Abstract
A convergent line of neuroscientific evidence suggests that meditation alters the functional and structural plasticity of distributed neural processes underlying attention and emotion. The purpose of this study was to examine the brain structural differences between a well-matched sample of long-term meditators and controls. We employed whole-brain cortical thickness analysis based on magnetic resonance imaging, and diffusion tensor imaging to quantify white matter integrity in the brains of 46 experienced meditators compared with 46 matched meditation-naïve volunteers. Meditators, compared with controls, showed significantly greater cortical thickness in the anterior regions of the brain, located in frontal and temporal areas, including the medial prefrontal cortex, superior frontal cortex, temporal pole and the middle and interior temporal cortices. Significantly thinner cortical thickness was found in the posterior regions of the brain, located in the parietal and occipital areas, including the postcentral cortex, inferior parietal cortex, middle occipital cortex and posterior cingulate cortex. Moreover, in the region adjacent to the medial prefrontal cortex, both higher fractional anisotropy values and greater cortical thickness were observed. Our findings suggest that long-term meditators have structural differences in both gray and white matter.
Keywords: cortical thickness, diffusion tensor imaging, medial prefrontal cortex, meditation
INTRODUCTION
One of the biggest challenges for modern neuroscience is the identification of the neuroanatomical correlates of experience-induced neuroplasticity. Experience-induced structural brain changes highlight the relationship between behavior and underlying cortical structures (Draganski et al., 2004; Driemeyer et al., 2008). Indeed, structural brain studies have demonstrated learning-dependent alterations in the adult human brain (Draganski et al., 2006), and the anatomical correlates of navigation (Maguire et al., 2000), arithmetic (Aydin et al., 2007), music acquisition (Han et al., 2009; Hyde et al., 2009), working memory (Takeuchi et al., 2010) and a board game called Baduk in Korea (Lee et al., 2010) have been identified.
Meditation can be conceptualized as a family of complex emotional and attentional regulatory training practices developed for various ends. Recently, the therapeutic use of meditation, including mindfulness-based techniques, has become increasingly important in the treatment of physiological and psychological conditions (Ludwig and Kabat-Zinn, 2008). Furthermore, a convergent body of neuroscientific evidence suggests that meditation alters the function and structure of distributed neural processes underlying attention and emotion (Brefczynski-Lewis et al., 2007; Pagnoni and Cekic, 2007; Lutz et al., 2008). In particular, the altered synaptic structure of the brain circuits associated with attention and emotion might be the one of the essential pathophysiological conditions underlying some major psychiatric disorders such as schizophrenia and depression (Goto et al., 2010).
Previous studies using structural magnetic resonance imaging (MRI) in meditators compared to controls have provided inconsistent results, especially in the frontal cortex (Lazar et al., 2005; Pagnoni and Cekic, 2007; Hölzel et al., 2008; Luders et al., 2009; Vestergaard-Poulsen et al., 2009). Luders et al. (2009) investigated 22 active practitioners of meditations, including Zazen, Samatha and Vipassana and found larger gray matter (GM) density in the orbitofrontal cortex (OFC), which is related to emotional regulation processing (Quirk and Beer, 2006). Lazar et al. (2005) compared 20 insight meditation practitioners to controls using cortical thickness analysis and detected greater cortical thickness in the middle and superior frontal cortices, which is associated with attention processing. There have been studies investigating the relationship between the duration of meditation practice and brain structure. Some studies demonstrated that there is an effect of the duration of meditation practice on local GM, suggesting longer meditation might induce greater changes in brain structure (Hölzel et al., 2008; Grant et al., 2010). However, other studies failed to find any significant correlation between local GM and meditation experience (Luders et al., 2009; Vestergaard-Poulsen et al., 2009). These mixed results may be due to relatively small sample sizes employed in previous studies. In an effort to overcome the limitations of previous studies, in the present study, a large sample of subjects were trained in an identical method of meditation, ‘Brain Wave Vibration (BWV)’. Recent studies have reported positive effects of BWV with respect to stress, emotional regulation and neurohormonal levels (Jung et al., 2010, 2012; Bowden et al., 2012).
Diffusion tensor imaging (DTI) is a quantitative method used to assess the integrity of anatomical connectivity in white matter (WM) through the examination of the degree of fractional anisotropy (FA). To our knowledge, only two studies have previously used DTI to examine WM changes in meditators (Tang et al., 2010; Luders et al., 2011). Recently, our group reported training-related alterations in functional connectivity using resting-state functional MRI. Specifically, we found greater default mode network (DMN) connectivity associated with meditation, particularly in the medial prefrontal cortex (MPFC) (Jang et al., 2011). As our previous findings indicated alterations in functional connectivity, we believed that investigating the impact of meditation on alterations in anatomical connectivity is crucial.
The primary focus of the present study was to determine structural brain differences between a group of experienced meditators and a meditation-naive group. Whole-brain cortical thickness analysis based on structural MRI was conducted to investigate GM differences between two groups. WM differences were also examined using DTI. The relationship between structural brain differences and individual characteristics, including practice duration, was conducted in the experienced meditation group to explore the reciprocal interplay between meditation training and underlying cortical structures. We hypothesized that experienced meditators would demonstrate structural differences in the MPFC, orbitofrontal cortex and parietal areas that associated with attention and emotional regulations.
METHODS
Participants
A total of 46 meditation practitioners (16 men and 30 women) and 46 control subjects (17 men and 29 women) participated in the study. Many of the current participants were subjects in our previous studies (Jung et al., 2010; Jang et al., 2011). The recruited meditation practitioners were individuals trained with BWV, a meditation practice that combines ancient Eastern philosophy with modern scientific methods to elevate human awareness (for detail see Bowden et al., 2012). Briefly, BWV is a kind of moving meditation that is designed to help quieting the thinking mind and releasing negative emotions through performing natural rhythmic physical movements and focusing on bodily sensations. For this, the meditation places importance on focusing attention on one’s own bodily sensations and emotions, and heightening the awareness of the movement of energy within the body. The mean duration of meditation practice was 41.23 ± 27.57 months. Control subjects had no previous experience with meditation or similar practices. The non-patient version of the Structured Clinical Interview for DSM-IV was used to assess psychiatric disorders in all participants. All subjects were right-handed (Annett, 1970). Exclusion criteria included a life-time history of psychosis, bipolar disorder, major depressive disorder, substance abuse or dependence, significant head injury, seizure disorder or mental retardation. The present study was approved by the Institutional Review Board at Seoul National University Hospital, and informed consent was obtained from all subjects following the explanation of the procedures.
Image acquisition
All data were acquired using a 1.5-Tesla Avanto scanner (Siemens, Erlangen, Germany). T1-weighted structural images covering the whole brain using a 3D magnetization-prepared rapid gradient echo sequence were acquired with the following parameters: TR/TE = 1160/4.76 ms, field of view = 23 cm, flip angle = 15°, voxel size = 0.45 × 0.45 × 0.90 mm3, slice thickness = 0.9 mm. Diffusion-weighted images (DWIs) were acquired with diffusion gradients (b-factor 1000 s/mm2) along 12 non-collinear directions. Ten images were acquired with no diffusion gradient (B0 images) to increase the signal-to-noise ratio. Other parameters were as follows: TR/TE = 9200/83 ms, 75 slices, field of view = 256 mm, voxel size = 2 × 2 × 2 mm3. All scans were judged by a neuroradiologist (C.H.C.) to be visually excellent without obvious artifacts, signal loss or gross pathology.
Cortical thickness analysis
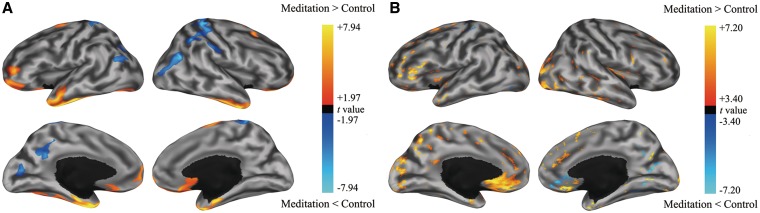
The FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/) was used to calculate cortical thickness on cortical surface models (see Fischl et al., 2002, 2004 for a full description of the methods). GM/WM boundary and pial surfaces were generated. The cortical thickness for each cortical location was then determined by calculating a distance between two vertices at the corresponding locations in GM/WM boundary and pial surface. Individual cortical thicknesses were mapped onto the N27 template brain-surface models (Mazziotta et al., 2001) using surface-based registration methods (SUMA, Saad and Reynolds, 2011). The spatial smoothing was performed on the GM/WM surface with 25-mm full-width-at-half-maximum (FWHM) of the heat kernel (Chung et al, 2005; Jo et al., 2007) (Supplementary Figure S1). To test the difference in cortical thickness between two groups, we performed an one-way analysis of covariance (ANCOVA) using AFNI package’s 3dttest++ to remove the covariate effects (age, sex and the duration of education) (see http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dttest++.html/). The statistical results were corrected for multiple comparisons using a Monte-Carlo simulation (corrected P < 0.01; |t| > 1.97, N > 214) (Supplementary Figure S2). For visual inspection and display purposes, the statistics results were mapped onto the inflated WM surfaces of the N27 template brain-surface models (Figure 1A).
Fig. 1.
Regional maps showing the statistical differences (A) in cortical thickness and (B) in FA between meditation practitioners and control subjects.
DTI analysis
DTI data were processed with the AFNI package (Cox, 1996, 2011). Each DWI image was transformed into the individual B0 image by AFNI’s registration tool with the ‘LPA’ cost function optimized for DTI applications (Saad et al., 2009). The image was then anisotropically smoothed by ‘3danisosmooth’ (Ding et al., 2005). Diffusion tensors were calculated voxel wisely by AFNI’s ‘Vecwarp’, a program that considers every transformation during vector calculations. Next, FA maps were produced from the eigenvalues and their means (Cox and Glen, 2006). Each individual FA map in individual volume space was normalized to the N27 brain template. To test the differences in FA between two groups, an ANCOVA was performed using AFNI package’s ‘3dttest++’ to remove the effects of covariates. The statistical results were corrected by the false discovery rate (FDR) control, and then the significance threshold was set (|t| > 3.4, P < 0.001, FDR q < 0.05). For visual inspection and display purposes, the statistical results were mapped onto the N27 template brain-surface models by mapping at the 2 mm deeper locations from the GM/WM surface along the normal vector of the two-surfaces method (i.e. GM/WM surface and pial surface) in SUMA (Saad and Reynolds, 2011) and visualized on the inflated WM surfaces (Figure 1B). In addition, we assessed not only the statistical differences in FA maps using the same Monte-Carlo simulation as that used in cortical thickness analysis, although it was not a rigorous statistical analysis, but also the statistical differences in cortical thickness using FDR correction (q < 0.05) to aid direct comparison between measures. The results from this analysis are given in Supplementary Figure S3 and S4.
Region-of-interest and correlation analyses
Further Region-of-interest (ROIs) and correlation analyses were conducted to investigate whether there were differences in FA values of the WM regions adjacent to the GM regions showing group differences in cortical thickness between two groups. For this purpose, FA values were extracted from the 2 mm deeper locations from the GM/WM surface along the normal vector of the two-surfaces because there exists a local conformal correspondence between points on pairs of two surfaces. The mean values in ROIs extracted from cortical thickness map and FA map of meditation practitioners were compared with those of control subjects separately. We employed Bonferroni correction (i.e. 0.05/15 = 0.0034) for multiple comparisons.
To investigate whether cortical thickness of meditation practitioners and their practice duration are significantly linked, we performed two correlations analyses. A correlation was calculated to establish the relationship between mean cortical thickness at each ROI and the duration of meditation practice. We employed Bonferroni correction (i.e. 0.05/15 = 0.0034) for multiple comparisons. We also conducted point-wise correlation analyses between whole-brain cortical thickness and the duration of meditation practice. The correlations between FA values and practice duration were also examined in the same manner as described above.
RESULTS
Demographic characteristics
The demographic characteristics of the subjects in each group are presented in Table 1. There were no major differences in sex, age or years of education between meditation practitioners and control subjects.
Table 1.
Demographic characteristics of meditation practitioners and control subjects
| Variable | Meditation practitioners | Control subjects | Analysis |
|
|---|---|---|---|---|
| (N = 46) | (N = 46) | t or χ2 score | P-value | |
| Demographic characteristics | ||||
| Sex (male/female) | 16/30 | 17/29 | χ2 = 0.05 | 0.83 |
| Handedness (right/left)a | 46/0 | 46/0 | χ2 = 0.00 | 1.00 |
| Age (years) | 25.35 ± 3.30 | 24.61 ± 3.53 | t = 1.04 | 0.30 |
| Education (years) | 14.63 ± 1.79 | 15.04 ± 1.21 | t = −1.30 | 0.20 |
| Duration of practice (months) | 41.23 ± 27.57 | – | ||
aHandedness was measured by Annett Hand Preference Questionnaire.
Cortical thickness results (GM differences between meditators and controls)
The majority of brain regions with significant group differences were areas that involved in the DMN (Table 2). Specifically, significantly greater cortical thickness in meditators compared to controls was found in the anterior parts of the brain located in the frontal and temporal areas, including bilateral ventromedial PFC, superior frontal cortex, temporal pole, middle and interior temporal cortices, and left fusiform cortex (Figure 1A). In contrast, significantly thinner cortical thickness was found in the posterior parts of the brain located in the parietal and occipital regions in meditators, including bilateral postcentral and inferior parietal cortices, right middle occipital cortex, left posterior cingulate cortex (PCC) and left cuneus (Table 2).
Table 2.
Brain regions showing group differences in cortical thickness between meditation practitioners and control subjects
| Brain region | Brodmann area | Area (mm2) | Mean, t-value | Mean, P-value | Peak, t-value | Peak, P-value | Talairach coordinates of peak vertex |
|---|---|---|---|---|---|---|---|
| Left hemisphere | |||||||
| Middle/inferior temporal cortexa | 20/38 | 3770.050 | 3.672 | <0.001 | 7.130 | <0.001 | −43, −4, −33 |
| Superior frontal cortex/OFCa | 10/47 | 1041.020 | 2.654 | 0.010 | 3.663 | <0.001 | −22, 55, −5 |
| Fusiform cortex | 37 | 902.250 | 2.520 | 0.014 | 3.404 | 0.001 | −30, −59, −13 |
| Ventromedial PFC/OFC | 11 | 643.150 | 2.317 | 0.023 | 3.138 | 0.002 | −20, 10, −12 |
| Middle frontal cortex | 10 | 563.790 | 2.370 | 0.020 | 3.072 | 0.003 | −39, 43, 12 |
| Postcentral cortexa | 5 | 472.820 | −2.570 | 0.012 | −3.885 | <0.001 | −14, −47, 68 |
| Posterior cingulate cortex | 31 | 411.460 | −2.431 | 0.017 | −3.026 | 0.003 | −10, −48, 27 |
| Angular gyrus | 39 | 387.460 | −2.177 | 0.032 | −2.418 | 0.018 | −37, −75, 27 |
| Cuneus cortex | 17 | 374.280 | −2.426 | 0.017 | −3.127 | 0.002 | −5, −74, 10 |
| PFC | 10 | 239.180 | 2.345 | 0.021 | 2.927 | 0.004 | −4, 60, 12 |
| Superior frontal cortex | 6 | 215.900 | 2.350 | 0.021 | 2.771 | 0.007 | −25, −6, 60 |
| Intraparietal cortexa | 7 | 214.070 | −2.719 | 0.008 | −3.627 | 0.001 | −20, −62, 28 |
| Right hemisphere | |||||||
| Middle/inferior temporal cortexa | 1/38 | 2483.600 | 3.468 | 0.001 | 6.336 | <0.001 | 35, 3, −35 |
| Inferior parietal cortex/ postcentral cortexa | 40/3 | 2001.300 | −2.753 | 0.007 | −4.620 | <0.001 | 35, −52, 34 |
| Ventromedial PFC/OFCa | 11 | 1910.640 | 2.689 | 0.009 | 4.976 | <0.001 | 17, 31, −15 |
| Middle occipital/temporal cortexa | 19/39 | 1038.670 | −2.784 | 0.007 | −4.030 | <0.001 | 46, −64, 26 |
| Supramarginal gyrusa | 40 | 423.600 | −2.871 | 0.005 | −3.941 | <0.001 | 51, −25, 27 |
| Superior frontal cortexa | 6 | 380.630 | 2.919 | 0.005 | 4.117 | <0.001 | 14, −6, 64 |
| Superior frontal cortex | 8 | 234.490 | 2.560 | 0.012 | 3.310 | 0.001 | 22, 11, 42 |
OFC, orbitofrontal cortex; PFC, prefrontal cortex. The brain regions in bold indicate areas showing significantly increased cortical thickness in meditation practitioners compared to controls. The statistical results were corrected for multiple comparisons using a Monte-Carlo simulation (corrected P < 0.01; |t| > 1.97, N > 214).
aThe regions that are significant under a corrected threshold of FDR q < 0.05 (please refer to Supplementary Figure S4)
DTI results (WM differences between meditators and controls)
Direct comparisons of FA values between the two groups revealed significant differences in the majority of the anterior portion of the brain, with higher FA values in the cuneus, precuneus and occipital regions and the ventromedial PFC in the meditator group (Figure 1B). In contrast, meditators showed lower FA values in the right MPFC, PCC and right occipital regions in comparison with controls. Supplementary Table S1 and Supplementary Figure S5 present WM regions showing the statistical differences in FA value between meditators and controls.
ROI-specific results
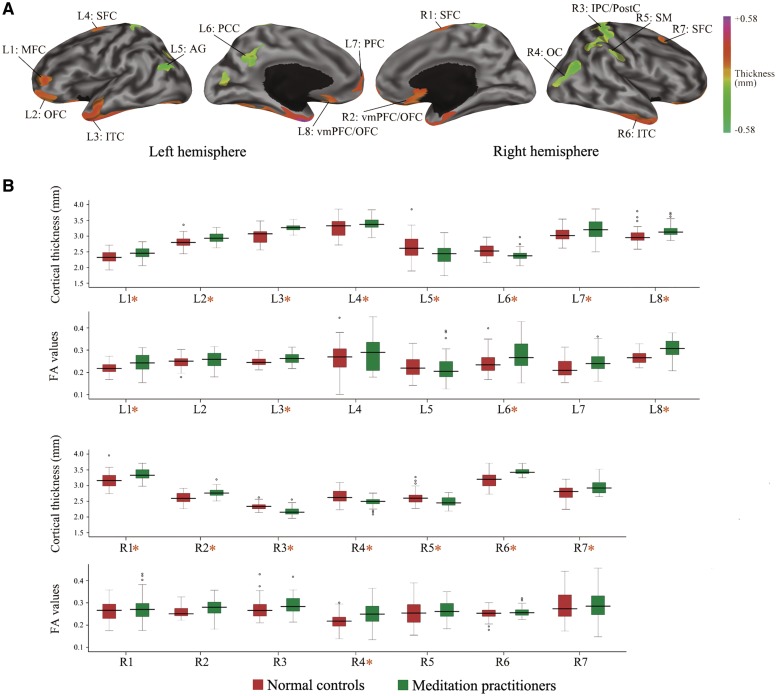
To further illustrate the magnitude of the cortical alterations and the differences in FA values of WM adjacent to these regions, mean cortical thickness and mean FA values in each ROI for meditators and controls are presented in Figure 2. Interestingly, the illustrated patterns for FA values were similar to those for cortical thickness, particularly in the anterior parts of the brain. Significant differences in mean FA values were observed between the two groups in several ROIs, including left ventromedial PFC, left lateral PFC, left temporal cortex, left PCC and right middle occipital cortex.
Fig. 2.
The mean differences between meditation practitioners and control subjects in each ROI. (A) Mean cortical differences between two groups in ROIs including the MFC (L1), OFC (L2), ITC (L3), SFC (L4), AG (L5), PCC (L6), PFC (L7), vmPFC/OFC (L8), SFC (R1), vmPFC/OFC (R2), IPC/PostC (R3), OC (R4), SM (R5), ITC (R6), and SFC (R7) (B) mean cortical thickness and mean FA values in each ROI. Red-colored boxes indicate control subjects and green-colored boxes indicate meditation practitioners. The x-axis shows brain regions and the y-axis indicates cortical thickness (millimeters) or FA values. The region showing statistical significant differences are marked with orange asterisk. L, left hemisphere; R, right hemisphere; ITC, inferior temporal cortex; AG, angular gyrus; vm, ventromedial; SFC, superior frontal cortex; IPC, inferior parietal cortex; PostC, postcentral cortex; OC, occipital cortex; SM, supramarginal cortex.
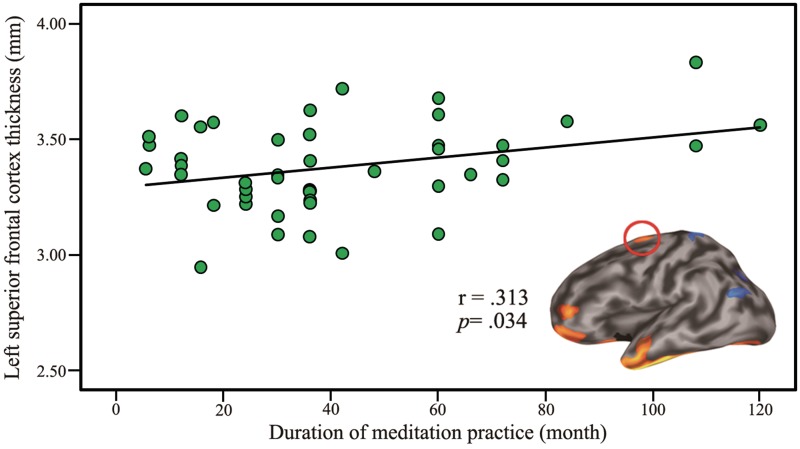
In ROI-specific correlation analysis, we found a positive but weak correlation between mean cortical thickness in the left superior frontal cortex adjacent to the primary motor cortex and the duration of meditation practice (r = 0.313, P = 0.034) (Figure 3). However, in point-wise correlation analysis, there was no significant correlation between whole-brain cortical thickness and the duration of meditation practice after correction for multiple comparisons. There were no significant correlations between FA values and practice duration in both ROI-specific analysis and point-wise analysis after correction for multiple comparisons.
Fig. 3.
Correlation between left superior frontal cortex thickness and duration of meditation practice.
DISCUSSION
To our knowledge, this is the first study to investigate structural brain differences in experienced meditators compared with meditation-naïve controls by combining surface-based cortical thickness and DTI analysis. The major findings of the present study were as follows: (i) meditators had thicker cortex in the anterior portions of the brain, located in the frontal–temporal region, including bilateral ventromedial PFC, superior frontal cortex and middle and interior temporal cortices than controls and (ii) meditators compared with controls had thinner cortex in the posterior portions of the brain, located in parietal–occipital region, including bilateral postcentral and inferior parietal cortices and left PCC. Additionally, the left superior frontal cortical thickness adjacent to the primary motor cortex in meditators showed a positive trend correlation with the duration of meditation practice. Regarding WM, DTI results showed higher FA values in the anterior part of the brain in meditators, namely in the MPFC as in the region with the greater cortical thickness and reduced FA values in some of MPFC, PCC and the occipital cortex in meditators compared with controls.
The most striking findings of the present study were greater cortical thickness in the MPFC/OFC regions and superior frontal cortex and lesser cortical thickness in the inferior parietal cortex and PCC. A possible explanation for present findings is the usage-dependent selective elimination of synapses (Huttenlocher and Dabholkar, 1997; Takeuchi et al., 2011a). Previous studies have repeatedly reported that increased cognitive functions after training are associated with decreased GM volumes (Kanai and Rees, 2011; Takeuchi et al., 2011a). Meditation training can enhance various cognitive processes, such as emotional regulation, executive control and attention, particularly sustained attention (Zeidan et al., 2010; Jung et al., 2010). Therefore, thinner cortical thickness of brain regions in meditators, including the lateral and medial parietal areas, may be associated with their enhanced cognitive functions through meditation training, such as improved attention and self-perception. Recent studies revealed the nonlinearity of training-induced GM changes, showing that an initial increase in regional GM volumes followed by a decrease in regional GM volumes (Driemeyer et al., 2008; Boyke et al., 2008), and suggested that these changes are affected by training length and intensity (Takeuchi et al., 2011a,b). In this context, although all brain regions showing thicker and thinner cortices are involved in meditation training we used (i.e. BWV), brain regions showing thinner cortices may be more strongly involved in meditation training used. In the present study, lesser cortical thickness in the meditators was observed in the regions related to self-referential processing. The inferior parietal region such as the angular gyrus is known to be involved in the integration of sensory information from different modalities and the regulation of attention. The inferior parietal cortex and the PCC are key regions involved in egocentric processing of one’s own body’s in spatial context (Vogeley and Fink, 2003). In contrast, meditators compared with controls showed thicker cortical thickness in lateral PFC, MPFC and temporal areas. Most of regions showing thicker cortical thickness in meditators are related with emotional processing. The MPFC is involved in emotional regulation through the coordination of behavioral and autonomic responses, which is critical for social adaptation. A number of behavioral and functional studies of meditation have previously reported the numerous beneficial effects of meditation on emotional regulation (Lutz et al., 2008; Jung et al., 2010). Interestingly, the observed structural differences of the MPFC in meditators are consistent with our previous findings that demonstrated greater MPFC functional connectivity in the DMN during a resting-state (Jang et al., 2011). Thus, these results indicate that the MPFC in mediators shows both structural and functional differences. Meditation practitioners showed thicker cortical thickness in the region adjacent to the primary motor area as well as a positive trend between cortical thickness in this region and practice duration. This region is involved in the control and execution of voluntary motor functions (Toma et al., 1999). It can be thus speculated that the rhythmic actions of the BWV protocol as well as the focusing of attention on the actions may be associated with the correlation that was observed in this study. We also found greater cortical thickness in the superior frontal cortex. The superior frontal cortices are regions of the brain that are typically involved in the regulation and monitoring of attention (Tang et al., 2007). Recent functional MRI studies have reported greater activation in these regions in experienced meditators during meditation (Brefczynski-Lewis et al., 2007; Hölzel et al., 2007). Taken together, it can be suggested that the meditation training we used, BWV, is involved primarily in attention processing and self-perception and secondarily in emotional processing.
Another alternative explanation for present findings is that each component involved in meditation training may affect a different part of the brain in different ways. A recent study demonstrated specific correlations between the executive and alerting components of attention and cortical thickness in anatomically relevant regions, showing positive correlations between the executive control component of attention and thickness in the ACC extending into the frontal pole and dorsolateral PFC, lateral PFC, inferior frontal cortex and temporal areas as well as negative correlations between the alerting component of attention and thickness in the lateral and medial aspects of the parietal areas (Westlye et al., 2011). Based on the findings from previous studies, we speculate that brain regions showing significantly thicker cortices in meditators than controls, such as the PFC and temporal areas, may be associated with their improved executive control. Meanwhile, brain regions showing significantly thinner cortices in meditators than controls, including the lateral parietal cortex and precuneus, may be associated with their increased vigilance. Further studies examining direct relationship between neuropsychological test performance and GM structures in meditators are warranted to clarify these hypotheses.
Interestingly, the majority of brain regions showing significant group differences in cortical thickness correspond to the region known as the DMN. The DMN is known to be involved in internal mentation or attention that is detached from the external world (Buckner et al., 2008), which is consistent with the meditative state utilized in the present study. The DMN consists of two subunits: the anterior DMN (aDMN) and the posterior DMN (pDMN) (Damoiseaux et al., 2008; Otti et al., 2010; Kim and Lee, 2011). The aDMN comprises primarily the MPFC, anterior cingulate and the precuneus, whereas the pDMN mainly includes the precuneus and the inferior parietal cortex. The area that showed greater cortical thickness in meditators mostly coincided with the aDMN, while the area with lesser cortical thickness was consistent with parts of the pDMN. As mentioned above, greater cortical thickness in the aDMN could be interpreted as enhanced regulation of emotional state, while thinner cortical thickness in the pDMN could be interpreted as improved self-referential processing. Given that the regulation of attention and emotion and self-referential processing is the central commonality across various meditation methods, our results suggest that meditation practice is associated with structural differences in regions that are typically activated during meditation and in regions that are relevant to the practice of meditation. However, as an adjunct to the cross-sectional design of this study, our findings should be confirmed using a longitudinal design with measures taken before and after meditation. Additionally, future studies using diverse data including neurohormonal and genetic factors and multimodal brain images would be helpful to clarify the mechanism underlying structural changes associated with meditation practices.
The effects of meditation observed in the present study may be associated with the plastic nature of the central nervous system, which allows the adaptation of existing neural connections and consistent neurogenesis in order to accommodate new information and experiences. FA values from the DTI may be considered the capacity of information flow within and between the brain regions. The results in the present study support this idea, as our results showed higher FA values in distributed regions in meditators, including the lateral PFC, MPFC, temporal pole, middle and inferior temporal areas and part of the parietal lobe, which is consistent with the results of other recent DTI studies. Tang et al. (2010) reported significant FA changes in the anterior corona radiate, and Luders et al. (2011) reported pronounced structural connectivity in the uncinate fasciculus as well as in the corticospinal tract and the temporal component of the superior longitudinal fasciculus. The uncinate fasciculus is a major WM tract connecting the anterior temporal lobe with the MPFC/OFC area. In the present study, higher FA values in the MPFC and temporal pole in meditators may be linked to their thicker cortical thickness in those regions. It is, thus, conceivable that meditation training might mediate information flow between these structures, which is implicated in emotional processing. Increased FA values in the dorsolateral PFC and part of the parietal lobe (Supplementary Figure S5), the regions involved in the so-called task-positive network (TPN), in the meditators is consistent with previous findings showing increased FA values in the superior longitudinal fasciculus, the main fronto-parietal fiber tract (Luders et al., 2011). Such increases in FA values may be associated with enhanced executive attention in meditators. Therefore, we suggest that meditation training can affect in both the DMN and TPN. The mechanism underlying the structural differences observed in the present study still remains largely unknown. However, our group recently found higher plasma dopamine levels in BWV practitioners compared with controls (Jung et al., 2010). The increase of dopamine associated with meditation training, through improved antioxidant status (Sharma et al., 2008), may contribute to dopaminergic neurogenesis. Interestingly, we also found reduced FA values in the anterior medial part, the ventromedial OFC and the posterior medial part, the PCC, in meditators compared with controls. These two regions are known as the key nodes of the DMN and are considered to act as important connector hubs in the brain (Hagmann et al., 2008). A possible explanation is that the decreased FA value may be due to increases in the number of crossing fibers in these regions as the hub. In this study, meditators compared with controls had increased FA values in most of brain regions. Based on our results, we speculate that meditators have increased crossing fibers in the hub regions and it may result in decreased FA values in the regions. Future studies are needed to reveal the underlying mechanism of such FA changes.
There were no significant correlations between brain structure and practice duration, except for the region adjacent to the primary motor area. Previous studies have reported inconsistent findings, showing positive correlation between GM volumes and training duration (Hölzel et al., 2008; Grant et al., 2010) or no correlations (Luders et al., 2009; Vestergaard-Poulsen et al., 2009). Luders et al. (2011) described that the lack of any significant correlations between brain structure and the amount of meditation experience might result from the confound effect of age and the inaccuracy of the indicators chosen for determining the actual extent/intensity of the individual training. That is, extrapolations of training duration are subjective rather than precise. In addition, even if all meditators practiced in one particular style, they are engaged differently in their mental exercises. Based on previous studies, training intensity may more heavily affect GM structures than the total number of years of practice. Clearly, longitudinal studies will be necessary to determine whether the differences of FA values and cortical thickness in meditators we found were actually induced by meditation, or whether they are inherent prerequisites for the beginning and continuation of meditation (Luders et al., 2009, 2011).
The present study has several limitations. First, this study was not a longitudinal design but a cross-sectional design study. As a result of the cross-sectional nature of this study, it is unclear that the brain structural differences we found were directly caused by meditation training. Second, we used DTI images of 12 diffusion directions. Recent studies suggested that larger number of diffusion gradient directions and using isotropic voxels may improve the estimation of the diffusion tensor (Ni et al., 2006). Future directions should include longitudinal studies with more directions of the diffusion gradient.
In summary, this study used cortical thickness and DTI to demonstrate structural differences in long-term meditators. Meditators showed thicker cortex in the anterior parts of the brain such as the lateral PFC, MPFC and temporal areas, and thinner cortex in the posterior parts of the brain, including the lateral and medial parietal regions. Additionally, DTI results showed greater WM integrity in the MPFC. Our findings demonstrate meditators, compared to controls, have structural brain differences in both GM and WM. These brain structural differences, particularly in the frontal cortex, may be associated with repeated practice of attentional and emotional regulations.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
CONFLICT OF INTEREST
None declared.
Supplementary Material
Acknowledgments
The authors give special thanks to Korea Institute of Brain Science for the assistance in the present study and Daniel Glen for his amazing support with data analysis. The authors also thank Ji Yeon Han for all her work in data acquisition, the clinical and nursing staff of the Clinical Cognitive Neuroscience Center for the help with recruitment, and Robert Cox and Ziad Saad for their development of AFNI and SUMA. This research was supported by Mid-career Research Program through National Research Foundation grant (20110015639) and by World Class University program through the Korea Science and Engineering Foundation (R31-10089) funded by the Ministry of Education, Science and Technology, Republic of Korea. Hang Joon Jo was supported by the National Institute of Mental Health (NIMH) and the National Institute of Neurological Disorders and Stroke (NINDS) Intramural Research Programs of the National Institutes of Health (NIH).
REFERENCES
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61(3):303–21. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucar A, Oguz KK, et al. Increased gray matter density in the parietal cortex of mathematicians: a voxel-based morphometry study. American Journal of Neuroradiology. 2007;28(10):1859–64. doi: 10.3174/ajnr.A0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D, Gaudry C, An SC, Gruzelier J. A comparative randomised controlled trial of the effects of Brain Wave Vibration training, Iyengar Yoga and Mindfulness on mood, well-being and salivary cortisol. Evidence-Based Complementary and Alternative Medicine. 2012;2012:234713. doi: 10.1155/2012/234713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. Journal of Neuroscience. 2008;28(28):7031–5. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences USA. 2007;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage. 2005;25(4):1256–65. doi: 10.1016/j.neuroimage.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Glen DR. 2006. Efficient, robust, nonlinear, and guaranteed positive definite diffusion tensor estimation. International Society of Magnetic Resonance in Medicine. In Proceedings of the ISMRM 14th Scientific Meeting, Seattle, WA. [Google Scholar]
- Cox RW. AFNI: What a long strange trip it's been. Neuroimage. in press doi: 10.1016/j.neuroimage.2011.08.056. doi: 10.1016/j.neuroimage.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the ‘default network’ in normal aging. Cerebral Cortex. 2008;18(8):1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26(23):6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Gore JC, Anderson AW. Reduction of noise in diffusion tensor images using anisotropic smoothing. Magnetic Resonance in Medicine. 2005;53(2):485–90. doi: 10.1002/mrm.20339. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning-revisited. PLoS One. 2008;3(7):e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological Psychiatry. 2010;67(3):199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10(1):43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yang H, Lv YT, et al. Gray matter density and white matter integrity in pianists' brain: a combined structural and diffusion tensor MRI study. Neuroscience Letters. 2009;459(1):3–6. doi: 10.1016/j.neulet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, et al. The effects of musical training on structural brain development: a longitudinal study. Annals of the New York Academy of Sciences. 2009;1169:182–6. doi: 10.1111/j.1749-6632.2009.04852.x. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487(3):358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Lee JM, Kim JH, et al. Spatial accuracy of fMRI activation influenced by volume- and surface-based spatial smoothing techniques. Neuroimage. 2007;34(2):550–64. doi: 10.1016/j.neuroimage.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Jung YH, Kang DH, Byun MS, et al. Influence of brain-derived neurotrophic factor and catechol O-methyl transferase polymorphisms on effects of meditation on plasma catecholamines and stress. Stress. 2012;15(1):97–104. doi: 10.3109/10253890.2011.592880. [DOI] [PubMed] [Google Scholar]
- Jung YH, Kang DH, Jang JH, et al. The effects of mind-body training on stress reduction, positive affect, and plasma catecholamines. Neuroscience Letters. 2010;479(2):138–42. doi: 10.1016/j.neulet.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12(4):231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kim DY, Lee JH. Are posterior default-mode networks more robust than anterior default-mode networks? Evidence from resting-state fMRI data analysis. Neuroscience Letters. 2011;498(1):57–62. doi: 10.1016/j.neulet.2011.04.062. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Park JY, Jung WH, et al. White matter neuroplastic changes in long-term trained players of the game of ‘Baduk’ (GO): a voxel-based diffusion-tensor imaging study. Neuroimage. 2010;52(1):9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–8. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57(4):1308–16. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. The Journal of the American Medical Association. 2008;300(11):1350–2. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS One. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences USA. 2000;97(8):4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356(1412):1293–322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. American Journal of Neuroradiology. 2006;27(8):1776–81. [PMC free article] [PubMed] [Google Scholar]
- Otti A, Guendel H, Läer L, et al. I know the pain you feel-how the human brain's default mode predicts our resonance to another's suffering. Neuroscience. 2010;169(1):143–8. doi: 10.1016/j.neuroscience.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging. 2007;28(10):1623–7. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–27. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44(3):839–48. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC. SUMA. Neuroimage. in press doi: 10.1016/j.neuroimage.2011.09.016. doi: 10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Datta P, Singh A, et al. Gene expression profiling in practitioners of Sudarshan Kriya. Journal of Psychosomatic Research. 2008;64(2):213–8. doi: 10.1016/j.jpsychores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. Journal of Neuroscience. 2010;30(9):3297–303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, et al. Effects of training of processing speed on neural systems. Journal of Neuroscience. 2011a;31(34):12139–48. doi: 10.1523/JNEUROSCI.2948-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One. 2011b;6(8):e23175. doi: 10.1371/journal.pone.0023175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences USA. 2007;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences USA. 2010;107(35):15649–52. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, et al. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. Journal of Neuroscience. 1999;19(9):3527–34. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van Beek M, Skewes J, et al. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20(2):170–4. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends in Cognitive Sciences. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral Cortex. 2011;21(2):345–56. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition. 2010;19(2):597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.