Abstract
Considerable research has disclosed how cognitive reappraisals and the modulation of emotional responses promote successful emotion regulation. Less research has examined how the early processing of emotion-relevant stimuli may create divergent emotional response consequences. Mindfulness—a receptive, non-evaluative form of attention—is theorized to foster emotion regulation, and the present study examined whether individual differences in mindfulness would modulate neural responses associated with the early processing of affective stimuli. Focus was on the late positive potential (LPP) of the event-related brain potential to visual stimuli varying in emotional valence and arousal. This study first found, replicating past research, that high arousal images, particularly of an unpleasant type, elicited larger LPP responses. Second, the study found that more mindful individuals showed lower LPP responses to high arousal unpleasant images, even after controlling for trait attentional control. Conversely, two traits contrasting with mindfulness—neuroticism and negative affectivity—were associated with higher LPP responses to high arousal unpleasant images. Finally, mindfulness was also associated with lower LPP responses to motivationally salient pleasant images (erotica). These findings suggest that mindfulness modulates neural responses in an early phase of affective processing, and contribute to understanding how this quality of attention may promote healthy emotional functioning.
Keywords: mindfulness, emotion regulation, event-related potentials, late positive potential
INTRODUCTION
Emotions play a key role in decision making, readying behavioral responses, enhancing memory for salient experiences and facilitating interpersonal interactions. However, emotions, particularly negative emotions, can also foster interpersonal conflict, poor decisions and mental health problems (Gross, 2007). Therefore, people often try to regulate their emotions, by changing the valence, intensity or duration of their responses (Rothbart and Sheese, 2007). To date, most emotion regulation research has been devoted to the study of cognitive reappraisals—changing how we think about a situation or our capacity to manage it—and the modulation of emotional responses; but increasingly researchers are examining how the ways in which people first process emotion-relevant stimuli may create divergent psychological responses. This topic is important because understanding how early attentional contact with stimuli becomes translated into cognitive, emotional and other responses can provide considerable leverage for changing the nature and time course of emotions, their associated physiological events, and long-term effects.
A key means to examine how attention to stimuli impacts later emotional responses is through the study of electrocortical activity in the brain, and scalp-recorded event-related potentials (ERPs) in particular. Affective processes are known to unfold rapidly after stimulus contact (over milliseconds), and the high temporal resolution of ERPs makes them well-suited to measuring the rapid affective and cognitive processes associated with the activation and initial regulation of emotions (Hajcak and Olvet, 2008). In particular, a component of the ERP called the Late Positive Potential (LPP) provides a useful window onto emotion regulatory processes. The LPP is a positive deflection of the ERP in the slow wave latency range (∼400–500 ms after stimulus onset) and appears most prominently in posterior and central midline scalp regions. The LPP reflects facilitated attention to emotional stimuli (Cuthbert et al., 2000; Schupp et al., 2000; Dennis & Hajcak, 2009). It is larger (more positive) for emotionally salient than for neutral visual stimuli, and especially for higher intensity (more arousing) pleasant and unpleasant stimuli (Cuthbert et al., 2000; Schupp et al., 2000, 2003; Keil et al., 2002). LPP amplitude has been correlated with subjective reports of arousal level (Cuthbert et al., 2000), providing convergent evidence that the LPP is a neural marker for emotional arousal. In short, the LPP seems to reflect preferential processing and encoding of motivationally relevant information.
Research has also shown the LPP to be sensitive to stimulus valence, with greater modulation of this component in response to unpleasant stimuli (Carretie et al., 2001, 2004; however see Weinberg and Hajcak, 2010). This ‘negativity bias’ is thought to reflect rapid amygdala processing of aversive information (LeDoux, 1995), so that attentional resources are engaged more readily for motivationally salient unpleasant stimuli than for neutral or pleasant stimuli to facilitate efficient, adaptive processing (e.g. Ito et al., 1998; Olafsson et al., 2008).
The LPP amplitude appears to be susceptible to top-down processing influences related to the evaluation of affective stimuli (Olofsson et al., 2008). Hajcak and colleagues have shown that the LPP elicited by unpleasant images is reduced when a more neutral interpretation of them is given (see Hajcak et al., 2010 for review). For example, Hajcak and Nieuwenhuis (2006) found that reappraisal instructions (reinterpreting images so that they no longer elicited a negative response) resulted in a reliably reduced LPP, the degree of which was positively related to reductions in self-reported emotional intensity.
Recently, theorists have suggested that ‘mindfulness’ may offer an important emotion regulatory advantage (Lutz et al., 2008), and this construct is relevant in the present context for two reasons. First, mindfulness concerns a receptive, non-evaluative form of attention marked by simple observation of what is taking place (e.g., Brown and Ryan, 2003). Mindfulness is an exemplar of the experiential mode of conscious processing (Teasdale, 1999) that can be contrasted with the conceptually driven mode of processing wherein occurrences are habitually filtered through appraisals, evaluations and other forms of cognitive manipulation (Brown and Cordon, 2009). As such, mindfulness is functionally similar to reappraisal in its dampened evaluation of stimuli, and may show similar, early influences on affective stimulus processing.
Second, there is evidence that mindfulness is associated with more benign responses to affective stimuli, particularly of an unpleasant or threatening nature. For example, dispositional mindfulness has been associated with cortical and limbic markers of emotional reactivity, including less amygdala activation at rest (Way et al., 2010) and during emotional threat (Creswell et al., 2007), as measured by functional magnetic resonance imaging. Creswell et al. (2007) also found trait mindfulness scores to be positively related both to higher activations in regions of the prefrontal cortex (PFC) during emotional threat, and a dampening of amygdala activation through this higher PFC activation in a way theorized to reflect better emotion regulation. But the study of mindful responses to affective stimuli in an early phase of attentional contact with those stimuli—for which the examination of ERPs are well-suited—has yet to be undertaken. Such study can inform our theoretical understanding of how specific modes of information processing become translated into ‘downstream’ emotional and other responses. Given the key role of emotion regulation in supporting mental health and other adaptive outcomes, such understanding may have important clinical relevance as well.
The present research
The present study was designed primarily to examine how dispositional mindfulness is associated with neural responses to affective stimuli in an early period of attentional engagement. Given past research showing that the LPP range of the ERP waveform is sensitive to stimulus evaluation and the downregulation of it (e.g. Foti and Hajcak, 2008), we specifically examined whether mindfulness, as a non-evaluative form of attention, would be related to lower LPP amplitudes elicited by affective images. Our interest was specifically in highly arousing images, given research showing that the LPP is particularly responsive to them over less arousing images.
Neuroimaging research (Creswell et al., 2007) suggests that mindfulness promotes lower neural reactivity to unpleasant or threatening stimuli, providing some basis to expect that mindfulness would be associated with lower LPP amplitudes elicited by such stimuli. However, the non-evaluative quality of mindful attention may also extend to the processing of pleasant stimuli, and we examined this here. Importantly in this regard, Weinberg and Hajcak (2010) found that erotic images elicited LPPs that were larger than other pleasant images (of exciting sports) and comparable to high arousal unpleasant images. We explored whether mindfulness modulated the LPP elicited by pleasant images in general and by erotic images specifically, which carry motivational salience similar to high arousal unpleasant stimuli.
This study had several other purposes as well. We contrasted the experiential, non-evaluative form of processing inherent in mindfulness with more conceptual, evaluative forms of processing inherent in such traits as neuroticism and negative affectivity to better understand the neural bases of these divergent styles of emotion-relevant processing. The latter traits, typically highly correlated, have been shown to correlate negatively with trait mindfulness (Giluk, 2009) and are marked by negative emotional reactivity to unpleasant life events (Goldberg, 1993) and neural reactivity to negative stimuli (Canli et al., 2001). We therefore anticipated that individuals higher in neuroticism and negative affectivity would show larger LPP amplitudes during unpleasant image viewing, in contrast to the lower LPP amplitudes anticipated of more mindful individuals.
Another contrast of interest here was that between mindfulness and attentional control. There is some debate about whether trait measures of mindfulness actually assess this subtle construct rather than simple attentiveness (Grossman, 2008). The present study provided a first test of the correlation between measures of these constructs and controlled for the role of attentional control in tests of the predictive role of mindfulness on LPP responses.
Finally, there is indication that the LPP can be meaningfully divided into separate time windows to permit the tracking of individual responsiveness to emotional material over time (Hajcak et al., 2010). Therefore, we examined whether mindfulness and contrasting traits are differentially associated with an earlier LPP reactivity to emotional stimuli vs a later, more actively regulated stage of affective processing (e.g. Hajcak and Nieuwenhuis, 2006).
METHODS
Participants
Participants were 46 introductory psychology students at a large mid-Atlantic university who earned course credit for participation. Participants with a history of neurological or psychiatric illness (n = 5) were excluded from the study. Four participants were excluded from analyses due to excessive electroencephalograph (EEG) artifact (rates higher than 70%), and three participants were excluded for procedural non-compliance. The remaining 34 participants [20 (61%) female; 1 undeclared] ranged in age from 18 to 59 years (M = 21.73, s.d. = 7.38). Most (46%) were Caucasian; the remainder were African-American (9%), Hispanic/Latino(a) (6%), Asian Indian (15%), or another race/ethnicity (24%) (1 undeclared).
Measures
Mindfulness
Two well-validated scales assessed trait mindfulness. The 15-item Mindful Attention Awareness Scale (MAAS; Brown and Ryan, 2003) assessed the frequency with which an individual is openly attentive to and aware of present events and experiences using a 6-point Likert scale (‘almost always’ to ‘almost never’). An example item is, ‘I could be experiencing some emotion and not be conscious of it until some time later’. Higher scores indicate higher mindfulness (sample α = 0.82). The 39-item Five-Factor Mindfulness Questionnaire (FFMQ; Baer et al., 2006) assessed ‘mindfulness skills’ with five subscales and using a 5-point Likert scale (‘never or very rarely true’ to ‘very often or always true’). Present interest was in the 8-item Acting with Awareness subscale; the other subscales appear to reflect skills used in mindfulness practice (Brown and Cordon, 2009). An example item is, ‘I am easily distracted’ (reversed). Higher scores indicate higher mindfulness (α = 0.89).
Attention control
The 20-item Attentional Control Scale (ACS; Derryberry and Reed, 2002) measured two major trait components of attention (focusing and shifting) on a 4-point scale (‘almost never’ to ‘always’). Higher scores indicate higher attention control (α = 0.77).
Neuroticism
The 12-item neuroticism subscale of the NEO-FFI (Costa and McCrae, 1992) assessed dispositional anxiety, hostility, depression, impulsiveness and vulnerability on a 5-point scale (‘strongly disagree’ to ‘strongly agree’). Higher scores indicate higher neuroticism (α = 0.84). The openness to experience and extroversion subscales were also administered, on the speculation that these traits might predict more receptive processing of affective stimuli (cf. Canli et al., 2001).
Negative affectivity
The 20-item Positive Affectivity Negative Affectivity Schedule (PANAS; Watson et al., 1988) assessed affective arousal over the past week. Present interest was in the 10-item negative affectivity (NA) subscale. Scores on the 7-point scale (‘not at all’ to ‘extremely’) indicate higher NA (α = 0.85).
Stimulus materials and presentation
Stimuli were 150 images from the International Affective Picture System (IAPS; Lang et al., 2008) that varied in valence and arousal. Stimuli were selected and categorized based on their normative valence and arousal ratings (Lang et al., 2008), and visual inspection (see Supplemental Materials). Of these, 30 images depicted pleasant, high arousal events (e.g. skydiving, erotica), 30 pleasant, low arousal (e.g. flowers, smiling people), 30 unpleasant, high arousal images (e.g. mutilations, corpses), 30 unpleasant, low arousal images (e.g. homeless people, pollution) and 30 neutral images (e.g. household objects, mushrooms). Erotic images were gender balanced. One-way analysis of variance tests indicated significant differences across stimulus categories in mean normative valence and arousal ratings (all Ps < 0.002).
The task was administered on a PC using Stim2 software (Neuroscan; El Paso, TX, USA) to control the timing, EEG synchronization, and stimulus display. Each image was displayed full screen in color on a 19″ flat-screen LCD monitor. Participants were seated approximately 34″ from the monitor with a vertical visual angle of 20°.
Procedure
After providing informed consent and meeting inclusionary criteria (see ‘Participants’ section), participants completed a battery of self-report measures, including those described in the ‘Measures’ section above. They were then fitted with an electrode cap for EEG recording. Participants were asked to refrain from moving during the recording period. For another study, we recorded resting EEG. Participants then began the passive viewing task, in which they were asked to look at each picture displayed on the monitor for the entire presentation duration. The 150 image trials were divided into 5 equal blocks, each separated by a 30-second break. The images were presented in a pre-arranged random order for 5 s each, with a random interstimulus interval between 2 and 4 s. Prior to the display of each image, a central fixation cross appeared on the screen for 2 s. After the passive viewing task, cap removal and a computer task (not discussed here) participants were debriefed, thanked and dismissed.
Electrophysiological recording and data reduction
EEG was recorded using 36 sintered Ag/AgCl electrodes mounted in a Quik Cap (Neuroscan). Electrodes positions were based on the 10–20 international system with a forehead ground and two monopolar references placed on the left and right mastoids. Continuous EEG was digitized at a sampling rate of 1000 Hz using a NuAmps Express digital amplifier (Neuroscan). Frequencies above 30 Hz were removed using an online low-pass filter. The electro-oculogram (EOG) was recorded with monopolar electrodes located below and on the outer canthus of each eye. Offline, the monopolar EOG channels were combined into bipolar channels prior to EOG artifact rejection. All EEG/EOG electrode impedances were below 10 k Ω (most were below 5 k Ω). After recording, gross artifacts were removed from the continuous EEG signal based on visual inspection. Single-trial epochs for each stimulus type were extracted separately offline for a period beginning −100 ms prior to stimulus onset and continued for 1100 ms. The raw EEG epochs were baseline-corrected by subtracting the average voltage of the 100 ms windows occurring before stimulus onset. Ocular artifacts with thresholds of −75 µV and +75 µV were automatically rejected. Stimulus-locked ERPs were averaged in the time domain for each stimulus category.
Visual inspection of the ERP waveform across electrode sites and stimulus conditions revealed that the LPP began, on average, ∼500 ms after stimulus onset and continued, on average, until ∼900 ms after onset. This signal window is generally consistent with past studies using passive picture viewing (e.g. Hajcak and Nieuwenhuis, 2006). Previous research of this kind has found strongest LPPs at central and parietal midline sites (e.g. Cuthbert et al., 2000; Schupp et al., 2000); therefore analyses of the LPP used the signal values at electrodes FCz, Cz, CPz and Pz. To simplify the presentation, we highlight results found at site CPz (cf. Hajcak and Nieuwenhuis, 2006), and briefly summarize results from the other three sites. Early and late windows of the LPP were created by splitting the full range of the LPP in half (500–700 ms, 701–900 ms) and averaging the signals within each window at each site.
RESULTS
Effects of stimulus condition and signal window on LPP amplitude
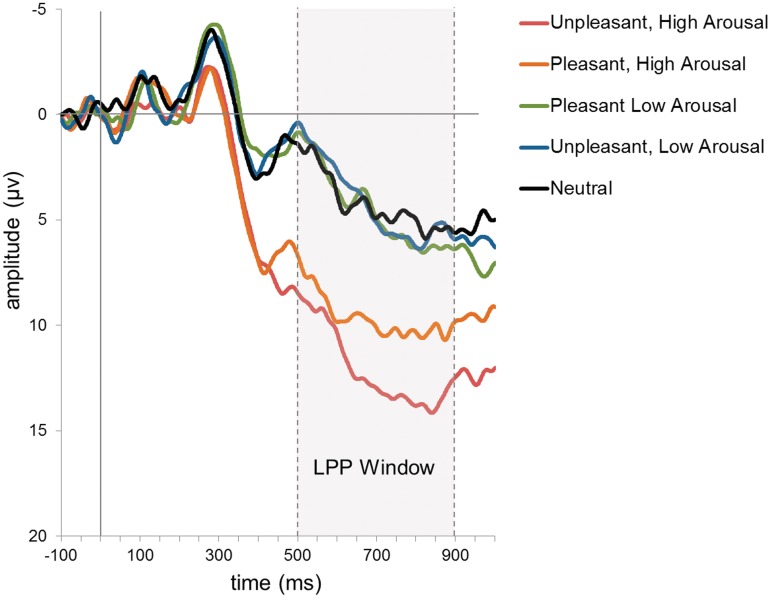
The ERP waveforms associated with high and low arousal pleasant and unpleasant stimuli and neutral stimuli at the CPz site in early and late windows of the LPP are presented in Figure 1. A 2 (signal window) × 5 (stimulus condition) repeated-measures mixed model using restricted maximum likelihood estimation (REML) (e.g. Bryk and Raudenbush, 1992) was tested to examine the role of each variable on the LPP amplitude. A main effect for both signal window [F(1, 33) = 33.84, P < 0.0001] and stimulus condition [F(4, 132) = 70.87, P < 0.0001] was found, but no interaction between them (P = 0.50). LPP amplitude was higher in the late window (M = 7.99, s.d. = 4.23) than in the early window (M = 5.50, s.d. = 5.20).
Fig. 1.
ERP waveforms at electrode site CPz associated with high and low arousal pleasant and unpleasant images and neutral images across a −100 ms (pre-stimulus) to 1000 ms (post-stimulus) recording period.
Tukey-Kramer post-hoc tests showed that across early and late windows, a larger LPP modulation was elicited to high arousal unpleasant stimuli (M = 11.96, s.d. = 7.02) and pleasant stimuli (M = 9.87, s.d. = 6.49) than to low arousal unpleasant and pleasant stimuli (M = 3.63, s.d. = 4.46 and M = 3.81, s.d. = 4.64, respectively) and to neutral stimuli (M = 3.77, s.d. = 5.48), all Ps < 0.0001. The LPPs associated with the two types of high arousal stimuli also differed from each other, P = 0.02. The LPPs associated with low arousal pleasant and unpleasant stimuli and neutral stimuli did not differ from each other (all Ps > 0.99). Generally consistent results were found at sites FCz, Cz and Pz. Specifically, main effects for signal window (except at Pz, P = 0.16) and stimulus condition were found (Ps < 0.0001), and a nearly identical pattern of arousal level differences, except that at Cz and Pz, the two high arousal stimulus class LPPs did not differ from each other, Ps > 0.06.
These results, particularly on stimulus condition, are consistent with prior research showing that the LPP is more sensitive to intensity than to valence of emotional stimuli, and somewhat consistent with research showing larger LPP amplitudes associated with unpleasant over pleasant stimuli (the negativity bias).
Modulation of LPP amplitude by psychological traits
The trait measures of experiential (mindful) and conceptual processing were intercorrelated as expected. The MAAS and FFMQ act with awareness measures of mindfulness were highly correlated (r = 0.92, P < 0.0001), as were neuroticism and NA (r = 0.50, P = 0.003). The MAAS and FFMQ measures correlated inversely with neuroticism (rs = − 0.44, P = 0.009 and −0.58, P = 0.0003, respectively) and with NA (rs = −0.35, P = 0.05 and −0.48, P = 0.005, respectively). Both MAAS and FFMQ mindfulness measures were correlated with attentional control (rs = 0.60, P = 0.0002 and 0.68, P = 0.0001, respectively).
Because the high arousal pleasant and unpleasant stimuli elicited significantly larger LPP amplitudes at all sites than the other stimulus types, only the former two types were retained for analyses of psychological trait relations to LPP amplitude. Four types of 2 (signal window) × 2 (stimulus condition) repeated-measures mixed models were tested, each covarying one trait: MAAS mindfulness, FFMQ awareness, NEO-FFI neuroticism and PANAS negative affectivity (NA).
The first analyses, testing the main and interactive role of MAAS mindfulness on LPP amplitude at CPz, showed, as in the previous model, main effects for both signal window [F(1, 33) = 8.64, P = 0.006] and stimulus condition [F(1, 33) = 12.15, P = 0.001].1 There was also a main effect for mindfulness on LPP amplitude [F(1, 32) = 7.35, P = 0.01], but more importantly, a significant mindfulness × stimulus condition interaction [F(1, 96) = 9.24, P = 0.003]. There were no mindfulness × signal window nor mindfulness × window × condition interactions (both Ps > 0.70). In the model covarying FFMQ mindfulness, the results were highly similar: the main effect of mindfulness was significant [F(1, 32) = 6.65, P = 0.02], as was the mindfulness × stimulus condition interaction [F(1, 96) = 11.69, P = 0.0009]. No other interactions with FFMQ mindfulness were significant (Ps > 0.46). The same pattern of results was found at the other three sites, and most importantly, the MAAS and FFMQ mindfulness × stimulus condition interactions (Ps < 0.005).
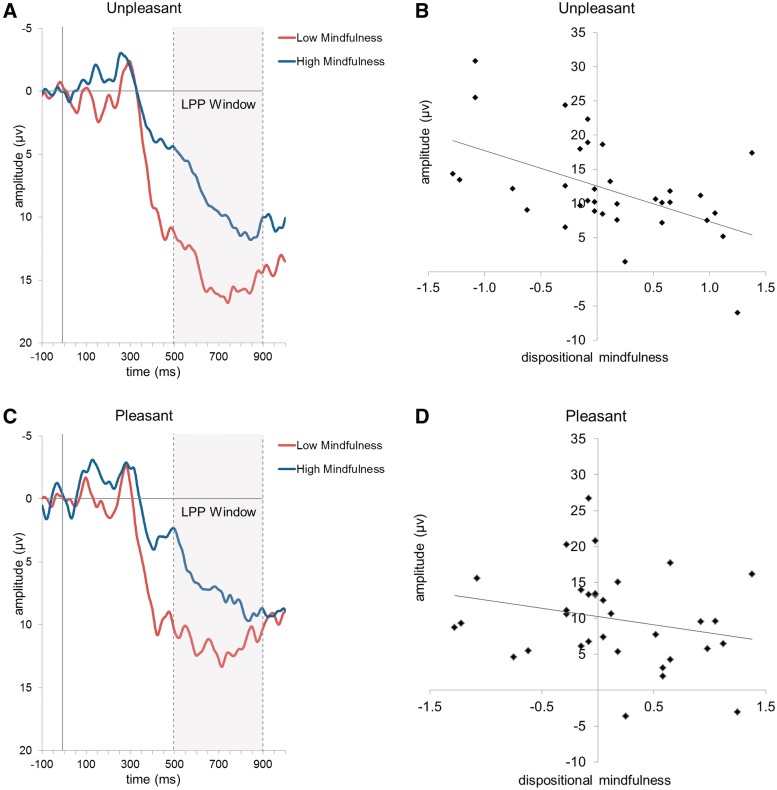
Table 1 shows that MAAS and FFMQ mindfulness were strongly inversely correlated with LPP amplitude at CPz (across the full 500–900 ms range) elicited by unpleasant images, while only weakly inversely correlated with the LPP associated with pleasant images. Figure 2 displays the average ERP signal at electrode site CPz elicited by high arousal pleasant and unpleasant images for participants higher and lower in MAAS mindfulness, as well as the bivariate correlations between MAAS score and average LPP evoked by these images. As is evident, those higher in mindfulness showed a dampened LPP response to unpleasant stimuli, in particular.2
Table 1.
Correlations between psychological traits and LPP amplitude at the CPz electrode site elicited by high and low arousal unpleasant and pleasant stimuli and neutral stimuli
| Stimulus type |
|||||
|---|---|---|---|---|---|
| Trait variable | High arousal unpleasant | High arousal pleasant | Low arousal unpleasant | Low arousal pleasant | Neutral |
| MAAS mindfulness | −0.52*** | −0.25 | −0.18 | −0.26 | −0.19 |
| FFMQ awareness | −0.52*** | −0.21 | −0.22 | −0.23 | −0.15 |
| NEO-FFI neuroticism | 0.44** | 0.27 | 0.31 | 0.24 | 0.14 |
| PANAS negative affectivity | 0.45** | 0.1 | 0.43** | 0.36* | 0.28 |
Notes. N = 34 (n = 33 for negative affectivity). LPP amplitude window = 500–900 ms. MAAS = Mindful Attention Awareness Scale; FFMQ = Five-Factor Mindfulness Questionnaire; NEO-FFI = Neuroticism Extroversion Openness-Five Factor Inventory; PANAS = Positive Affectivity Negative Affectivity Schedule.
*P < 0.05 **P < 0.01 ***P < 0.005
Fig. 2.
Left panel: LPP waveforms at electrode site CPz associated with (A) high arousal unpleasant images and (C) high arousal pleasant images, shown separately for high and low MAAS mindfulness groups, created by median split. In the right panel, scatterplots depict the correlation of MAAS dispositional mindfulness (using centered scores) with the LPP amplitudes elicited by (B) high arousal unpleasant images (r = −0.52, P = 0.002) and (D) high arousal pleasant images (r = −0.25, P = 0.16) at electrode site CPz.
To address the dispute about whether trait measures of mindfulness differ from measures of attentiveness, we repeated the mixed model analyses just reported while controlling for the main effect of ATS attention control.3 In both MAAS and FFMQ mindfulness-based models, the mindfulness x stimulus condition interactions remained significant at CPz (P = 0.003 and P = 0.0009, respectively). Similar results were found at FCz, Cz and Pz (MAAS model Ps < 0.005; FFMQ model Ps < 0.002). Attention control did not predict the LPP in these models (Ps > 0.17).
In the models covarying PANAS NA, the results were somewhat consistent with those of the mindfulness-based models, though in the opposite direction. In the CPz model covarying NA, there was no main effect of this trait on LPP amplitude (P = 0.07) but there was a significant NA × stimulus condition interaction [F(1, 93) = 15.94, P < 0.0002]. Similar interaction results were found at the other three sites (Ps < 0.05). In models covarying NEO-FFI neuroticism, a main effect on LPP amplitude was found at CPz [F(1, 32) = 6.11, P = 0.02] and at Cz and Pz (Ps < 0.05), but not at FCz (P = 0.21). No neuroticism × stimulus condition interactions were found at any site (Ps > 0.06). Table 1 shows that NA in particular was positively correlated with the LPP amplitude at CPz elicited by unpleasant images, while less correlated with the pleasant images-elicited LPP (see Supplementary Tables S2–S4 for all trait correlations at FCz, Cz and Pz).4
Modulation of motivational salience-relevant LPP amplitude by psychological traits
To test whether the trait modulation of LPP amplitude elicited by high arousal unpleasant, but not pleasant stimuli reported here was determined by the motivational relevance of pleasant stimuli, we repeated the primary analyses in this section using only erotic image LPPs to represent high arousal pleasant stimulus responses. Except at site FCz, the condition main effect remained at all sites (Ps < 0.05). But except at site Pz, where the mindfulness × stimulus condition interaction remained (Ps < 0.002), only main effects for mindfulness were found (Ps < 0.004). MAAS mindfulness—erotic image LPP correlations ranged across sites from −0.22, P = 0.20 (Pz) to −0.35, P = 0.04 (CPz) [FFMQ rs = −0.18, P = 0.32 (Pz) to −0.32, P = 0.06 (CPz)]. The mixed model results for neuroticism and NA were generally unchanged from those already reported.
DISCUSSION
Accumulating research is showing that mindfulness, and training to enhance it, is associated with adaptive behavioral, psychological and physical health outcomes (Brown et al., 2007). Researchers have also begun to uncover what may prove to be important neural mechanisms for these salutary outcomes of mindfulness (e.g. Davidson et al., 2003; Tang et al., 2007). The present research examined the role of mindfulness in neural dynamics associated with the regulation of emotion, a process key to numerous adaptive outcomes (Gross and Munoz, 1995). Specifically, we sought to disclose how dispositional mindfulness is related to neural processes associated with emotional processing that unfolds quickly after stimulus contact.
Examining the LPP component of the ERP response to emotional visual stimuli, this study first replicated past research showing that highly arousing pictorial stimuli, and high arousal unpleasant stimuli in particular, elicited a larger LPP response than low arousal and neutral stimuli (Olafsson et al., 2008). However, trait mindfulness, as measured by two different instruments, modulated the LPP response to high arousal unpleasant images, such that the LPP amplitude was lower for more mindful individuals than for those less mindful. These results held across four midline sites (FCz, Cz, CPz and Pz) and after controlling for self-reported attentional control, a construct that, while distinct from mindfulness, may be confused with it operationally (Grossman, 2008).
Further, the modulation of the LPP to unpleasant images among more mindful individuals also extended to pleasant images carrying similar motivational salience (erotica; Weinberg and Hajcak, 2010). This accords with the notion of mindfulness as a broadband, non-evaluative mode of attention (e.g. Brown et al., 2007). The study also found that constructs somewhat antithetical to mindfulness, neuroticism and negative affectivity, were positively associated with LPP amplitudes elicited by high arousal unpleasant images across superior-posterior midline sites in particular.
This study contributes to efforts to characterize interindividual variability in affective ERP responses. Brain imaging research has pointed to systematic differences in affective perception linked to genetic and personality factors (e.g. Canli et al., 2001) but until recently individual differences in ERP variability have received less consideration (but see Hirsh and Inzlicht, 2008; Foti et al., 2010; Olvet and Hajcak, in press). Olofsson et al. (2008) suggest that testing correlations with psychological traits (and demographic factors) are important first steps toward a theoretical characterization of affective ERP variability. The present findings contribute to our growing understanding of how psychological traits that have known implications for emotional functioning are associated with the early (<1 s) processing of affective stimuli.
The results also help to inform our theoretical understanding of how two primary forms of processing (Teasdale, 1999)—experiential (exemplified by mindfulness)—and conceptual (inherent in neuroticism and NA)—have divergent associations with the LPP, a neural marker for an early phase of emotion regulation, in which attention is engaged by stimuli about which appraisals or evaluations are often made. These findings are the first known to us suggesting that mindfulness may temper the early response to unpleasant and other motivationally salient affective stimuli before a subsequent emotional response has opportunity to arise. This may provide insight into how mindfulness provides the emotion regulatory advantages thought to accrue from it (Lutz et al., 2008). Conversely, the fact that neuroticism and NA predicted elevated early neural responses to unpleasant stimuli may offer clues as to why these traits present emotion regulatory difficulties (John and Gross, 2007).
Recent neuroimaging studies have demonstrated that training in mindfulness can produce changes in the neural networks associated with attention (Tang et al., 2007), affective processing (Lutz et al., 2008), and emotion regulation (Goldin and Gross, 2010). Directly relevant to the current study, Sobelowski et al. (in press) showed that experienced mindfulness trainees showed lower LPP deflections in response to unpleasant images, with no difference from controls in the LPP response to pleasant images. Yet many forms of mindfulness training are multimodal, and it has been unclear whether enhanced mindfulness per se is responsible for the functional neural responses observed. Recently, Modinos et al. (2010) showed that trait mindfulness accentuated activity in neural systems involved in cognitive reappraisal of affective stimuli, namely regions of the prefrontal cortex, the anterior cingulate cortex and the amygdala (Oschner and Gross, 2005). The present findings increase our knowledge in this area by first showing, consistent with Modinos et al. (2010), that mindfulness itself is functionally related to a key neural marker of emotion regulation, and second, by showing that this regulatory role manifests in an early phase of affective stimulus processing. In so doing, this research offers insight into how mindfulness may operate as a top-down attentional mechanism that acts to regulate emotion by dampening the arising of emotions before they have opportunity to impact well-being (cf. Barnes et al., 2007).
Limitations and future research
This study was designed in part to examine how preexisting differences in mindfulness translate into LPP responses, but without an experimental manipulation of mindfulness, the relations observed here could be due to some other variable(s) associated with mindfulness, rather than mindfulness itself. We showed that trait mindfulness was associated with LPP modulation after controlling for attentional control, but other variables correlated with mindfulness could be responsible for the relations observed. Research is needed to corroborate the present results using designs permitting causal inferences using, for example, brief mindfulness inductions (e.g. Arch and Craske, 2006). Also, to help ensure that mindfulness (and other traits) do predict ERP responses, periodic checking of participants’ attention to the stimuli would help to eliminate the possibility that attention is selective. Finally, behavioral measures of mindful attention will help to validate the theoretical interpretation of the LPP responses made here by revealing the functional significance of the ERP modulations observed (cf. Olofsson et al., 2008).
CONCLUSION
This study found that individual differences in mindfulness were associated with lower deflections in the LPP component of the ERP signal at central and parietal midline regions of the cortex whose activity has been associated with attention to and appraisal of emotional stimuli. The lower deflection of the LPP among more mindful individuals was largest in response to highly arousing unpleasant stimuli, consistent with the conception of mindfulness as promoting receptivity to unpleasant or threatening stimuli (Baer, 2003). But mindfulness was also associated with less deflection of the LPP in response to motivationally salient pleasant stimuli (erotica), suggesting that this trait facilitates non-evaluative processing of self-relevant stimuli more generally. The findings also support the search for individual differences in the neural dynamics of affective processing, and shed light on how mindfulness may support effective emotion regulation. This knowledge may inform clinical efforts to enhance affective functioning.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank the following for research assistance: James Ballard, Shayla Cashwell, Philippa Childress, Brian Haver, Jacqueline Hoyt, Arturo Iglesias, Glendon Kemp, Holly Muller, Sheri Oyan, and Elizabeth Sadock.
Footnotes
1These main effects were also found in the other trait models reported in this section, all Ps < 0.006.
2Correlations between the other four FFMQ subscales and the LPP amplitude elicited by high arousal unpleasant images were weaker, ranging from r = 0.06, p P = 0.61 (Observe) to r = -−0.42, P = 0.01 (Describe) at CPz. Similar correlations were found for high arousal pleasant image-elicited LPP amplitude across sites. PANAS PA scores were uncorrelated with the LPP in both stimulus conditions across sites, Ps > 0.31, and similarly for NEO openness to experience and extroversion, Ps > 0.12.
3Preliminary mixed model analyses showed no attention control × signal window nor attention control × stimulus condition interactions in predictions of the LPP (all Ps > 0.05).
4Participant gender was covaried in preliminary analyses to explore relations with the LPP amplitudes, but across sites did not consistently predict LPP either as a main effect nor in interaction with LPP time window and/or stimulus condition.
In mixed models including both mindfulness and NA as main effects and in interaction with condition, the MAAS and FFMQ interactions with condition remained significant across all sites (Ps < 0.04). NA × condition was significant at CPz and Pz only (Ps < 0.05). In models including the neuroticism main effect, the mindfulness interactions were significant across sites (Ps < 0.005). Neuroticism was not a significant predictor (Ps > 0.14).
REFERENCES
- Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Barnes S, Brown KW, Krusemark E, Campbell WK, Rogge RD. The role of mindfulness in romantic relationship satisfaction and responses to relationship stress. Journal of Marital and Family Therapy. 2007;33:482–500. doi: 10.1111/j.1752-0606.2007.00033.x. [DOI] [PubMed] [Google Scholar]
- Brown KW, Cordon SL. Toward a phenomenology of mindfulness: subjective experience and emotional correlates. In: Didonna F, editor. Clinical Handbook of Mindfulness. New York: Springer; 2009. pp. 59–81. [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18:21–37. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications; 1992. [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross JJ, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: Neural correlates. Human Brain Mapping. 2004;22:290–9. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO- FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumaker J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50:1373–83. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–36. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–88. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti MA, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–20. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Giluk TL. Mindfulness, big five personality and affect: A meta-analysis. Personality and Individual Differences. 2009;47:805–11. [Google Scholar]
- Goldberg LR. The structure of phenotypic personality traits. American Psychologist. 1993;48:26–34. doi: 10.1037//0003-066x.48.1.26. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–64. [Google Scholar]
- Grossman P. On measuring mindfulness in psychosomatic and psychological research. Journal of Psychosomatic Research. 2008;64:405–8. doi: 10.1016/j.jpsychores.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Macnamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology. 2010;35:129–55. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective and Behavioral Neuroscience. 2006;6:291–7. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8:250–5. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, Inzlicht M. The devil you know: Neuroticism predicts neural response to uncertainty. Psychological Science. 2008;19:962–7. doi: 10.1111/j.1467-9280.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Individual differences in emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 351–72. [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–9. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in Mental Health Care Delivery Systems. Norwood, NJ: Ablex Publishing; 1980. pp. 119–37. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annual Review of Psychology. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis JA, Johnstone T, Davidson RJ. Voluntary regulation of the neural circuitry of emotion by compassion meditation: Effects of expertise. PLoS One. 2008a;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008b;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Social Cognitive and Affective Neuroscience. 2010;5:369–78. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) relates to sadness following mood induction among individuals with high neuroticism. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr007. doi:10.1093/scan/nsr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE. Temperament and emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 331–50. [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–61. [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14:1107–10. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Sobolewski A, Holt E, Kublik E, Wróbel A. Impact of meditation on emotional processing—a visual ERP study. Neuroscience Research. 2011;71:44–8. doi: 10.1016/j.neures.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behaviour Research and Therapy. 1999;37:53–77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: Correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10:12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.