |
| R | 2489 | 50 | −22 | 14 | −5.38 | ||
| Caudate | L | 193 | 14 | 16 | 0 | −4.55 | |
Fig. 6.
Representative single-subject plots of functional connectivity in VS and insula. Activity in VS and insula from two different subjects compared to ACC activity in MindfulSmoking and LookSmoking conditions. Functional coupling between ACC and insula (A) and VS (B) was greater in the LookSmoking condition than in the MindfulSmoking condition.
DISCUSSION
Mindful attention and reduced reactivity to craving
To our knowledge, the present study is the first to examine the neural pathways linking mindful attention to reduced cigarette craving. Our self-report results provide supporting evidence that mindful attention can reduce craving even among smokers who have no formal meditation experience. Furthermore, our imaging results suggest that mindful attention decreases craving-related activity in sgACC, and may reduce functional connectivity between the sgACC and other craving-related regions.
With the fMRI data, we directly tested two competing pathways by which mindful-attention may reduce craving. Our findings lend support to the ‘reduced reactivity’ account, which suggests that mindfulness acts as a ‘bottom-up’ attention to one's present moment experience (‘bare attention’, cf. Brown et al., 2007). We found activity in subgenual ACC increased for smoking images during passive looking, but decreased activity to smoking images during mindful attention. This suggests that nonjudgmental attention to one's craving-related experience lessens not only the subjective experience of craving, but also its neural correlates. Conversely, we found no evidence supporting a ‘regulatory’ pathway in this study as there was no evidence for increased recruitment of lateral PFC during mindful attention in this meditation-naïve sample. The results of the PPI analysis further support the ‘reduced reactivity’ account. We found that activity in sgACC seed region was less functionally connected to other craving-related regions during mindful attention, and not more strongly connected to any lateral PFC regulatory regions.
Thus far, the ‘reduced reactivity’ account has received relatively less consideration in the mindfulness literature. However, we believe this is because most studies examining the relationship between mindfulness and emotion have included active self-regulatory tasks. For instance, Creswell et al. (2007) used an affect-labeling technique, while the paradigm employed by Farb et al. (2007) involved reappraisal of words. Both of these tasks could be presumed to activate regions involved in active self-regulation. On the other hand, the current work compliments that of Zeidan et al. (2011) and Kober et al. (2011), in which participants are asked to notice and accept—but not ‘reappraise’—experiences such as pain. In both cases, mindfulness was associated with reductions in self-reported pain and pain-related brain activity, without concomitant increases in lateral-PFC regions, as predicted by the ‘active regulation’ account. Consistently, we trained participants to ‘notice and accept’ any feelings, sensations, etc., but not to reappraise, let go of, or distance themselves from any such perceptions. We feel that this approach is similar to the deployment of mindful attention used during meditation, and also encouraged in mindfulness-based interventions. As other authors have pointed out (Davidson, 2010; Williams, 2010; Chiesa et al., 2011), this observation underscores the importance of specifying how ‘mindfulness’ is defined and experimentally manipulated in research studies.
Our finding of reduced activity in sgACC during mindful attention is consistent with what is known about the functional significance of this region to substance use disorders and craving. Subgenual ACC appears to be directly involved in craving (Kober et al., 2010; Heatherton and Wagner, 2011) as well as more generally in emotion (Kober et al., 2008) and in mood psychopathology (Etkin and Wager, 2007; Drevets et al., 2009). The most direct evidence for the role of sgACC in cue-induced craving has been from Brody and colleagues, who found in several studies that this region was more metabolically active in heavy smokers during cue-induced craving (Brody et al., 2002), but this neural effect, along with self-reported craving, was blunted by treatment with buproprion (Brody et al., 2002). The decrease in sgACC activity we observed during mindful attention also extended to VMPFC, including the medial BA10 region. BA10 is posited to encode the subjective value of goods, such as an appetitive snack or monetary gamble (Damasio, 1994; Kable and Glimcher, 2007; Hare et al., 2008).
In addition to decreased activity in sgACC during mindful attention, we also found decreased functional connectivity between this region and a network of other craving-related areas including caudate, VS, premotor cortex and insula. All of these have been related to cigarette craving and smoking behavior in prior research (Naqvi and Bechara, 2010; Kühn and Gallinat, 2011).VS, in particular, is a known substrate of reward-related processing (Volkow et al., 2006; Franklin et al., 2009) and has been associated with dependence on nicotine as well as alcohol and cocaine (Kühn and Gallinat, 2011). Caudate and premotor cortex have both been reported in prior studies with cue-induced craving, are associated with severity of nicotine dependence (McClernon et al., 2005, 2007) and are implicated in the relationship between craving and smoking (Berkman et al., 2011). These regions are involved in motor planning, and it has been suggested that responses here reflect associations between smoking cues and motivated actions learned through repeated exposure (e.g. the act of smoking; Smolka et al., 2006). Finally, insula appears to play a particularly important role in substance use disorders, as damage to this region disrupts addiction to cigarettes (Naqvi et al., 2007). It has been hypothesized that this region may represent the interoceptive effects of craving (Gray and Critchley, 2007; Garavan, 2010).
Strengths and limitations
Major strengths of the study include the large neuroimaging sample size, the analytic approach, and the ecological and face validity of our task and participants. Our sample of smokers was abstinent and treatment seeking. They were also naïve to meditation practices, which is representative of most of the 46 million US adult smokers, only ∼14% of whom report having used mind body therapies such as mindfulness (Tindle et al., 2005). Furthermore, our mindful attention instructions were simple and fast to teach, making them similar to what a clinician might use in a brief intervention setting. Finally, the greatest strength of our study was the use of fMRI to elucidate the regulatory vs reduced reactivity mechanisms linking mindfulness to reduced cue-induced craving.
We did not collect information on psychiatric comorbidity within our sample, with the exception of the BDI-II (Beck et al., 1996). We feel that our decision not to exclude based on psychiatric comorbidity rendered our sample more generalizable to smokers in the USA. Specifically, current estimates indicate that 44% of all cigarettes sold in the US are smoked by individuals with diagnosed psychiatric disorders (Lasser et al., 2000). However, having more information about the psychiatric profile of our participants by way of a diagnostic interview would have provided more information about the psychiatric comorbidities in our sample, which is a limitation of the present work.
Given our design, one potential limitation is the possibility of an expectancy effect caused by our mindful attention instructions (i.e. our participants may have expected their craving to decrease, and therefore reported likewise in spite of their actual experiences). However, we think this is unlikely because we intentionally did not describe mindful attention as a craving–reduction strategy. Additionally, evidence from prior research indicates that self-reporting of affective changes in similar study designs is not linked to measures of social desirability (Ochsner et al., 2002). If anything, participants might expect the task to ‘increase’ their craving, following the general expectation that directing attention toward a given experience will increase the strength of that experience (Lieberman et al., 2011).
We were limited in the number of conditions we were able to assess while participants underwent neuroimaging. For example, we did not include a condition exploring how mindful attention affects neural and self-reported craving response to neutral images. Previous research suggests that mindful attention may increase self-reported positive affect to neutral stimuli (Arch and Craske, 2006), suggesting the possibility (to be assessed in future studies) that mindful attention may affect craving responses to neutral images in smokers. It is possible that carry-over neural activity may have contributed to the present results (Siegle et al., 2002), although we attempted to minimize this possibility through counterbalancing the order of task trials across participants. Finally, the present work describes the benefits of a brief mindful attentional state on craving, and the extent to which the observed effects may change or evolve over time is unclear. A randomized controlled trial of mindfulness in comparison to a rigorous control therapy would be required to fully elucidate the effects of mindfulness training interventions on neural and self-reported craving in smoking populations.
Summary
Overall, the present work suggests that mindful attention can reduce craving, and does so by decreasing activity and functional connectivity in regions of the brain known to subserve cigarette craving. Our work provides a potential neural mechanism for how mindfulness-based treatments improve smoking cessation (Bowen and Marlatt, 2009; Brewer et al., in press), and suggests that the mechanism of action may be via reduced reactivity rather than active self-regulation via lateral PFC. Thus, the present results suggest that mindful attention might be qualitatively distinct compared to cognitive regulation strategies that have been studied thus far in smokers (e.g. Kober et al., 2010). Although more research is needed to explore these neural craving pathways in mindfulness training randomized controlled trials, the present work corroborates Buddhist accounts of mindfulness and the hindrance of craving (Fronsdal, 2005), suggesting that mindful attention can reduce self-reported and neural reactivity to cue-induced craving.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to acknowledge the following individuals for advice and help at various stages of this project: Judd Brewer, James Bursley, Fadel Zeidan, the Pittsburgh Brain Imaging Research Center and the Pittsburgh Mind Body Center; and to Jill Delaney and Courtney Watson for their research assistance. C.W. would especially like to thank Dr Jennifer Silk for her guidance and support.
This work was supported in large part by the Pittsburgh Foundation Charles and Nancy Emmerling Fund to HT and by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (KL2 RR024154-05 to H.T.). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.; as well as a grant from the Pittsburgh Mind Body Center to HT, Mind and Life Institute Varela Awards to H.T. and C.W., the Pittsburgh Life Sciences Greenhouse Opportunity Fund to G.T. and D.C., and T32 MH17140 to G.T.
REFERENCES
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine and Tobacco Research. 2008;10(1):35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Altner N. Mindfulness practice and smoking cessation: The Essen hospital smoking cessation study (EASY) Journal for Meditation and Meditation Research. 2002;1:9–18. [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, et al. Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–241. [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology. 1989;57(3):443–9. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- Bowen S, Marlatt A. Surfing the urge: Brief mindfulness-based intervention for college student smokers. Psychology of Addictive Behaviors. 2009;23(4):666–71. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Mindfulness-based treatments for co-occurring depression and substance use disorders: what can we learn from the brain? Addiction. 2010;105(10):1698–1706. doi: 10.1111/j.1360-0443.2009.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness Training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119:72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, et al. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Research. 2004;130:269–81. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, et al. Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry. 2007;62(6):642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–37. [Google Scholar]
- Catley D, O'Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychology of Addictive Behaviors. 2000;14(1):73–6. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–40. [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Cigarette Smoking Among Adults – United States 2007. Morbidity and Mortality Weekly Report. 2008;57(45):1221–6. [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29:560–72. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Research. 2011;187(3):441–53. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Brambilla P, Serretti A. Functional neural correlates of mindfulness meditations in comparison to psychotherapy, pharmacotherapy and placebo effect: is there a link? Acta Neuropsychiatrica. 2010;22(3):104–17. doi: 10.1111/j.1601-5215.2010.00460.x. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical Psychology Review. 2011;31(3):449–64. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, et al. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature Neuroscience. 2011;14:426–27. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural Correlates of Dispositional Mindfulness During Affect Labeling. Psychosomatic Medicine. 2007;69(6):560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason and the Human Brain. New York: Avon Books, Inc; 1994. [Google Scholar]
- David SP, Munafò MR, Johansen-Berg J, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and non-smokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58(6):488–94. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion. 2010;10(1):8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. Complementary and Alternative Medicine. 2007;7:2. doi: 10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2009;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due D, Huettel S, Hall W, Rubin D. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J-L, Preston KL. Real-time electronic-diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66(1):88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive Behaviors. 2010;35(4):318–24. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3(34):235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback LO, Arendt M, Ørnbøl E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy—a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124(2):102–19. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fronsdal G. The Dhammapada: A New Translation of the Buddhist Classic With Annotations. Boston, MA: Shambhala Publications, Inc; 2005. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Structure and Function. 2010;214(5-6):593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International Smoking Image Series (With Neutral Counterparts), version 1.2. Carbondale, Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmagology. 1999;7(4):427–43. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology. 2002;70(1):142–52. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54(2):183–6. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, et al. DAT genotype modulates brain and behavioural responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–28. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare. TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation. Trends in Cognitive Sciences. 2011;15(3):132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67(8):722–9. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AP, Krompiger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(2):109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5(2):137–42. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions and emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107(33):14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Buhle J, et al. Mindfulness Modulates Pain and Negative Emotion: Evidence from Self Report and fMRI. Garrison, NY: Mind and Life Summer Research Institute; 2011. [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity for brain response. European Journal of Neuroscience. 2011;33:1318–26. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. Journal of the American Medical Association. 2000;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (fMRI) study of cue-induced smoking craving in virtual environments. Applied Psychophysiology and Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ. Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion. 2011;11(3):468–80. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover GH, Reiss AL. Methods and Software for fMRI Analysis for Clinical Subjects. San Francisco, CA: Human Brain Mapping; 2009. [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence of the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessations: results of a preliminary functional magnetic resonance imaging study. Addiction Biology. 2007;12(3–4):503–12. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214(5–6):435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Schwartz JE, Gerkovich MM, Marjorie B, Shiffman S. Playful and rebellious states vs. negative affect in explaining the occurrence of temptations and lapses during smoking cessation. Nicotine and Tobacco Research. 2004;6(4):661–674. doi: 10.1080/14622200410001734049. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104(10):1610–6. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles of discouragement: two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology. 1998;107(2):238–51. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin J-L, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207(2):291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogojanski J, Vettese LC, Antony MM. Coping with cigarette cravings: comparison of suppression versus mindfulness-based strategies. Mindfulness. 2011;2(1):14–26. [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;62:373–86. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Siegle G, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Responses to smoking cues are relevant to smoking and relapse. Addiction. 2009;104(10):1617–22. doi: 10.1111/j.1360-0443.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shumaker SA, Abrams DB, et al. Models of smoking relapse. Health Psychology. 1986;5(Suppl):13–27. [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, et al. A day at a time: predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106(1):104–16. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li C-SR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Bühler M, Klein S, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184(3–4):577–88. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Tiffany S. The continuing conundrum of craving. Addiction. 2009;104(10):1617–22. doi: 10.1111/j.1360-0443.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- Tindle HA, David RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Alternative Therapies in Health and Research. 2005;11(1):42–9. [PubMed] [Google Scholar]
- United States Public Health Service. Treating Tobacco Use and Dependence: 2008 Update. 2008. U. S. P. H. S. Department of Health and Human Services. Rockville, MD, U.S. [Google Scholar]
- van den Hurk PAM, Janssen BH, Giommi F, Barendregt HP, Gielen SC. Mindfulness meditation associated with alterations in bottom-up processing: Psychophysiological evidence for reduced reactivity. International Journal of Psychophysiology. 2010;78:151–7. doi: 10.1016/j.ijpsycho.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Businelle MS, Cinciripini P, et al. Associations of mindfulness with nicotine dependence, withdrawal, and agency. Substance Abuse. 2009;30(4):318–27. doi: 10.1080/08897070903252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26(24):6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way B, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG. Mindfulness and psychological process. Emotion. 2010;10(1):1–7. doi: 10.1037/a0018360. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA, Walker D. Mindfulness-based relapse prevention for alcohol and substance use disorders. Journal of Cognitive Psychotherapy. 2005;19(3):211–28. [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology. 2010;78(3):362–74. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. 2011;31(14):5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]