Abstract
Mindfulness-based interventions are effective for reducing depressive symptoms. However, the psychological and neural mechanisms are unclear. This study examined which facets of trait mindfulness offer protection against negative bias and rumination, which are key risk factors for depression. Nineteen male volunteers completed a 2-day functional magnetic resonance imaging study. One day utilized a stress-induction task and the other day utilized a mindful breathing task. An emotional inhibition task was used to measure neural and behavioral changes related to state negative bias, defined by poorer performance in inhibiting negative relative to neutral stimuli. Associations among trait mindfulness [measured by the Five Facet Mindfulness Questionnaire (FFMQ)], trait rumination, and negative bias were examined. Non-reactivity scores on the FFMQ correlated negatively with rumination and negative bias following the stress induction. Non-reactivity was inversely correlated with insula activation during inhibition to negative stimuli after the mindful breathing task. Our results suggest non-reactivity to inner experience is the key facet of mindfulness that protects individuals from psychological risk for depression. Based on these results, mindfulness could reduce vulnerability to depression in at least two ways: (i) by buffering against trait rumination and negative bias and (ii) by reducing automatic emotional responding via the insula.
Keywords: mindfulness, non-reactivity, rumination, negative bias, insula, fMRI
INTRODUCTION
With our increasing knowledge of the significant impact of stress on depression and other mental disorders, it becomes more and more essential to develop methods for reducing stress and depression vulnerability. A number of mindfulness-based interventions are effective in reducing stress and promoting mental health (Hofmann et al., 2010). Mindfulness refers to the self-regulation of attention as well as an orientation of openness, curiosity, and acceptance to all experiences (Bishop et al., 2004). Individuals who are more mindful in daily life (high in trait mindfulness) demonstrate better psychological health (Keng et al., 2011). In addition, numerous clinical studies have shown that Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy are effective for alleviating symptoms of medically related stress (Speca et al., 2000; Sephton et al., 2007; Rosenzweig et al., 2010), depression (Ramel et al., 2004; Pradhan et al., 2007; Bondolfi et al., 2010; van Aalderen et al., 2011) and anxiety (Craigie et al., 2008; Evans et al., 2008; Kim et al., 2009), with comparable efficacy as antidepressant medication (Teasdale et al., 2000; Kuyken et al., 2008; Segal et al., 2010) in preventing or delaying depression relapse. However, effect sizes for mindfulness-based interventions ranges from low to high depending on the population studied and the outcome measure used (Bohlmeijer et al., 2010; Hofmann et al., 2010).
Variability in the efficacy of mindfulness-based interventions may be related to a number of factors, including individual differences in trait mindfulness (Shapiro et al., 2011), varied responses to diverse mindfulness practices (Feldman et al., 2010), as well as inconsistency in measurement and conceptualization of trait mindfulness (Kuyken et al., 2008; Deyo et al., 2009; Grossman et al., 2010). Specific facets of trait mindfulness may be effective through distinct psychological and neural mechanisms (Holzel et al., 2011b). Though mindfulness is often conceptualized as a unified (Brown and Ryan, 2003; Walach et al., 2006; Chadwick et al., 2008) or two-part (Bishop et al., 2004) construct, consistent subcomponents have been identified (Baer et al., 2006). Neuroimaging studies often use a unified score to measure mindfulness and diverse cognitive and affective paradigms to examine neural mechanisms of mindfulness, which makes it difficult to interpret findings across studies. As a result, a wide range of regions have been identified, such as the dorsolateral prefrontal cortex (dlPFC) and anterior cingulate (Farb et al., 2007; Short et al., 2010), posterior cingulate, inferior or superior parietal lobe, insula (Brefczynski-Lewis et al., 2007; Hölzel et al., 2011a) and amygdala (Goldin and Gross, 2010), which highlights the need of studies exploring neural mechanisms of the subcomponents of mindfulness.
Discovering which components underlie the cognitive and emotional benefits mindfulness confers is vital to improving existing interventions or developing new interventions. The Five Facet Mindfulness Questionnaire (FFMQ) (Baer et al., 2006) is a well-received measure of trait mindfulness. Its subscales, as identified by factor analysis, include the following: non-reactivity to inner experience (non-reactivity), observing sensations/thoughts/feelings (observe), acting with awareness and concentration (act with awareness), describing experiences with words (describe) and non-judging of inner experience (non-judge) (Baer et al., 2006). In addition to being interrelated, each of the five factors may be associated with unique cognitive skills. One recent study found that participants high in non-reactivity compared with participants low in non-reactivity had better performance on a cognitive control flexibility task, and participants high in observe compared with low in observe did better on two tasks measuring perceptual ability (Anicha et al., 2012). However, it is unclear whether any of the facets of mindfulness exert a role in protecting against depression vulnerability.
Two frequently used measures of depression vulnerability are rumination and negative bias. Rumination refers to repetitive thoughts focusing on one’s symptoms, causes, meanings, and consequences of depressive symptoms. Trait rumination is a core psychopathological feature of depression and anxiety, which predicts onset and maintenance of depression (Nolen-Hoeksema, 2000). Several studies have found a decrease in rumination following a mindfulness-based intervention (Ramel et al., 2004; Jain et al., 2007; Frewen et al., 2008; Shapiro et al., 2008; Deyo et al., 2009; Raes et al., 2009; Dobkin and Zhao, 2011). The inverse relationship between trait mindfulness and rumination (Frewen et al., 2008; Raes et al., 2009; Bränström et al., 2011; Raes and Williams, 2010) also suggests that mindfulness may work by reducing rumination. In addition to rumination, depressed patients show preferential bias for negative content in attention, memory and interpretation of stimuli, known as negative bias or cognitive bias (Gotlib et al., 2004; Fritzsche et al., 2010). Experimentally, faster processing of negative stimuli and difficulty in disengaging from or in inhibiting response to negative stimuli (Joormann and Siemer, 2004; Joormann and Gotlib, 2007) has often been referred to as an indication of negative bias. Negative bias has been well documented in Beck’s cognitive theory (Beck, 1987) and supported (Lyubomirsky and Nolen-Hoeksema, 1995; Nolen-Hoeksema, 1987, 1991; Lyubomirsky et al., 1998; Joormann et al., 2010;) as a behavioral marker of depression vulnerability. Individuals who are vulnerable to depression tend to develop negative bias under mild stress (Bolger and Schilling, 1991; Kendler et al., 2004; Wichers et al., 2007). We reason that if mindfulness has a protective effect against depression vulnerability, individuals with high mindfulness skills may have low trait rumination and show less negative bias following a mild stressor.
In this study, we used a 2-day design to examine the impact of trait mindfulness and rumination on negative bias during an emotional inhibition task following stress vs mindfulness tasks. The go/no-go task is one of the most frequently used paradigms in studying inhibition processing (Simmonds et al., 2008). The task requires participants to press a button to a go stimulus and withhold pressing to a no-go stimulus. Because the go stimuli appear very frequently, participants typically develop a tendency to respond to each stimulus. As a result, effort is needed to withhold the button when the infrequent no-go stimulus appears. The emotional go/no-go (EGNG) task can examine the ability to inhibit responses to negative relative to neutral stimuli by measuring relative inhibition accuracy, i.e. how accurate a participant is in withholding a response to negative vs neutral no-go stimuli (Feder et al., 2011; Gopin et al., 2011). Negative bias, defined as poorer performance in inhibiting responses to negative relative to neutral stimuli, has been observed in depressed patients (Eugene et al., 2010; Joormann et al., 2010). Our goal is to understand which facets of trait mindfulness confer protection from rumination and stress-induced negative bias and whether those facets are effective through ‘top-down’ effortful inhibition associated with greater activation in the right inferior frontal cortex (IFC), a region that has been associated with cognitive inhibition of negative stimuli (Aron and Poldrack, 2005; Dolcos et al., 2006), or through a lesser response to negative stimuli associated with reduced activation in the affective system (i.e. amygdala and insula). The results of the study will help clarify the psychological and neural mechanisms of mindfulness and provide direction for improving existing mindfulness therapies designed to treat and prevent depression and other psychological disorders.
METHOD
Participants
Because of the known variation of stress sensitivity across the menstrual cycle (Ossewaarde et al., 2010), only male subjects were recruited in the study. Nineteen healthy male participants completed the study with mean (s.d.) age of 27.05 (7.21) years. Participants were recruited from the subject registry at the Duke-UNC Brain Imaging and Analysis Center. Individuals with magnetic resonance imaging (MRI) contraindications, current or history of neurological and psychiatric disorders, drug abuse, and current medication use were excluded from the study. The study was approved by the Duke University Health System Institutional Review Board. All participants provided written consent.
Procedures
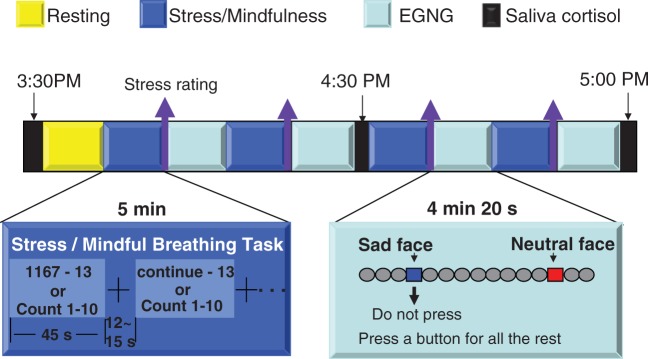
The experiment took place over two days separated by 7–10 days. A stress induction task was administered on one day and a mindful breathing task was administered on the other day. The order of stress and mindfulness tasks was counterbalanced among the participants. Each day was composed of a pre-scan session and a functional MRI (fMRI) scan session. In the pre-scan session, participants completed the questionnaires (see the questionnaire section below), and practiced the stress or mindful breathing task as well as the EGNG task. The scanning session was composed of an anatomical scan, a resting state scan, and four pairs of stress (or mindful breathing) task and EGNG task runs (Figure 1). To evaluate stress level, changes in heart rate, respiration rate, and cortisol level were measured during the stress and mindful breathing tasks. In addition, self-ratings of stress were obtained immediately after the completion of each stress or mindful breathing task run. Salivary cortisol levels were measured at the beginning, middle, and end of each fMRI scan session. We tried to minimize the factors affecting cortisol variation by asking subjects to abstain from caffeine, smoking and exercise 2 h prior to scanning. All fMRI scans were completed in the late afternoon because the cortisol level is relatively low and stable during these hours and is therefore more susceptible to stimulation (Jansen et al., 1998).
Fig. 1.
Illustration of the task flow. Overview of the fMRI sequence of paired stress/mindfulness tasks and the EGNG (emotional go/no-go) task runs.
Questionnaires
The Beck Depression Inventory II (Beck et al., 1996) was used to screen for depression. Participants who scored above 13 were excluded to ensure all participants had minimal depression symptoms (Beck et al., 1996) in order to reduce any confounding effects of significant depression symptoms on stress reactivity or negative bias. Additional questionnaires included the following: the FFMQ (Baer et al., 2006), as described in the introduction, a 39-item self-report questionnaire measuring trait mindfulness; the Ruminative Response Scale (RRS) (Nolen-Hoeksema, 1991), a 22-item self-report questionnaire to measure trait rumination and the Perceived Stress Scale (PSS, Cohen et al., 1983) to measure perceptions of life stress and coping ability. The PSS has been used in studies assessing the effectiveness of stress-reduction interventions (Holzel et al., 2010) and has been found to predict increased risk for depression (Carpenter et al., 2004). To ensure baseline mood and state anxiety were stable between the 2 experimental days, on each experimental day prior to the fMRI session, the Positive Affect and Negative Affect Scale (PANAS) (Watson et al., 1988) and the Spielberger State and Trait Anxiety Inventory (STAI-state) (Spielberger et al., 1983) were administered.
Experimental design
The task for the scanning sessions was composed of four stress induction or mindfulness task runs paired with four EGNG task runs on each day (Figure 1).
Stress induction task
We used a mental arithmetic (Soufer et al., 1998; Wang et al., 2005) paradigm to induce stress similar to the Trier Social Stress Test (Kirschbaum et al., 1993). At the beginning of a run, participants were given a four-digit starting number and a two-digit integer to serially subtract from the starting number. These instructions were presented for 5 s. Participants subtracted continuously during the run which was broken into five 45 s blocks. The subtraction was temporarily paused when a fixation cross (jittered from 12 s to 16 s) was presented. Each run lasted for 5 min. At the completion of the run, participants reported the final subtraction value. Each run started with a different number and participants subtracted a different integer from the starting number during each run. Subjects were instructed to rate their stress level according to a 1–4 analogue bar (with 1 being the lowest and 4 being the highest) at the end of each induction.
Mindful breathing task
In the mindful breathing task, participants were instructed to (i) focus your attention on the bodily sensations of breathing and count breaths from 1 to 10; (ii) notice if your mind has wandered and return to counting when your mind wanders and (iii) do not be frustrated when your mind wanders, but simply return attention to breathing. These instructions mirror a commonly used mindfulness meditation practice (Hanh, 1976). Participants practiced the mindful breathing task before beginning the scan session and were given the opportunity to ask questions or receive feedback on the task before the scan. For both tasks, participants paused from the stress or mindfulness tasks when a fixation cross was displayed on the screen.
Emotional inhibition task
There were three types of stimuli in the EGNG task, shown in Figure 1: emotionally neutral face images, emotionally negative face images, and scrambled images of the negative and neutral face images. Emotional images were taken from the International Affective Picture System (Lang et al., 1999), our previous experiments, and the Internet (http://www.lifestockphotos.com). In an EGNG run, the frequency of scrambled images was 80%, negative images 10%, and neutral images 10%, with negative and neutral images randomly distributed in a run. Each scrambled image was presented for 1.8 s and each negative and neutral picture was presented for 2.6 s. The task for subjects was to press a button with their right index finger (go trials) for all scramble pictures and one type of emotional face images (negative or neutral) and inhibit their response to the other type of emotional face images depending on the instructions at the beginning of the run. The duration between two face images (i.e. our interested events) was jittered from 5.4 s to 10.8 s pseudo-randomly. The jittered timing duration was the same for both negative and neutral stimuli across runs and across all participants. The run order (i.e. no-go negative or no-go neutral first) was counterbalanced across days and across participants. Each EGNG run lasted for a total duration of 4.3 min. Subjects rated the valence of all the face pictures (neutral or negative) at the completion of the scan. Overall, participants’ ratings matched our a priori picture categories well, with group mean (s.d.) matching rates of 92% (0.07) for negative and 97% (0.06) for neutral pictures across the 2 days. There was no significant rating difference between days for either negative (t18 = –0.850, P = 0.41) or neutral (t18 = –1.202, P = 0.246) pictures. Picture rating data from three participants were lost due to technical problems.
Biochemical and physiological measures
Salivary cortisol was collected during three points in our protocol: before the participant entered the scanner, at the midpoint of the scanning session and immediately after the participant exited the scanner. Participants were given a Salivette (Sarstedt AG & Co., Germany) and were instructed to place it in their mouth for 90 s. Salivettes were sealed immediately after each collection and placed in frozen storage at the end of the scanning session. Samples were freed from mucopolysaccharides and other residuals by three freeze–thaw cycles followed by centrifugation. Salivary cortisol levels were assessed with solid-phase Coat-A-Count 125I radioimmunoassays for Cortisol (TKCO) provided by Siemens Healthcare Diagnostics (Los Angeles, CA, USA). The procedures were identical to our previous work (Schultheiss and Stanton, 2009; Stanton et al., 2009). Assay reliability was evaluated by including control samples with known hormone concentrations in each assay (Bio-Rad Lyphochecks from Bio-Rad Laboratories, Hercules, CA, USA). Analytical sensitivity (B0 −3 s.d.) was 0.02 ng/ml. The intra-assay cortisol coefficients of variability (CV) for samples of known concentration was 14.4% (1.5 ng/ml) and 4.1% (3.5 ng/ml). Participants’ three saliva samples were counted in duplicate and had a mean intra-assay CV of 5.96%.
Heart rate and respiration rate were continuously monitored during scanning using a pulse oximeter and a chest belt, respectively (Biopac Systems, Goleta, CA, USA).
Image acquisition and analysis
All images were acquired with a 3.0 Tesla GE MR750 scanner at the Duke-UNC Brain Imaging and Analysis Center. After an initial localizer scan was completed, a T1-weighted spoiled gradient-recalled echo anatomical image (matrix = 256 × 256 × 180, 1 mm3) was acquired with slices in the horizontal plane parallel to the anterior and posterior commissures (AC-PC) line. The 5 min resting state and stress/mindfulness induction tasks were acquired with an arterial spin labeling sequence to investigate individual differences in baseline perfusion level (results were not included here). For the functional (EGNG task) runs, we acquired 34 slices of images in the AC-PC plane using a SENSE inverse-spiral pulse sequence. Our sequence was composed of time to echo (Echo Time = 30 ms, Repetition Time = 2000 ms, Field of View = 15.5 cm2, matrix = 64 × 64 × 34, 3.8 mm3).
All analysis was carried out using FMRI Expert Analysis Tool Version 5.92, part of the FSL analysis package (FMRIB’s Software Library; www.fmrib.ox.ac.uk/fsl). The following standard preprocessing steps were taken: removal of non-brain signal outside the head using the Brain Extraction Tool, slice-time correction, co-registration, motion correction, normalization, spatial smoothing (5 mm FWHM) and high-pass filtering (1/60 Hz). The general linear model (GLM) was used at the first-level analysis including the following explanatory variables (EVs) with scrambled image trials as the baseline: correct go trials, error go trials, correct no-go trials and error no-go trials. Our data analyses in higher levels were focused on the correct EV constructed contrasts: negative vs neutral go and negative vs neutral no-go. We also subsequently analyzed response to negative go and negative no-go to ensure that significant results were induced by negative rather than neutral stimuli. The within-subject between-day differences (induction effect) for each EV were computed at the second level using a fixed-effect model. The induction effect for each subject was input for the third-level group analysis using random effect model (FLAME1). To examine the association of self-reported mindfulness (a FFMQ facet) and rumination (RRS) with the blood-oxygenation-level-dependent (BOLD) signal, we also input each subject’s demeaned value for these measures as regressors in the GLM model. For all analyses, significance was determined using a voxel significance level of z > 2.3, with a whole-brain-corrected cluster significance threshold of P < 0.05.
Each significant cluster from our regression analyses in the third-level analysis was extracted as Region-of-Interest (ROI). Given that the significant clusters of bilateral insula extended to IFC, only voxels of significant cluster within anatomically defined insula region (Harvard–Oxford probability Atlas with probability of insula > 25%) were used for the insula ROI. The mean signal strength with each ROI for each subject was calculated using FSL’s featquery tool. The ROI values were used for illustrative purposes from the whole-brain analysis and to test for significant relationships on a different data set (e.g. defining ROIs from the mindfulness day analysis and doing significance testing on the stress day).
RESULTS
Task validation and behavioral results
Validation of the stress induction and mindfulness tasks
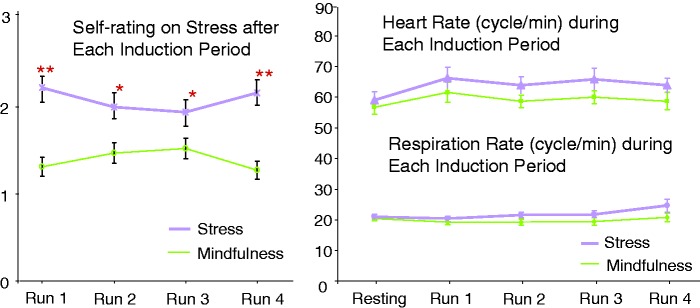
There was no pre-scan difference between the 2 days in positive affect as measured by the PA scale of the PANAS, t18 = –0.04, P = 0.97, negative affect as measured by the NA scale of the PANAS, t18 = –0.15, P = 0.88, or state anxiety as measured by the state STAI, t18 = –0.79, P = 0.44. The physiological measures during the induction period and self-ratings validated our stress and mindfulness tasks. Specifically, average salivary cortisol and heart rate across the three time points were higher for stress induction than the mindfulness task (Table 1). As expected, stress ratings were higher following the stress task than the mindful breathing task (Table 1; Figure 2).
Table 1.
Physiological and Behavioral Data During and After the Stress and Mindful Breathing Tasks
| Measure | Mindfulness session mean (s.d.) | Stress session mean (s.d.) | Stress vs mindfulness (paired t-test) |
|---|---|---|---|
| Physiological responses | |||
| Respiration (breaths per min) | 19.34 (4.77) | 21.98 (4.53) | t = –1.34, P = 0.20 |
| Heart rate (beats per min) | 59.61 (0.15) | 64.88 (0.20) | t = –2.91, P = 0.01 |
| Cortisol (ng/ml) | 1.30 (0.40) | 1.70 (0.60) | t = –3.67, P < 0.01 |
| Stress rating | 1.46 (0.38) | 2.08 (0.54) | t = –6.06, P < 0.01 |
| Performance accuracy on the EGNG task post stress and mindfulness | |||
| Nogo Neg | 0.87 (0.09) | 0.84 (0.09) | t = 0.59, P = 0.56 |
| Nogo Neu | 0.89 (0.10) | 0.93 (0.08) | t = –1.09, P = 0.29 |
| Negative Bias Nogo (Neu-Neg) | 0.08 (0.12) | 0.11 (0.11) | t = 1.23, P = 0.24 |
| Go Neg | 0.90 (0.10) | 0.88 (0.06) | t = 0.75, P = 0.47 |
| Go Neu | 0.91 (0.10) | 0.93 (0.06) | t = –1.3, P = 0.21 |
Note: Accuracy ratings reflect the proportion of trials correctly inhibited (nogo trials) or responded to (go trials.) Neg = negative images, Neu = neutral images.
Fig. 2.
Stress levels following the stress and mindful breathing tasks across runs (left) and heart rate (upper right) as well as respiratory rate (lower right) during the stress and mindful breathing tasks. *paired t-test between the stress and mindful breathing task within a run, P < 0.005; **paired t-test between the stress and mindful breathing task within a run, P < 0.001.
Behavioral performance on the inhibition task and the influence of trait mindfulness and rumination
Task performance accuracy on the experimental days is reported in Table 1. Repeated measures analysis of variance on task performance accuracy using day (stress and mindfulness), emotional valence (negative and neutral) and task (go and no-go) as predictors revealed a significant emotional valance effect (F1,36 = 40.13, P < 0.01). Participants had worse behavioral performance (i.e. poorer inhibitory control) to negative than neutral stimuli (Bonferroni post hoc test, t = –6.37, P < 0.01) across task conditions and across the 2 days, without task or day interactions.
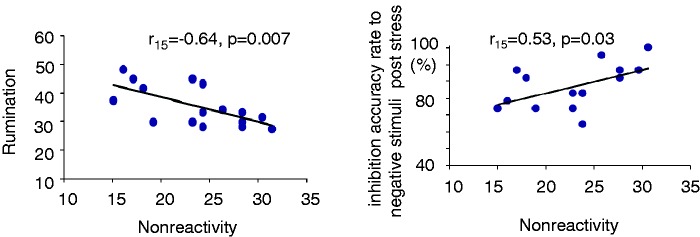
Multiple regression analysis revealed that among the five facets of the FFMQ, only non-reactivity was significantly and inversely correlated with rumination and perceived stress (Tables 2 and 3; Figure 3). Therefore, we further studied whether non-reactivity demonstrated a protective effect from stress and negative bias, particularly from poor inhibition accuracy to no-go negative stimuli. First, individuals higher in non-reactivity showed slower respiration rate during performing the stress induction task (Table 3). Second, non-reactivity was inversely correlated with negative bias (no-go neutral—negative, r16 = –0.55, P = 0.03) following the stress task but not following the mindful breathing task (no-go neutral > negative, r16 = 0. 21, P = 0.45). Third, after both stress and mindful breathing tasks, higher non-reactivity was correlated with better inhibition accuracy rate for negative images (stress, r16 = 0.53, P = 0.03; mindfulness, r16 = 0.50, P = 0.05) but not for neutral images (stress, r16 = –0.06, P = 0.83; mindfulness, r16 = –0.09, P = 0.73). Therefore, the inverse correlation between non-reactivity and negative bias scores under stress was due to improved accuracy for negative images, not impaired performance for neutral images. In summary, our subtle stressful task vs mindfulness task did not support a significant day × emotional valence × task interaction effect on negative bias. Rather, we found an effect of individual differences associated with non-reactivity on negative bias under stress.
Table 2.
Means and Intercorrelations between Measures of Mindfulness, Rumination and Perceived Stress
| Measure | Mean (s.d.) | Correlation with RRS using the general linear regression model |
|
|---|---|---|---|
| F or t value | P value | ||
| FFMQ total | 129.81 (19.60) | F = 3.242 | 0.06 |
| Observe | 26.38 (5.18) | t = –1.738 | 0.116 |
| Describe | 27.56 (5.89) | t = –1.432 | 0.186 |
| Act with awareness | 22.31 (6.50) | t = –1.493 | 0.170 |
| Non-judge | 26.75 (7.48) | t = 1.701 | 0.123 |
| Non-reactivity | 23.39 (5.09) | t = –2.394 | 0.040 |
| PSS | 9.81 (3.71) | — | — |
| RRS | 32.56 (8.38) | — | — |
Note: FFMQ = Five Factor Mindfulness Scale; PSS = Perceived Stress Scale; RRS = Ruminative Response Scale.
Table 3.
Correlation of Behavioral Measures with Non-reactivity (Pearson’s Coefficient Correlation)
| Measure | r value | P value |
|---|---|---|
| Rumination | –0.64 | <0.01 |
| Perceived stress | –0.55 | 0.03 |
| Respiration rate under stress | –0.63 | 0.01 |
| Negative bias post mindfulness | –0.21 | 0.45 |
| Negative bias post stress | –0.55 | 0.03 |
Fig. 3.
Scatterplots revealing the relationship between non-reactivity and trait rumination (left) as well as the relationship between non-reactivity and inhibition accuracy for negative no-go trials (right).
To further explore individual differences in performance of inhibition control, we compared negative bias (the inhibition accuracy difference between neutral and negative no-go stimuli) between individuals with high non-reactivity and individuals with low non-reactivity using a median split of non-reactivity scores. Indeed, participants with high non-reactivity had less negative bias than those with low non-reactivity under the stress condition (two-sample t-test, t14 = 2.72, P = 0.02), but not under the mindfulness condition (two-sample t-test, t14 = 1.54, P = 0.15). These findings together demonstrated a protective effect of non-reactivity on stress-induced negative bias.
Neuroimaging results
Main effect of the emotional inhibition task and main effect of stress induction and mindfulness tasks
The primary contrasts of interest were negative > neutral go trials (i.e. reactivity to negative stimuli) and negative > neutral no-go trials (i.e. inhibition and/or reactivity to negative stimuli). Across the 2 days, the following brain regions showed a main effect of activation to the negative > neutral go contrast: dorsomedial prefrontal cortex, bilateral inferior-orbital frontal area (IFC/OFC, BA47), bilateral anterior insula and right visual cortex area. For the negative > neutral no-go contrast, activation was found in bilateral inferior frontal (IFC) and inferior frontal–orbital area (IFC/OFC, BA47), bilateral anterior insula, bilateral middle temporal cortex and occipital-temporal junction area (supplementary Table1). The activation to negative > neutral go and no-go contrasts overlapped in bilateral insula (supplementary Figure 1) indicating an association of the insula with negative information processing.
We did not find any significant difference in brain activation following stress vs mindfulness task with either of the contrasts. Given our prior interest in affective-processing-related regions, we conducted an exploratory ROI analysis on structurally defined amygdala using the Harvard–Oxford probability Atlas (voxels with probability >25% as amygdala). The analysis revealed that following the stress task, amgydala activation to negative > neutral go contrast was significantly greater than activation to negative > neutral go contrast following the mindful breathing task (neg-neu go contrast, paired t-test, right amygdala, t16 = 2.32, P = 0.03; left amygdala, t16 = 0.57, P = 0.57, supplementary Figure 2). There was no significant difference in amygdala activation in response to negative > neutral no-go stimuli following stress vs mindful breathing task.
Correlation of non-reactivity and rumination with the emotional inhibition task
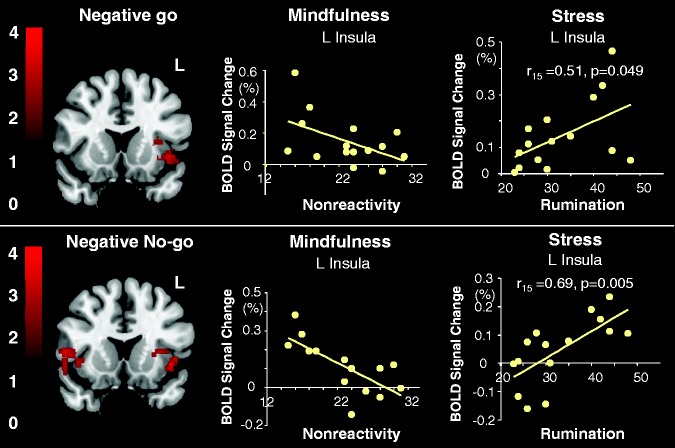
To understand the association of non-reactivity and rumination with neural responses to inhibition accuracy, we conducted regression analyses on negative and neutral stimuli independently. Following the mindful breathing task, whole-brain voxelwise regression analyses revealed that higher non-reactivity was not associated with activation in the IFC, rather it was negatively correlated with brain activation in the bilateral anterior insula in response to the negative no-go trials and in the left insula in response to negative go trials during the EGNG task (Figure 4; Table 4). The scatter plot from the ROI analysis confirmed that the regression was not driven by outliers (Figure 4). On the contrary, rumination was correlated positively with activation in bilateral anterior insula for negative go trials. Following the stress task, whole-brain analyses did not reveal any correlations between brain activation with non-reactivity or rumination. Given our interest in stress-induced neural responses, we used the significant clusters of the left and right insula identified post mindful breathing task as ROIs to conduct regression analyses following the stress task. We found that rumination was correlated positively with activation in the left anterior insula for negative no-go trials (Figure 4). No significant correlation was found between non-reactivity or rumination with brain activation in response to neutral go or neutral no-go stimuli. Furthermore, using a multiple regression model, we found that the correlation of trait rumination with activation to negative no-go stimuli, but not with activation to neutral no-go stimuli, explains the significance of the regression (F1,15 = 5.33, P = 0.02; negative no-go, t = 3.26, P = 0,007; neutral, t = 1.04, P = 0.31). No significant differences were found between negative and neutral contrasts in response to go stimuli following either the stress or mindful breathing tasks.
Fig. 4.
The relationship among insular activation, trait non-reactivity, and trait rumination. Top panel image (left): significant clusters from voxelwise whole-brain analysis revealing a negative correlation between non-reactivity and activation in the left insula while engaging in negative stimuli (negative go) after the mindful breathing task. Top panel scatterplots: results from ROI analyses to illustrate correlation between non-reactivity and left insula activation after mindfulness (middle) and correlation between rumination and left insula activation after stress (right). Bottom panel image (left): significant clusters from whole-brain voxelwise analysis revealing negative correlation between non-reactivity and activation during inhibiting negative stimuli (negative no-go) after the mindful breathing task. Bottom panel scatterplots: results from ROI analyses to illustrate correlation between non-reactivity and left insula activation after mindful breathing (middle) and correlation between rumination and left insula activation after stress (right). Note that the left insula ROI in each scatterplot was the significant cluster in the whole-brain analysis revealing a negative correlation between non-reactivity and activation in the left insula while engaging in both negative go and negative no-go stimuli after the mindful breathing task (top left brain image). Only the voxels of the significant cluster within anatomically defined insula region (Harvard–Oxford probability Atlas with probability of insula >25%) were used as the insula ROI. The mean signal strength within this left insula ROI for each task condition for each subject was calculated using FSL’s featquery tool. The Pearson’s correlation coefficients were computed for the correlation between insular activation and non-reactivity/rumination using a statistical threshold of r > 0.5 and P < 0.05 (two-tailed).
Table 4.
Regions Correlating with Non-reactivity or Rumination During the Voxel-Based Whole Brain Analysis (Cluster Corrected, z > 2.3, P < 0.05, Coordinates Are in MNI Space)
| Contrast | Region | Brodman’s area | Peak voxel co-ordinates (x, y, z) MNI | Cluster size | Zpeak value |
|---|---|---|---|---|---|
| Negative correlations with non-reactivity post mindful breathing task | |||||
| Neg Go | Left insula and IFC | 13, 44 | –42, 6, 14 | 311 | 3.33 |
| Neg Nogo | Right insula and IFC | 13, 44 | 48, 10, 10 | 284 | 3.73 |
| Left insula | 13 | –32, 24, 6 | 281 | 3.88 | |
| Positive correlations with rumination post stress task | |||||
| Neg Go | Right insula and IFC | 13, 45 | 36, 26, 8 | 471 | 3.77 |
| Left insula and IFC | 13, 44, 45 | -28, 14, 6 | 456 | 3.6 | |
Note: IFC = inferior frontal cortex.
DISCUSSION
The aim of the study was to examine whether and how specific facets of mindfulness play a protective role against depression vulnerability. We found that, among the five facets of the FFMQ, higher non-reactivity was inversely correlated with depression vulnerability, indicated by low rumination and less negative bias (i.e. better ability to inhibit a behavioral response to negative emotions). On the neural level, we did not find a significant correlation between non-reactivity and activation in the right IFC. Instead, non-reactivity was negatively correlated with activation in the left anterior insula during inhibiting and engaging in negative stimuli after the mindful breathing task, whereas rumination was positively correlated with activation in bilateral anterior insula activation after the stress task. These findings indicate that trait non-reactivity is a critical component of mindfulness that could protect against negative bias by reducing automatic emotional responding to negative stimuli reflected by reduced anterior insula activation under stress. Taken together, the data suggest plausible psychological and neural mechanisms that could explain how a specific facet of mindfulness—non-reactivity to negative stimuli—might buffer vulnerability to depression.
There are studies which have found greater cortical thickness in meditators compared with non-meditators in the right insula (Lazar et al., 2005; Hölzel et al., 2008) and other regions. Using different cognitive and affective paradigms, increased and decreased insular activation has also found to be associated with dispositional mindfulness or post-intervention mindfulness (Kumar et al., 2008; Ives-Deliperi et al., 2010; Slagter et al., 2011; Zeidan et al., 2011). To our knowledge, this study is the first to examine the neural mechanisms for subcomponents of mindfulness in protection against negative bias. The majority of neuroimaging studies on mindfulness in the literature used a unified score to measure mindfulness and have found increases in activation in attentional and executive function regions such as the superior/inferior parietal lobe (Brefczynski-Lewis et al., 2007), dlPFC and dorsal anterior cingulate cortex (Farb et al., 2007; Ives-Deliperi et al., 2010; Manna et al., 2010). However, in our examination of facets of mindfulness as measured by the FFMQ, we did not find a correlation between non-reactivity and activation in the executive control regions (dlPFC or IFC). Rather, non-reactivity was correlated with less activation to negative stimuli in the insula. Non-reactivity is the tendency to notice thoughts and emotions without getting engrossed in them and without reacting automatically (Baer et al., 2006). We did not find support for a relationship between non-reactivity and effortful ‘top-down’ regulation of negative bias. Non-reactivity may reflect less automatic emotional response via less activation in the anterior insula.
There is ample evidence supporting the insula as the interoceptive cortex representing emotional arousal, feelings, empathy and internal body state and reflecting visceral states associated with emotional experiences (Damasio et al., 2000; Craig, 2003; Critchley et al., 2004; Singer et al., 2009). Low insula activation to negative stimuli in our study suggests that individuals with high non-reactivity scores may possibly use interoception to regulate automatic emotional responding. This result is consistent with recent experimental evidence linking trait mindfulness and decreased emotional reactivity (e.g. Brown et al., 2012). The amygdala is often activated by emotionally salient stimuli and has been associated with emotional arousal. The fact that non-reactivity was associated with insula activation but not amygdala activation also supports our speculation that non-reactivity is effective through interoception to regulate automatic emotional responding.
Our overarching hypothesis is that different mindfulness skills are related to different cognitive processes as they relate to emotional responding (Slagter et al., 2011). Each facet of mindfulness may have its own neural mechanism and confer different cognitive or emotional benefits. Our study does not imply that non-reactivity is superior to other facets of mindfulness. Rather, we recognize that non-reactivity was uniquely related to rumination and negative bias in this relatively small sample of healthy young males, which indicates its potential usefulness protecting against stress and depression vulnerability. Our findings warrant future studies in both males and females to confirm these results.
The major limitation of the study is that although we found a significant correlation between non-reactivity and insula activation to negative go and no-go stimuli, but not to neutral go or no-go stimuli, we did not find the correlation using the direct negative > neutral contrast in the whole-brain voxelwise analysis. Rather, the inverse relationship we found between nonreactivity and activation in the insula in the whole-brain voxelwise analysis was confirmed in a post hoc multiple regression analysis with the insula ROI (F1,15 = 6.19, P = 0.01; negative no-go, t = 3.27, P = 0.007; neutral, t = 1.35, P = 0.20). Because the post-hoc test on the insula activation can increase type I error, our finding that nonreactivity influences processing of negative but not neutral stimuli needs to replicated. Therefore, to further confirm whether non-reactivity was associated with negative bias on the behavioral level, future studies using larger sample size are necessary.
Another caveat of the study is that although we requested participants abstain from smoking (which might increase participants stress level for smokers), we did not include formal smoking measures. The study also lacked ratings of stress at baseline before the stress or mindful breathing tasks. This omission prohibited us from drawing conclusions about a specific stress-inducing effect of the stress task and/or stress-reducing effect of the mindful breathing task. However, our measures of positive and negative affect, state anxiety, heart rate and respiratory rate and cortisol were all comparable at baseline, which indicated that the pre-task stress levels were likely comparable between the two task sessions.
We did not formally collect information regarding prior mindfulness meditation experience, although the majority of study participants informally mentioned that they were meditation naive. Future studies should consider measuring the relationship between past mindfulness experience, trait mindfulness, and task-based measures of negative bias. In addition, future studies could compare training in mindfulness skills (e.g. non-reactivity) vs other emotion regulation skills, such as reappraisal, in novices to clarify the neural mechanisms associated with different pathways to reducing negative bias.
In summary, this study is unique in that it suggests the trait non-reactivity facet of mindfulness offers cognitive protection from rumination and negative bias on a task explicitly involving the interaction of emotion and cognition, and does so using a region of the brain traditionally involved with interoceptive awareness. These results suggest that cultivating non-reactivity through formal meditation practice or other mindfulness training techniques could offer protection from depression. Thus, current or new interventions may benefit from adding or increasing components that foster non-reactivity through mindfulness practices.
Conflict of Interest
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Paul B. Beeson Career Development Awards (K23-AG028982) from the National Institute on Aging (NIA) and a National Alliance for Research in Schizophrenia and Depression Young Investigator Award (L.W.).
M.J.S.’s effort was supported by K23-MH087754 from the National Institute of Mental Health (NIMH). J.M.G.’s effort was supported by R00-AT004945 from the National Center for Complementary & Alternative Medicine (NCCAM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NIMH, NCCAM, or the National Institutes of Health.
REFERENCES
- Anicha CL, Ode S, Moeller SK, Robinson MD. Toward a cognitive view of trait mindfulness: distinct cognitive skills predict its observing and nonreactivity facets. Journal of Personality. 2012;80(2):255–85. doi: 10.1111/j.1467-6494.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Baron Short E, Kose S, Mu Q, et al. Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evidence-Based Complementary and Alternative Medicine. 2010;7(1):121–7. doi: 10.1093/ecam/nem163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bishop SR, Lau M, Shapiro S, et al. Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice. 2004;11(3):230–41. [Google Scholar]
- Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. Journal of Psychosomatic Research. 2010;68(6):539–44. doi: 10.1016/j.jpsychores.2009.10.005. [doi: DOI: 10.1016/j.jpsychores.2009.10.005] [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59(3):355–86. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, der Linden MV, et al. Depression relapse prophylaxis with Mindfulness-Based Cognitive Therapy: replication and extension in the Swiss health care system. Journal of Affective Disorders. 2010;122(3):224–31. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränström R, Duncan LG, Moskowitz JT. The association between dispositional mindfulness, psychological well-being, and perceived health in a Swedish population-based sample. British Journal of Health Psychology. 2011;16(2):300–16. doi: 10.1348/135910710X501683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Goodman RJ, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Social, Cognitive, and Affective Neuroscience. 2012 doi: 10.1093/scan/nss004. doi: 10.1093/scan/nss004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84(4):822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29(4):777–84. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Chadwick P, Hember M, Symes J, Peters E, Kuipers E, Dagnan D. Responding mindfully to unpleasant thoughts and images: reliability and validity of the Southampton mindfulness questionnaire (SMQ) The British Journal of Clinical Psychology. 2008;47(Pt 4):451–5. doi: 10.1348/014466508X314891. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–96. [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craigie MA, Rees CS, Marsh A, Nathan P. Mindfulness-based cognitive therapy for generalized anxiety disorder: a preliminary evaluation. Behavioural and Cognitive Psychotherapy. 2008;36(5):553–68. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. [10.1038/nn1176] Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? Explore (New York, N.Y.) 2009;5(5):265–71. doi: 10.1016/j.explore.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Dobkin PL, Zhao Q. Increased mindfulness—the active component of the mindfulness-based stress reduction program? Complementary Therapies in Clinical Practice. 2011;17(1):22–7. doi: 10.1016/j.ctcp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17(15):1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Eugène F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Research. 2010;181(1):30–5. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. Journal of Anxiety Disorders. 2008;22(4):716–21. doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Skipper J, Blair JR, et al. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biological Psychiatry. 2011;69(8):804–7. doi: 10.1016/j.biopsych.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman G, Greeson J, Senville J. Differential effects of mindful breathing, progressive muscle relaxation, and loving-kindness meditation on decentering and negative reactions to repetitive thoughts. Behaviour Research and Therapy. 2010;48(10):1002–11. doi: 10.1016/j.brat.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Evans EM, Maraj N, Dozois DJA, Partridge K. Letting go: mindfulness and negative automatic thinking. Cognitive Therapy and Research. 2008;32(6):758–74. [Google Scholar]
- Fritzsche A, Dahme B, Gotlib IH, et al. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychological Medicine. 2010;40(5):815–26. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopin CB, Burdick KE, Derosse P, Goldberg TE, Malhotra AK. Emotional modulation of response inhibition in stable patients with bipolar I disorder: a comparison with healthy and schizophrenia subjects. Bipolar Disorders. 2011;13(2):164–72. doi: 10.1111/j.1399-5618.2011.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):121–35. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141–9. doi: 10.1212/WNL.0b013e3181f4d80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanh TN. The Miracle of Mindfulness: An Introduction to the Practice of Meditation. Boston, MA: Beacon Press; 1976. [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5(1):11–7. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research. 2011a;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Lazar SW, Gard T, Shuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Sciences. 2011b;6:537–59. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Social Neuroscience. 2010;6:231–42. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden JA, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophrenia Research. 1998;33(1–2):87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116(1):80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Nee DE, Berman MG, Jonides J, Gotlib IH. Interference resolution in major depression. Cognitive Affective and Behavioral Neuroscience. 2010;10(1):21–33. doi: 10.3758/CABN.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: difficulties in repairing sad mood with happy memories? Journal of Abnormal Psychology. 2004;113(2):179–88. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. American Journal of Psychiatry. 2004;161(4):631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review. 2011;31(6):1041–56. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Lee SH, Choi TK, et al. Effectiveness of mindfulness-based cognitive therapy as an adjuvant to pharmacotherapy in patients with panic disorder or generalized anxiety disorder. Depression and Anxiety. 2009;26(7):601–6. doi: 10.1002/da.20552. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test' a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kumar S, Feldman G, Hayes A. Changes in mindfulness and emotion regulation in an exposure-based cognitive therapy for depression. Cognitive Therapy and Research. 2008;32(6):734–44. [Google Scholar]
- Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–78. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Lang PJ, et al. Gainesville: University of Florida, Center for Research in Psychophysiology; 1999. International affective picture system (IAPS): Technical manual and affective ratings. [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality and Social Psychology. 1998;75(1):166–77. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology. 1995;69(1):176–90. doi: 10.1037//0022-3514.69.1.176. [DOI] [PubMed] [Google Scholar]
- Manna A, Raffone A, Perrucci MG, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Research Bulletin. 2010;82(1–2):46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychologial Bulletin. 1987;101(2):259–82. [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–82. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–11. [doi:10.1037/0021-843X.109.3.504] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Pradhan EK, Baumgarten M, Langenberg P, et al. Effect of mindfulness-based stress reduction in rheumatoid arthritis patients. Arthritis and Rheumatism. 2007;57(7):1134–42. doi: 10.1002/art.23010. [DOI] [PubMed] [Google Scholar]
- Raes F, Dewulf D, Van Heeringen C, Williams JM. Mindfulness and reduced cognitive reactivity to sad mood: evidence from a correlational study and a non-randomized waiting list controlled study. Behaviour Research and Therapy. 2009;47(7):623–7. doi: 10.1016/j.brat.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Raes F, Williams J. The relationship between mindfulness and uncontrollability of ruminative thinking. Mindfulness. 2010;1(4):199–203. [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, McQuaid JR. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognitive Therapy and Research. 2004;28(4):433–55. [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. Journal of Psychosomatic Research. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [doi: DOI: 10.1016/j.jpsychores.2009.03.010] [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Stanton SJ. Assessment of salivary hormones. In: Harmon-Jones E, Beer JS, editors. Methods in Social Neuroscience. New York: Guilford Press; 2009. pp. 17–44. [Google Scholar]
- Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry. 2010;67(12):1256–64. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Salmon P, Weissbecker I, et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis and Rheumatism. 2007;57(1):77–85. doi: 10.1002/art.22478. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Brown KW, Thoresen C, Plante TG. The moderation of mindfulness-based stress reduction effects by trait mindfulness: results from a randomized controlled trial. Journal of Clinical Psychology. 2011;67(3):267–77. doi: 10.1002/jclp.20761. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Oman D, Thoresen CE, Plante TG, Flinders T. Cultivating mindfulness: effects on well-being. Journal of Clinical Psychology. 2008;64(7):840–62. doi: 10.1002/jclp.20491. [DOI] [PubMed] [Google Scholar]
- Short EB, Kose S, Mu Q, et al. Regional brain activation during meditation shows time and practice effects: an exploratory FMRI study. Evidence-Based Complementary and Alternative Medicine. 2010;7(1):121–127. doi: 10.1093/ecam/nem163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Davidson RJ, Lutz A. Mental training as a tool in the neuroscientific study of brain and cognitive plasticity. Frontiers in Human Neuroscience. 2011;5:17. doi: 10.3389/fnhum.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufer R, Bremner JD, Arrighi JA, et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proceeings of the National Academy of Sciences of the United States of America. 1998;95(11):6454–9. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic Medicine. 2000;62(5):613–22. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the Statie-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Stanton SJ, Beehner JC, Saini EK, Kuhn CM, Labar KS. Dominance, politics, and physiology: voters' testosterone changes on the night of the 2008 United States presidential election. PLoS One. 2009;4(10):e7543. doi: 10.1371/journal.pone.0007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68(4):615–23. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- van Aalderen JR, Donders AR, Giommi F, Spinhoven P, Barendregt HP, Speckens AE. The efficacy of mindfulness-based cognitive therapy in recurrent depressed patients with and without a current depressive episode: a randomized controlled trial. Psychological Medicine. 2012;42(5):989–1001. doi: 10.1017/S0033291711002054. [DOI] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmüller V, et al. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI) Personality and Individual Differences. 2006;40(8):1543–55. [Google Scholar]
- Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17804–9. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wichers M, Myin-Germeys I, Jacobs N, et al. Genetic risk of depression and stress-induced negative affect in daily life. The British Journal of Psychiatry. 2007;191:218–23. doi: 10.1192/bjp.bp.106.032201. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. 2011;31(14):5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.