Abstract
Mindfulness meditation—the practice of attending to present moment experience and allowing emotions and thoughts to pass without judgment—has shown to be beneficial in clinical populations across diverse outcomes. However, the basic neural mechanisms by which mindfulness operates and relates to everyday outcomes in novices remain unexplored. Focused attention is a common mindfulness induction where practitioners focus on specific physical sensations, typically the breath. The present study explores the neural mechanisms of this common mindfulness induction among novice practitioners. Healthy novice participants completed a brief task with both mindful attention [focused breathing (FB)] and control (unfocused attention) conditions during functional magnetic resonance imaging (fMRI). Relative to the control condition, FB recruited an attention network including parietal and prefrontal structures and trait-level mindfulness during this comparison also correlated with parietal activation. Results suggest that the neural mechanisms of a brief mindfulness induction are related to attention processes in novices and that trait mindfulness positively moderates this activation.
INTRODUCTION
Mindfulness meditation (MM) is an ancient practice traditionally taught within the context of Buddhism and other religions. Practitioners deliberately bring their attention to an object or experience in their awareness (e.g. the sensations of breathing) with openness, curiosity and non-attachment (Kabat-Zinn, 1994).
In recent years, mindfulness has become increasingly popular in the West and interest in the clinical and general well-being applications of MM has grown within the scientific community. MM has been successfully employed in clinical and non-clinical contexts and shows promise as an intervention for general stress reduction (Baer, 2003), prevention of depression and substance abuse relapse (Marlatt et al., 1984; Zgierska et al., 2009), reducing chronic panic and related impairment (Kabat-Zinn, 1984; Kabat-Zinn et al., 1985; McCracken et al., 2007; Zeidan et al., 2010), enhancing immune function (Davidson et al., 2003; Witek-Janusek et al., 2008), promoting adaptive emotion regulation (Gifford et al., 2004; Goldin and Gross, 2010) and improving attentional control (e.g. Jha et al., 2007; Zylowska et al., 2007). Such studies generally are conducted with participants who have undergone several months of relatively intensive MM training, although there is also some evidence that a brief MM induction can be beneficial for untrained participants (Arch and Craske, 2006).
Despite clinical evidence of the salutary effects of mindfulness training, our knowledge of the underlying mechanisms of mindfulness, while increasing, is still limited. Specifically, examination of the elementary neural underpinnings of basic mindfulness—of MM or induced mindfulness independent from other constructs, such as emotion regulation, acceptance and social support—have not been broadly investigated. This may be in part due to the complexity of isolating the basic processes underlying mindfulness. A fundamental understanding of the neural mechanisms of MM per se in isolation from any other process requires a task that investigates mindfulness in a neutral context, absent emotionally evocative external stimuli. Such a task should use a tight control condition that involves attention-related instructions but without the present experience focus unique to mindfulness in order to separate mindfulness from attention. Finally, neuroimaging tools such as functional magnetic resonance imaging (fMRI) can be used to reveal the brain mechanisms of basic mindfulness in real time. Existing studies (reviewed below) have often employed one or more of these procedures, but none to date has used them all in a sample of novice individuals practicing mindfulness in a neuroimaging environment.
Previous work on brief mindfulness inductions
Several behavioral (i.e. non-neuroimaging) studies on mindfulness in first-time beginners (Broderick, 2005; Arch and Craske, 2006) indicate that brief, one-time mindfulness inductions can result in enhanced emotion regulation relative to a variety of control conditions. For example, Broderick (2005) found that participants who were assigned to a MM condition (as opposed to a rumination or distraction condition) reported lower levels of negative mood during a dysphoric state induction. Similarly, Arch and Craske (2006) found that a brief mindfulness induction temporarily decreased intensity and negative emotional responses to emotionally valenced pictures. Participants in the focused breathing ‘FB’ condition not only reported reduced negative affect to unpleasant slides, but were also willing to view the negative pictures for a longer period of time than participants that paid attention to their thoughts in a non-mindful way. These behavioral studies are important in demonstrating the potential outcomes of MM—even among novice practitioners—but they are not ideal for understanding the mechanisms by which mindfulness leads to those outcomes.
Previous work on the neural systems of mindfulness
To understand the mechanisms underlying the behavioral results, several neuroimaging studies have sought to identify the process by which mindfulness can reduce or regulate negative emotional responses in novice (or non-) meditators. For example, Creswell and colleagues (2007) found that individuals high in trait mindfulness showed increased prefrontal and reduced limbic activation during an emotion labeling task. These findings and related others (Farb et al., 2007; Goldin and Gross, 2010) support the behavioral findings noted above and have important implications for understanding the affective consequences of mindfulness training and trait mindfulness in real-world situations. However, these studies investigate the effects of mindfulness on other processes (e.g. emotion reactivity or regulation) instead of the basic processes of mindfulness itself.
Other neuroimaging studies attempt to examine the neural changes generated by mindfulness practice by examining the brain activation of expert or highly trained mindfulness practitioners (Hölzel et al., 2007) or by comparing activation in expert meditators to less experienced or non-MM practitioners (Brefczynski-Lewis et al., 2007; Jha et al., 2007; Manna et al., 2010). Some of these studies vastly differ in their results, perhaps due to the type of control conditions or samples employed. For example, Hölzel and colleagues (2007) compared expert meditators to non-meditators during a mindfulness task, in which participants focused on their breath, as compared to an arithmetic task. They found only the rostral anterior cingulate cortex (rACC) and the dorsal medial prefrontal cortex to be active. Other studies found divergent results (e.g. a fronto-parietal network instead of rACC) when using a resting baseline as a comparison for the mindfulness condition (Brefczynski-Lewis et al., 2007; Short et al., 2007). The discrepancy of these results underscores the sensitivity of results to the specific comparison condition that is used, especially in neuroimaging which uses subtractive logic to make deductions about neural processes. That is, neural activation for a specific process is inferred by subtracting the activation from one condition that does not rely upon that process from the activation from another condition that does. Nonetheless, to the extent that there is consistency in the mindfulness neuroimaging literature, it is in the association between mindfulness practice (or in individuals who choose to practice long-term MM) and attentional networks (see Ivanovski and Malhi, 2007; Lutz et al., 2008; Manna et al., 2010).
Recently, a rigorous study extended previous findings of attentional mechanisms possibly underlying the process of mindfulness in long-term meditators (Hasenkamp et al., 2012). Practitioners engaged in a common mindfulness technique, in which they focused their attention on the breath, and signaled when their mind wandered by a button press. The imaging data were divided into different mindfulness components: mind wandering (MW), in which attention drifted away from the breath; the awareness of MW, denoted by their signal of becoming aware their mind wandered; shifting attention back to the breath; focus, which involved complete focus on the breath. The authors found that MW recruits regions associated with self-related thought, termed the default mode network. Additionally, when meditators became aware that their mind wandered away from the breath, regions associated with salience and conflict monitoring became active. Regions associated with executive attention and re-orienting attention were active as practitioners reallocate their attention back to their breath. Finally, focus appears to be maintained with activity in the dorsolateral prefrontal cortex (Hasenkamp et al., 2012). This study suggests that different components of attention control play different roles in mindfulness. Whether or not some, all, or none of the regions active in this study are present during the initial process of mindfulness induction (i.e. among novices) has yet to be fully examined.
Which neural regions might be involved in basic MM in beginners? Each of the studies reviewed above captures a piece of the answer, but none addresses it directly. Given that a fundamental definition of mindfulness centers on attention and awareness (Brown and Ryan, 2003), it follows that basic mindfulness would recruit neural regions associated with attention. This hypothesis is supported indirectly by neuroimaging studies that compare expert meditators to non-meditators, which have found preliminary evidence that neural attentional networks are closely associated with mindfulness (Ivanovski and Malhi, 2007; Lutz et al., 2008) and that mindfulness training enhances performance on attentional tasks such as the Stroop interference task, the d2 concentration and endurance task (Moore and Molinowski, 2009) and the Attention Network Task (Jha et al., 2007; Tang et al., 2007).
Furthermore, more recent studies examining long-term meditators hint at possible underlying mechanisms in novices. In light of Hasenkamp et al.’s (2012), four component model of focused attention, it is possible that the mechanism of mindfulness is different in experts than it is in novices. Manna and colleagues (2010) found that focused attention leads to activation of the medial frontal areas and a reduction in lateral prefrontal activation in expert meditators as compared to controls. However, while our knowledge of the significant effects of MM on the brain is expanding greatly, the initial underlying mechanisms (i.e. in novices) of mindfulness as it occurs in real time have not completely been examined.
The current study
In summary, one fundamental question has been overlooked: which neural systems support basic MM in those who are not experienced practitioners? In addressing this question, the current study sought to identify the neural processes of one basic form of MM—focused attention using the breath as the object of focus—among novice practitioners. To isolate this basic process at the core of many mindfulness practices, we induced mindfulness in a neutral (i.e. non-emotional) context. We compare FB to a tightly matched control condition—unfocused attention, in which participants attend to their thoughts in an unfocused manner (cf Arch and Craske, 2006). We expected to find increased blood oxygenated level-dependent (BOLD) signal in regions associated with attentional regulation, such as parietal–frontal activations and that regions associated with the default mode network would be more active in the MW task. Specifically, because novices experience difficulty with sustaining attention (Lutz et al., 2008), we did not expect to find activity of regions that are typically associated with this ability, such as lateral prefrontal regions. Additionally, because we were interested in understanding how individual differences in mindfulness altered these mechanisms, we assessed the interaction between the effect of the FB task on BOLD signal and trait mindfulness. We hypothesize that people high in trait mindfulness are more likely to recruit regions related to re-shifting and sustaining attention than those who are lower in trait mindfulness.
METHODS
Participants
Thirty-one participants (15 female) were recruited for an fMRI study (mindfulness was not mentioned in any recruitment materials) from professionally led group-based smoking cessation programs in Los Angeles (American Lung Association’s Freedom from Smoking). They varied in age from 28 to 69 years (M = 46, SD = 9.7). To account for this large variation, all analyses reported below are adjusted for age. Participants were ethnically diverse: 52% were Caucasian, 26% Hispanic, 19% African American and 3% other. They were each given $80 for compensation. All participants provided written informed consent that was approved by the University of California, Los Angeles Institutional Review Board (IRB).
Procedure
Participants came to the Ahmanson-Lovelace Brainmapping Center at least 2 and no more than 7 days prior to their target quit date. Upon arrival, participants completed a consent form for the scanner. They were screened for use of following illicit drugs: amphetamines, cocaine, marijuana, opiates and PCP and baseline ad libitum cigarette smoking was assessed by carbon monoxide (CO) levels in expired air (Micro-smokerlyzer; Bedfont Scientific Ltd, Kent, UK). Since brain functioning of smokers only differs from that of non-smokers when in withdrawal (Azizian et al., 2009), participants smoked a cigarette within 1 h of the beginning of the scan. Next, participations received verbal instructions about the focused attention and MW tasks (see below for detail). Participants were then situated in the scanner for the duration of the 10-min task. Foam padding was placed around participants’ heads to reduce motion. Stimuli were presented on LCD goggles and responses were recorded on a magnet-safe button box placed in the right hand. Following the task, participants completed a questionnaire packet and were compensated for their time.
Materials
Mindfulness meditation task
We adapted Arch and Craske’s (2006) MM task for use in the scanning environment. In this task, MM is operationalized using a FB manipulation and a MW condition is used as a control. In the FB condition, participants were instructed to focus on the physical sensations of breathing. Specifically, the instructions for the condition were ‘focus on the actual sensations of breath entering and leaving the body. There is no need to think about the breath—just experience the sensations of it. When you notice that your awareness is no longer on the breath, gently bring your awareness back to the sensations of breathing’. We modified the task to take place across several 50-s blocks so that it would be compatible for the scanner. Brief instructions were displayed on the screen that read ‘Breathe in and out … Focus on the sensation of breathing’ to remind participants of the condition. A tone indicated the end of the block.
In the MW control condition, participants were instructed to allow their mind to wander. They were told to ‘let your mind take you wherever it goes as you normally would throughout the day’. Like the FB exercise, participants were shown instructions, ‘Think about whatever comes to mind, Go wherever your mind takes you’ before the task began and were notified about the end of each block by a tone. Each MW block was 50 s in duration. Following each block, participants rated five questions about their subjective experience as a manipulation check.
Trait mindfulness scale
The Mindful Attention Awareness Scale (MAAS) consists of 15 items that were designed to reflect mindfulness and mindlessness in everyday experiences including awareness and attention to actions, thoughts, emotions and physical states. Items were rated on a six-point Likert scale from 1 (almost never) to 6 (almost always). A typical item is ‘I find myself doing things without paying attention’. There is evidence that the MAAS is a valid and reliable scale (Brown and Ryan, 2003), although there is some controversy surrounding measurement of this scale (Grossman, 2011; Brown et al., 2011). Additionally, there was a significant difference in MAAS scores between Zen meditators and a community sample (Brown and Ryan, 2003). Recent evidence has further supported the notion that the MAAS is a valid, though indirect, measure of mindfulness (Brown et al., 2011b).
fMRI data acquisition and analysis
Brain imaging data were acquired on a 3T Siemens Trio scanner at the UCLA Ahmanson-Lovelace Brain Mapping Center. High-resolution structural T2-weighted echo-planar images (spin-echo; TR = 5000 ms; TE = 34 ms; matrix size 128 × 128; 34 sagittal slices; FOV = 192 mm; 4 mm thick) were acquired coplanar with the functional scans. Two functional runs each acquiring 174 whole-brain volumes and lasting 5:48 were acquired during the task (echo-planar T2*-weighted gradient-echo, TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix size 64 × 64, 34 axial slices, FOV = 192 mm; 4 mm thick), totaling 348 functional volumes.
The imaging data were analyzed using a combination of FSL tools (FMRIB Software Library; Oxford University, Oxford, UK) and SPM8 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, UK). The pre-processing stream for the images was as follows. All images were brain extracted using BET (FSL’s Brain Extraction Tool) and realigned within runs using MCFLIRT (FSL’s Motion Correction using FMRIB’s Linear Image Registration Tool), then checked for residual motion and noise spikes using a custom automated diagnostic tool (thresholded at 2 mm motion or 2% global signal change from one image to the next). In SPM8, all functional and anatomical images were reoriented to set the origin to the anterior commissure and the horizontal (y) axis parallel to the line between the inferior surface of the anterior corpus collosum and the inferior surface of the occipital lobe. Also in SPM8, functional images were corrected for slice acquisition timing differences within volumes, realigned within and between runs to correct for residual head motion and co-registered to the matched-bandwidth structural scan using a six-parameter rigid body transformation. The co-registered structural scan was then normalized into the Montreal Neurological Institute (MNI) standard stereotactic space and these parameters were applied to all functional images. Finally, the normalized functional images were smoothed using an 8-mm full-width at half-maximum Gaussian kernel.
Each block began with 3 s of instructions for the condition followed by 50 s of the actual task. Afterwards, participants responded to five questions about their experience during the previous block as manipulation checks: ‘I was able to follow the instructions’, ‘I felt calm and relaxed’, ‘I felt spiritual’, ‘I felt in control’ and ‘I enjoyed this experience’. Each question was displayed for 5 s, and responses were provided on a 4-point scale: ‘Disagree strongly’, ‘Disagree’, ‘Agree’ and ‘Agree strongly’. A fixation cross was displayed for 12 s between blocks. Runs consisted of 2 blocks of FB and 2 blocks of MW. The order was counterbalanced across subjects by randomly assigning each subject to complete either the FB–MW–FB–MW or the MW–FB–MW–FB block first. Both runs together took ∼10 min.
The task was modeled using a blocked design in SPM8. The focused attention and MW conditions were convolved with the canonical hemodynamic function and entered as predictors with rest as an implicit baseline. Additionally, we included the post-block questions as covariates, though the responses to these questions did not alter the results. We normalized each functional scan by subtracting the grand mean of that scan from each voxel in order to correct for global differences in signal between the FB condition and the MW condition. Our primary analysis was the linear contrast of FB compared to MW. We also examined whether individual differences in the MAAS scores moderated the effects of the task by examining the interaction between MAAS scores and the two linear contrasts (FB > MW and MW > FB). Both of these analyses adjust for the large variation in participant age by including age as a covariate in the second-level models. Family-wise false discovery rate (FDR) was set at 0.05 using a Monte–Carlo simulation as implemented in AlphaSim (AFNI; Cox, 1996), which yielded a joint voxel-wise threshold of 0.005 and cluster extent of 33 voxels. All functional imaging results are reported in MNI coordinates.
RESULTS
We first examined the mean of the item, ‘I was able to follow the instructions’ as a manipulation check to ensure that participants were able to engage in the FB exercise. Participants reported a mean of 3.3 of 4 (SD = 0.85), indicating they were able to follow the directions between ‘most of the time’ and ‘all of the time’.
FB vs MW
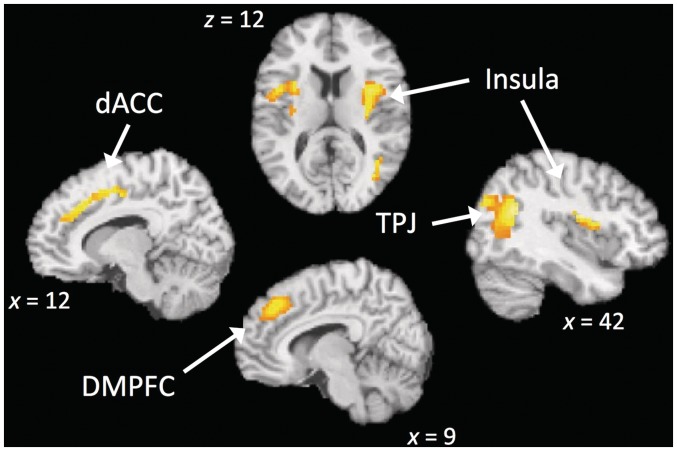
To identify the neural systems that support the fundamental mechanisms of a basic form of MM, a contrast comparing FB to control (MW) was performed (i.e. FB > MW). Consistent with our hypothesis and with the core notion of mindfulness, the FB condition recruited several components of the attention network (Figure 1 and Table 1). Significant increases were found in a set of fronto-parietal regions involved in attentional control, such as the superior parietal lobule (SPL), temporal–parietal junction (TPJ), pre-supplementary motor area and dorsal anterior cingulate gyrus which are specifically thought to mediate attention to sensory information and response selection (Corbetta and Shulman, 2002). There was additional activation in the insula, a region often involved in awareness of bodily sensations, has also been implicated in attentional control (Corbetta et al., 2008; Menon and Uddin, 2010). All of the regions mentioned above are involved in a fronto-parietal attention network (Corbetta et al., 2008; Toro et al. 2008), suggesting that FB predominantly recruits attentional networks. Additionally, we found that subcortical structures (i.e. hippocampus and caudate) showed an increase in activation during FB (Table 1).
Fig. 1.
Comparison of FB > MW reveals regions involved in attention (TPJ, ACC, DMPFC) and somatosensory awareness (anterior insula). dACC = dorsal anterior cingulate cortex; TPJ = temporal–parietal junction; preSMA = pre-supplementary motor area
Table 1.
Regions showing differential activation during MM (FB) and control (MW)
| Region | Hemisphere | x | y | z | Cluster size | t |
|---|---|---|---|---|---|---|
| FB > MW | ||||||
| Mid-insula | R | 39 | −7 | 22 | 40 | 6.47 |
| Caudate | R | 18 | 26 | 3 | 41 | 6.12 |
| Angular gyrus | R | 42 | −58 | 25 | 46 | 5.3 |
| Superior frontal gyrus | R | 24 | 2 | 52 | 41 | 4.84 |
| Mid-insula | L | −36 | −19 | 25 | 43 | 4.73 |
| Central operculum | L | −45 | 5 | 10 | 44 | 4.63 |
| Dorsal anterior cingulate gyrus | R | 12 | 26 | 40 | 58 | 4.52 |
| Parahippocampal gyrus | L | −30 | −40 | −10 | 36 | 4.22 |
| Middle occipital gyrus | R | 42 | −79 | 31 | 36 | 4.15 |
| Hippocampus | R | 30 | −25 | −5 | 30 | 4.04 |
| Dorsomedial PFC BA8 | R | 9 | 38 | 43 | 38 | 4.02 |
| Superior parietal lobule | R | 36 | −52 | 43 | 41 | 3.82 |
| Frontal pole BA 9 | R | 21 | 38 | 34 | 34 | 3.5 |
| MW > FB | ||||||
| Superior frontal gyrus BA10 | midline | −3 | 50 | −8 | 123 | 6.04 |
| Medial prefrontal cortex/ rACC | midline | −3 | 50 | 7 | 157 | 5.98 |
| Inferior frontal gyrus BA47 | L | −48 | 14 | −8 | 131 | 5.77 |
| Middle frontal gyrus BA6 | L | −42 | 11 | 52 | 60 | 5.37 |
| Para-cingulate gyrus | L | −6 | 38 | −8 | 67 | 5.14 |
| Superior parietal lobule | L | −39 | −61 | 58 | 42 | 5.11 |
| Precuneus BA7 | L | −6 | −67 | 64 | 52 | 5.08 |
| Precuneus BA7 | midline | 0 | −46 | 61 | 110 | 4.99 |
| Superior frontal gyrus | midline | 0 | 8 | 64 | 96 | 4.96 |
| Posterior cingulate gyrus | L | −9 | −46 | 1 | 51 | 4.86 |
| Angular gyrus | L | −30 | −64 | 58 | 51 | 4.76 |
| Lateral occipital cortex | L | −27 | −73 | 49 | 75 | 4.73 |
| Inferior frontal gyrus BA47 | R | 51 | 17 | −2 | 138 | 4.68 |
| Frontal pole BA10 | L | −33 | 59 | 7 | 53 | 4.59 |
| Posterior cingulate gyrus | midline | 0 | −22 | 55 | 83 | 4.48 |
| Precentral gyrus | L | −42 | −4 | 58 | 45 | 4.11 |
| Parahippocampal gyrus | L | −12 | −37 | −2 | 38 | 4.06 |
| Middle frontal gyrus BA8 | midline | −3 | 23 | 37 | 66 | 3.97 |
| Superior temporal gyrus BA22 | R | 60 | −13 | 10 | 52 | 3.86 |
| Precuneus BA7 | R | 3 | −76 | 46 | 84 | 3.67 |
| Dorsomedial PFC BA 8 + B55 | L | −3 | 26 | 43 | 51 | 3.4 |
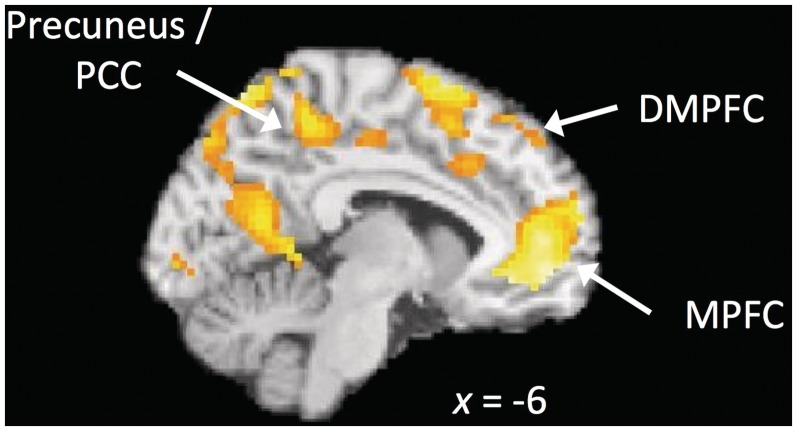
Next, we compared MW to FB (i.e. MW > FB). A coherent subset of default mode network regions including MPFC, dorsomedial PFC, angular gyrus and precuneus (Raichle et al., 2001) was active in this contrast (Figure 2 and Table 1). This network has been observed in previous work to be activated during rest periods and is thought to index cognitive activities such as self-related thought, daydreaming and free association (Spreng et al., 2009) as well as during attention lapses (Weissman et al., 2006) and MW (Hasenkamp et al., 2012). This result indicates that default mode network activity was greater in the MW condition than FB, despite FB possibly having periods of MW in it (Hasenkamp et al., 2012). This provides support that by using MW as a control condition we were able to isolate the awareness and attentive aspects of mindfulness, while controlling for aspects of MW that occur during FB.
Fig. 2.
Comparison of MW > FB reveals default mode network regions (MPFC, precuneus/posterior cingulate). MPFC = Medial Prefrontal Cortex.
Interaction with MAAS (i.e. correlation between MAAS and the main effect of condition)
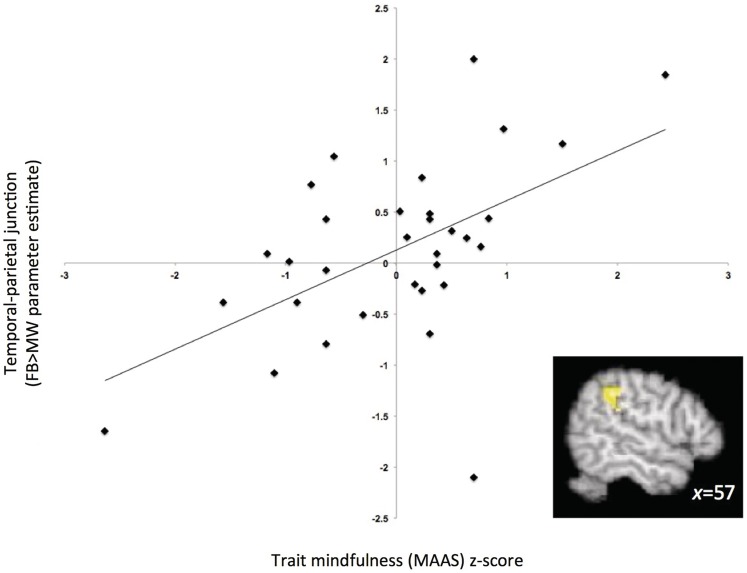
We investigated how brain activation during the FB induction (relative to MW) was moderated by individual differences as assessed by the MAAS. To examine this interaction, we performed a whole-brain search for regions that were correlated with dispositional mindfulness as measured by the MAAS within the contrast of FB greater than MW. This analysis identifies regions that show differential activation between the task conditions (FB and MW) depending on the level of the trait measure. We found that scores on the MAAS moderated the main effect of condition in several foci. Regions in the frontal and parietal lobes, specifically SPL, TPJ and dorsolateral PFC, correlated positively with MAAS scores (Table 2 and Figure 3). These regions did not overlap with those observed in the main effect of FB > MW described above, but both parietal activations are adjacent to the activations found in the TPJ and SPL (distance between peaks were 23 mm and 18 mm, respectively). Dorsolateral PFC, SPL and TPJ activations are associated with attentional orienting (e.g. Corbetta and Shulman, 2002; Raz and Buhle, 2006). Specifically, dorsolateral PFC is thought to be heavily involved in executive attention as well as shifting attention (Kondo et al., 2004; Raz and Buhle, 2006). Additionally, the SPL is thought to help sustain attention and signal distractions in goal-directed tasks (Behrmann et al., 2004; Dong et al., 2010), while the TPJ is specifically attributed to disengaging from a particular location and re-shifting attention back to the salient stimulus (Corbetta and Shulman, 2002; Ciaramelli et al., 2008; Raz and Buhle 2006). This was consistent with our expectations that individual differences in MAAS would interact with the condition manipulation in areas involved in attention.
Table 2.
Regions correlated with trait mindfulness (MAAS) during FB > MW
| Region | Hemisphere | x | y | z | Cluster size | t |
|---|---|---|---|---|---|---|
| FB > MW correlated with MAAS | ||||||
| Superior parietal lobule | R | 24 | −55 | 73 | 44 | 4.42 |
| Supramarginal gyrus/TPJ | R | 57 | −37 | 37 | 43 | 4.14 |
| Superior frontal gyrus BA 10 | R | −24 | 23 | 46 | 45 | 3.38 |
| MW > FB correlated with MAAS | ||||||
| Frontal pole | R | 39 | 47 | 16 | 43 | 3.77 |
| Superior frontal gyrus BA6 | R | 15 | 38 | 52 | 71 | 3.6 |
Note. All clusters are corrected for FDR = 0.05 using a voxel-wise threshold of P < 0.005 and a cluster extent of 33 voxels. Analyses adjusted for age.
BA, Brodmann’s Area.
Fig. 3.
Correlation of MAAS scores with neural activity during FB (relative to MW) in the temporal-parietal junction (43-voxel cluster centred at x = 57, y = −37, z = 37, FDR-corrected P < 0.05).
The parallel analysis on the contrast MW > FB revealed no significant clusters. In other words, the main effect, MW > FB, was not moderated by MAAS in any region.
DISCUSSION
To gain insight into the neural networks involved in a basic mindfulness induction, we compared neural activity during a common form of MM (FB) to a control task that engaged attention but not in a focused or deliberate manner (MW). Relative to the control condition, FB recruited an attention network consisting of the anterior cingulate, insula and frontal–parietal regions including TPJ, SPL, superior frontal gyrus, pre-supplementary motor area and dorsomedial PFC. Additionally, there were significant decreases in areas implicated in the default mode network. Further, we examined the correlations between neural activity during the mindfulness induction and individual differences in mindfulness. During a mindfulness induction, scores on the MAAS were positively related to activation in part of the dorsolateral PFC, TPJ and SPL (all of which have been implicated in attentional control).
Together, these findings have implications for understanding the neural mechanisms that underlie the early stages of MM practice, especially given the basic form of the MM induction used and the novice and non-practitioner sample. Relative deactivation of the self-referential default mode network during the mindfulness induction provides further support for the active engagement of attention in that condition (Mason et al., 2007) and extends similar findings in expert meditators to novice participants (Brefczynski-Lewis et al., 2007; Taylor et al., 2011). Finally, neural activation in attention regions during MM was correlated with MAAS scores, suggesting that the brain systems involved in mindfulness are meaningfully related to outcomes beyond the experimental setting. Taken together, the present results are consistent with behavioral results in suggesting that in the early stages of MM practice, a simple mindfulness induction recruits neural regions associated with attentional engagement.
The goal of this study was to identify the neural regions that support basic mindfulness in a neural context and with a novice (non-practitioner) sample. We found that FB, relative to MW, recruited a network of fronto-parietal regions and others largely implicated in attention and awareness, including the pre-supplementary motor cortex, TPJ, anterior insula and ACC (Lazar et al., 2000; Raz and Buhle, 2006; Posner and Rothbart, 2007; Lutz et al., 2008).
We observed an increase in right TPJ activation during focused attention relative to MW. These findings are consistent with the presumed role of this region in re-shifting attention back to a target following distractions and in integrating somatosensory information (Corbetta and Shulman, 2002; Ciaramelli et al., 2008). Additionally, recruitment of the dorsal portion of the ACC, a region typically involved in goal conflict (Bush et al., 2000), is consistent with conflict between attention to breath and to distractions (e.g. MW). We also observed activation in the insula during MM, which has also been implicated in attention to and awareness of physical sensations (Eckert et al., 2009). Together, these activations are consistent with the instructions to participants to be attentive to and aware of the physical sensations of their breath, and suggest that non-trained meditators are capable of maintaining focus on their breath by recruiting attentional regions that reflect the specific demands of MM.
Additionally, we found evidence that engaging in mindfulness reduces default mode network activity relative to MW. One measure of progress in FB (Lutz et al., 2008) is the frequency of MW during the focused attention period. It has been hypothesized that training in attention during MM would likely result in better control of MW (Braboszcz et al., 2010). This may be indexed by relative de-activations in the default mode network, as activation in these regions is observed during lapses of attention (Weissman et al., 2006). Our data clearly support this hypothesis. The studies that do address the cognitive control of MW using MM suggest that the ability develops only over time with meditation practice (Farb et al., 2007). For example, Taylor and colleagues (2011) found that viewing emotional pictures in a mindful way induced a deactivation of default mode network in expert meditators as compared to novices (Taylor et al., 2011), whereas novices showed deactivation in the amygdala. The authors indicated that novices engaged in a more active process of emotion regulation, whereas expert meditators experience emotional attenuation by mindful experiencing emotional processes without interference from internal thoughts or judgments. Our results extend these findings. In the present study, we found that a very simple and brief induction with little prior training was sufficient to suppress default mode network activity, suggesting that presence of this control of MW is inherent to mindfulness and does not develop over time. However, the level of this ability and its ability to influence other processes, such as emotion regulation is what develops over time. The current study in conjunction with prior evidence implies that the extent of deactivation of the default mode network activity may become stronger with practice, and thus better able to influence other processes. Paying attention to MW in the context of MM appears to be critical for future work.
The interaction between regions of brain activity during FB and ratings on the MAAS presents more evidence that MM is related to attentional control processes. We found that the extent of the increase in the dorsolateral PFC, TPJ and SPL, regions implicated in attention (Corbetta and Shulman, 2002; Singh-Curry and Husain, 2009), was associated with higher MAAS scores. Even though this part of the fronto-parietal attention network was not significantly active at the group level, those high in trait mindfulness recruited these regions in addition to other regions observed at the group level. Particularly, people who have higher MAAS scores recruit attentional processes more typically associated with orienting and sustaining attention than do novices who have lower MAAS scores. For example, the dorsolateral PFC is typically activated during goal-oriented attentional tasks and is thought to contribute to sustaining attention as well updating goal-relevant information in switching paradigms (Wager et al., 2004). In line with this, Hasenkamp et al. (2012) found activation in the dorsolateral PFC when experienced meditators shifted their attention back to their breath, and was the only region associated with sustaining focus on the breath. Additionally, this region is reported to show an inverted U-shaped curve, in which expert meditators with the lowest amount of practice showed activation in the dorsolateral PFC, while novices and experts with the highest amount of meditation practice did not (Brefczynski-Lewis et al., 2007), suggesting at the highest levels of expertise meditation may be less effortful. It is possible that higher scores on the MAAS represents people for whom FB may utilize the most cognitive resources, whereas people who scored lower on the MAAS may not recruit these areas because they are less able to sustain and continually shift attention to the breath and thus less mindful (as the MAAS indirectly assesses). Further research would be needed to confirm this hypothesis.
Additionally, people who scored higher on the MAAS recruited the SPL and TPJ, both of which are involved in the orienting attention (Shulman et al., 2010). Similar to the dorsolateral PFC, the SPL is involved in maintaining attention and in signaling attention shifts when attention is goal directed (Hopfinger et al., 2000; Behrmann et al., 2004; Dong et al., 2010). This suggests that people who scored higher on the MAAS may be more goal oriented in MM than people who are less mindful. On the other hand, the TPJ is considered to be involved in bottom–up attentional processes, in which the TPJ is heavily involved in shifting attention to task relevant stimuli (e.g. Ciaramelli et al., 2008). It is possible that the portion of the parietal lobe that was significantly recruited for MM across subjects is necessary to engage attention on the breath, while the SPL, along with the dorsolateral PFC come online to orient and sustain that attention by avoiding distractions in a goal-directed way. When those distractions become more salient than their current focus, the TPJ rapidly intervenes to bring attention back to the breath. Thus, it might be that the less mindless a person tends to be, the better they can maintain focus on the breath, perhaps by being more aware when their mind wandered and thus, shifting their attention back to the breath, implicated by the recruitment of DLPFC and SPL.
Furthermore, the fact that these regions seems to be sensitive to individual variability has implications for future studies on mindfulness. Many studies investigating mindfulness advertise as such, which may result in self-selection of individuals who perceive themselves to be high in mindfulness, or at least not mindless. Since our sample was not recruited in that way, it is possible that we have a more representative sample of variations in attributes associated with attentional processing during mindfulness. The activation of these regions also provides an interesting link between the exclusively breath-focused mindfulness task and the daily-attending focus of the MAAS. It is possible that the link between formal meditation and the everyday capacity to attend to the ‘here and now’ (as assessed by the MAAS) involves the capacity to integrate sensory information and attend to a range of stimuli. This new insight warrants further investigation. In summary, the results of this interaction suggest that people with higher MAAS scores are recruiting a more top–down attentional process, as well as a bottom up process as denoted by the main effect and the TPJ correlation.
We view the MAAS here as an individual difference in mindfulness, there may be some limitations to this interpretation. The MAAS has been criticized for a possible discrepancy between definitions of mindfulness of the scales and original Buddhist definitions, questions regarding the validity of the mindfulness construct (i.e. perhaps it is measuring the attention lapses) and issues concerning reverse coding (Grossman, 2011). However, recent evidence provides support for the validity of the MAAS as an indirect measure of mindfulness (Brown et al., 2011). The MAAS correlates strongly with direct measures of mindfulness (Brown et al., 2011) and is only moderately correlated with attentional control, suggesting that attention is a separate construct than what is measured by the MAAS (Brown et al., 2012). Regardless, the implication of our data remain the same: individual differences on the MAAS are related to specific attentional processes recruited during a mindfulness induction, such that increases in MAAS scores promote more goal-directed attention processes.
The fact that participants were regular smokers may limit the generalizability of this study. However, in terms of functional activation, there are few differences between non-smokers and smokers who are not in withdrawal during cognitive tasks (Xu et al., 2005). One study found that nicotine administration to smokers reduced activation during a working memory task relative to placebo administration (Ernst et al., 2001). This result suggests that our results may be a conservative estimate of the magnitude of the neural effects of the task, and that the activations may be even larger in non-smokers or abstinent smokers.
This study examined a specific type of MM—one in which participants focused on the physical sensations of breathing. It is important to note that this type of meditation is only one of many types of meditation practices. Other practices common in mindfulness interventions (e.g. MBSR), for example ‘open monitoring’ (see Lutz et al., 2008), are practiced only after one has mastered focused attention because they involve a more complex set of tasks than focused attention alone. Whereas FB largely involves sustaining attention, open monitoring involves attending to thoughts and emotions ‘openly’ or non-reactively and non-judgmentally, for the purpose of recognizing emotional and cognitive patterns. The present study intentionally focused exclusively on sustained attention rather than other pieces of MM such as acceptance because of its centrality to many different meditation practices. Future research should consider examining the neural process progress of focused attention to open monitoring to examine other factors (e.g. acceptance).
The current study is the first to investigate the basic processes involved in MM, separate from the characteristics of those who self-select into long-term meditation training and from the effects of other phenomena (e.g. emotion regulation). As such, it expands our current knowledge of MM by providing a bridge from its basic processes to the clinical benefits that have been documented elsewhere. First, we now have evidence that MM recruits neural regions involved in attention and interoceptive awareness, specifically an orienting attention network (Corbetta and Schulman, 2002). This finding validates and supports the attentional benefits of practicing MM and provides insight into its clinical mechanisms. Second, we found reduced default mode network activation compared to the control (MW). This finding is consistent with the notion that reductions in default mode activity may be intrinsic to the task itself (and are not limited to individuals who are disposed to initiate MM on their own), and may happen as an immediate consequence of attentional control. Third, correlations with trait mindfulness further support the notion that during the beginning stages, mindfulness relies heavily on attentional control. In summary, our results support the basic definition of mindfulness as being ‘rooted in the fundamental activities of consciousness: attention and awareness’ (Brown et al., 2007).
Conflict of Interest
None declared.
Acknowledgments
This research was supported by the National Institutes of Health with grant number F31DA024904 to E.T.B.
REFERENCES
- Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology. 2009;35:775–82. doi: 10.1038/npp.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10(2):125–43. [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Current Opinion in Neurobiology. 2004;14(2):212–7. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Braboszcz C, Hahusseau S, Delorme A. Meditation and neuroscience: from basic research to clinical practice. In: Carlstedt RA, editor. Integrative Clinical Psychology, Psychiatry and Behavioral Medicine: Perspectives, Practices and Research. New York: Springer Publishing; 2010. pp. 1910–29. [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of the Sciences of the United States of America. 2007;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick PC. Mindfulness and coping with dysphoric mood: contrasts with rumination and distraction. Cognitive Therapy and Research. 2005;29(5):501–10. [Google Scholar]
- Brown KW, Goodman R, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss004. doi: 10.1093/scan/nss004 [Advance access published on 17 January 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84(4):822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–37. [Google Scholar]
- Brown KW, Ryan RM, Loverich TM, Biegel GM, West AM. Out of the armchair and into the streets: measuring mindfulness advances knowledge and improves interventions: reply to Grossman (2011) Psychological Assessment. 2011a;23:1041–6. [Google Scholar]
- Brown KW, West AM, Loverich TM, Biegel GM. Assessing adolescent mindfulness: validation of an adapted Mindful Attention Awareness Scale in adolescent normative and psychiatric populations. Psychological Assessment. 2011b;23:1023–33. doi: 10.1037/a0021338. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Neurosciences. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of cognitive neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological bulletin. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Carriere JS, Cheyne JA, Smilek D. Everyday attention lapses and memory failures: the affective consequences of mindlessness. Consciousness and Cognition. 2008;17(3):835–47. doi: 10.1016/j.concog.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady C, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46(7):1828–51. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69(6):560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65(4):564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Dong M, Kanai R, Bahrami B, Rees G. The anatomy of superior parietal cortex links everyday distractibility with attentional capture. Journal of Vision. 2010;10(7):124. [Google Scholar]
- Eckert MA, Menon V, Walczak A, et al. At the heart of the ventral attention system: the right anterior insula. Human Brain Mapping. 2009;30(8):2530–41. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, et al. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the Natinal Academy of Science United States of America. 2001;98(8):4728–33. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, et al. Attending to the present: meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, et al. Applying a functional acceptance based model to smoking cessation: an initial trial of acceptance and commitment therapy. Behavior Therapy. 2004;35:689–705. [Google Scholar]
- Goldin P, Gross J. Effect of mindfulness meditation training on the neural bases of emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–4. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4259–4. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology's (re)invention of mindfulness: comment on Brown et al (2011) Psychological Assessement. 2011;23(4):1034–40. doi: 10.1037/a0022713. [DOI] [PubMed] [Google Scholar]
- Hölzel B, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59(1):750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3(3):284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Neuropsychiatrica. 2007;19(2):76–91. doi: 10.1111/j.1601-5215.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime M. Mindfulness training modifies subsystems of attention. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(2):109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. ReVISION. 1984;7(1):71–2. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go there you are: mindfulness meditations in everyday life. New York: Hyperion; 1994. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine. 1985;8(2):163–90. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23(2):670–9. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–5. [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Science. 2008;12(4):163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna A, Raffone A, Perrucci, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Research Bulletin. 2010;82(1–2):46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Pagano RR, Rose RM, Marques JK. Effects of meditation and relaxation training upon alcohol use in male social drinkers. In: Shapiro DH, Walsh RN, editors. Meditation: Classic and contemporary perspectives. New York, NY: Aldine; 1984. pp. 105–20. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Gauntlett-Gilbert J, Vowles KE. The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain. 2007;131(1–2):63–9. doi: 10.1016/j.pain.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, MacRae CN. The link between social congition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Moore A, Molinowski Meditation, mindfulness and cognitive flexibility. Consciousness and Cognition. 2009;18(1):176–86. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Medical Hypotheses. 2003;61(2):282–91. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58(1):1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of the Sciences of the USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nature Reviews Neuroscience. 2006;7(5):367–79. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Short EB, Kose S, Mu Q, et al. Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evidence-based Complementary and Alternative Medicine. 2010;7(1):121–7. doi: 10.1093/ecam/nem163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. Journal of Neuroscience. 2010;30(10):3640–51. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh- Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–48. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57(4):1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. [Meta-Analysis] Cerebral Cortex. 2008;18(11):2553–9. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia. 2008;46(12):3014–8. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. [Meta-Analysis] Neuroimage. 2004;22(4):1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Rambo Chroniak K, Chroniak C, Durazo-Arvizu R, Matthews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women with newly diagnosed with early stage breast cancer. Brain, Behavior, and Immunity. 2008;22(6):969–81. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biological Psychiatry. 2005;58(2):143–50. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Gordan NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. The Journal of Pain. 2010;11(3):199–209. doi: 10.1016/j.jpain.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness meditation for substance use disorder: a systematic review. Substance Abuse. 2009;30(4):266–94. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylowska L, Ackerman DL, Yang M, et al. Mindfulness meditation training in adults and adolescents with Attention Deficit Hyperactivity Disorder—a feasibility study. Journal of Attention Disorders. 2008;11(6):737–46. doi: 10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]