Abstract
The ability to accurately infer others’ mental states from facial expressions is important for optimal social functioning and is fundamentally impaired in social cognitive disorders such as autism. While pharmacologic interventions have shown promise for enhancing empathic accuracy, little is known about the effects of behavioral interventions on empathic accuracy and related brain activity. This study employed a randomized, controlled and longitudinal design to investigate the effect of a secularized analytical compassion meditation program, cognitive-based compassion training (CBCT), on empathic accuracy. Twenty-one healthy participants received functional MRI scans while completing an empathic accuracy task, the Reading the Mind in the Eyes Test (RMET), both prior to and after completion of either CBCT or a health discussion control group. Upon completion of the study interventions, participants randomized to CBCT and were significantly more likely than control subjects to have increased scores on the RMET and increased neural activity in the inferior frontal gyrus (IFG) and dorsomedial prefrontal cortex (dmPFC). Moreover, changes in dmPFC and IFG activity from baseline to the post-intervention assessment were associated with changes in empathic accuracy. These findings suggest that CBCT may hold promise as a behavioral intervention for enhancing empathic accuracy and the neurobiology supporting it.
Keywords: meditation, compassion, empathic accuracy, emotion recognition, theory of mind, social cognition
INTRODUCTION
A fundamental goal of most meditative practices is to enhance compassionate thoughts, feelings and behaviors toward others (The Dalai Lama, 1995; Wallace, 2001). In certain practices, this goal is implicit as compassion is considered to arise spontaneously as a result of enhanced mindful awareness. Other practices, such as Tibetan Buddhist mind training (in Tibetan: lojong), utilize meditative techniques to specifically promote empathic feelings and behaviors toward others as initial steps toward developing a sense of universal compassion for all people.
While little is known regarding whether meditation training actually enhances empathic behavior in daily life, increasing evidence suggests that meditation may positively impact a range of factors that—while not sufficient for compassionate behavior in and of themselves—are nonetheless known to impact empathic mental processes and their underlying neural correlates. For example, even relatively short-term training programs for novices enhance positive regard for others (Hutcherson et al., 2008) and prosocial behavior toward strangers (Leiberg et al., 2011). Recent work indicates that these types of emotional and behavioral effects are associated with changes in neural circuitry known to be of central importance for empathy. Interestingly, changes in neural activity seen after brief compassion meditation training are reminiscent of more powerful changes in the same brain areas observed in advanced practitioners of compassion meditation (Lutz et al., 2008, 2009, Klimecki et al., 2012).
Another factor of relevance for empathy is psychological stress, given evidence that psychosocial adversity in the form of social exclusion reduces subsequent prosocial behavior (Twenge et al., 2007), which is strongly correlated with empathy for others (Batson, 1998). Recent evidence suggests that increased activity in brain areas known to aggravate negative emotionality in response to social exclusion is highly correlated with inflammatory responses to a standardized laboratory psychosocial stressor [Trier Social Stress Test (TSST)] (Slavich et al., 2010). These findings are of great interest to the exploration of potential mechanisms whereby meditation may enhance empathy, given findings from our group that the practice of a secularized program of compassion meditation [cognitive-based compassion training (CBCT)] reduced innate immune inflammatory and emotional distress responses to the same laboratory psychosocial stressor (i.e. the TSST). Taken together, these findings suggest that meditation may enhance empathy in part by reducing deleterious central nervous system and peripheral stress responses in situations of psychosocial threat or adversity.
Yet another factor repeatedly shown to be important for empathy and—by extension—compassion is the ability to accurately identify and understand the mental states of other people. Understanding the neural mechanisms that support this ability is a major goal within social cognitive neuroscience (Lieberman, 2007), and deficits in mental state recognition are a core feature of multiple psychiatric conditions associated with impairments in empathy, most notably autism (Baron-Cohen, 1995; Yirmiya et al., 1998; Hill and Frith, 2003). Mental state recognition, or empathic accuracy (Ickes, 2009), has also been identified as an important component of positive relationship outcomes in healthy populations (Simpson et al., 2003; Gleason et al., 2009; Ickes, 2009). Given the importance of mental state recognition for empathy, it is surprising that no study has yet to directly examine whether behavioral interventions in general, or meditation in particular, have the potential to impact empathic accuracy and its underlying neurobiology in healthy populations.
As a first attempt to address this issue, the current study utilized a randomized longitudinal design to examine whether 8 weeks of CBCT would enhance empathic accuracy and its underlying neural correlates when compared to participation in an active control condition consisting of a health education discussion class. To probe empathic accuracy, we administered a well-validated empathic accuracy task that is increasingly used to investigate mental state deciphering, the ‘Reading the Mind in the Eyes Test’ (RMET) (Baron-Cohen et al., 2001). The RMET has been called an advanced test of theory of mind (Baron-Cohen et al., 2001), as it involves processing subtle social stimuli in a way that allows one to infer the mental states of others. In addition, the RMET activates neural regions important for theory of mind [the medial prefrontal cortex (PFC), the posterior superior temporal sulcus (STS) and the temporal poles], limbic regions such as the amygdala, and portions of the putative mirror system, in particular the inferior frontal gyrus (IFG) (Baron-Cohen et al., 1999, 2006; Russell et al., 2000; Adams et al., 2010).
In addition to assessing task accuracy, we examined changes in task-related neural activity in regions most often implicated in RMET performance, including bilateral IFG (Baron-Cohen et al., 1999, 2006; Russell et al., 2000), and STS (Adams et al., 2010). Beyond their known involvement in the RMET and the fact that reduced activity in these regions has been found in groups with compromised task performance (Baron-Cohen et al., 1999, 2006; Adams et al., 2010), the IFG and STS are consistently recruited during face processing tasks. The IFG is part of a putative mirror system and appears to be integral for inferring others’ mental states based on their facial expressions (Carr et al., 2003; Schulte-Ruther et al., 2007; Keuken et al., 2011). The posterior STS is active during early perceptual processing of faces (Haxby et al., 2000) and is thought to be a particularly important site for integration of visual social information (Allison et al., 2000; Haxby et al., 2000; Iacoboni and Dapretto, 2006). We also investigated neural regions known to be important for thinking about others’ mental states, including dorsomedial PFC (dmPFC) and the temporal poles (Lieberman, 2007).
MATERIALS AND METHODS
To investigate the effects of CBCT on empathic accuracy, this study used a longitudinal, randomized and controlled design in which participants received functional MRI (fMRI) scans while completing the RMET prior to randomization to either 8 weeks of CBCT or a health discussion class control condition and again following completion of these interventions. This was part of a larger study assessing the effects of CBCT on empathy and compassion and, as such, the scanner session included other tasks discussed elsewhere (Mascaro, et al., manuscript in review). The RMET consists of 36 black and white photographs depicting the eye region of an equal number of male and female Caucasian adults. Accompanying each photograph participants see four mental state choices, one correct answer and three foil words, and are asked to judge what the person in the photograph is thinking or feeling. For the current study, the original RMET was modified for use in the fMRI scanner. Thirty of the original stimuli were used for the emotion task, and 30 matching control items were created using the same 30 photographs used in the emotion task, with gender choice replacing the mental state words. The order of the gender choice was randomly alternated in order to better control for the reading demands of the emotion task. The 60 items (30 emotion/30 gender) were grouped into 6 alternating blocks, with 10 items per block and a 20-s rest period between each block. Subjects were given up to 8 s to view the eyes and choose their answer, after which the stimulus disappeared and their answer choice was displayed for 1 s. In order to maintain task difficulty and reduce the risk of a ceiling effect, which would decrease the likelihood that subjects could increase their scores with meditation practice, we preserved the original four-choice design for the emotion task rather than using a two-choice design as employed in previous studies (Baron-Cohen et al., 1999, 2006; Adams et al., 2010). RMET scores were calculated as the number of items answered correctly for the emotion and gender tasks.
Participants
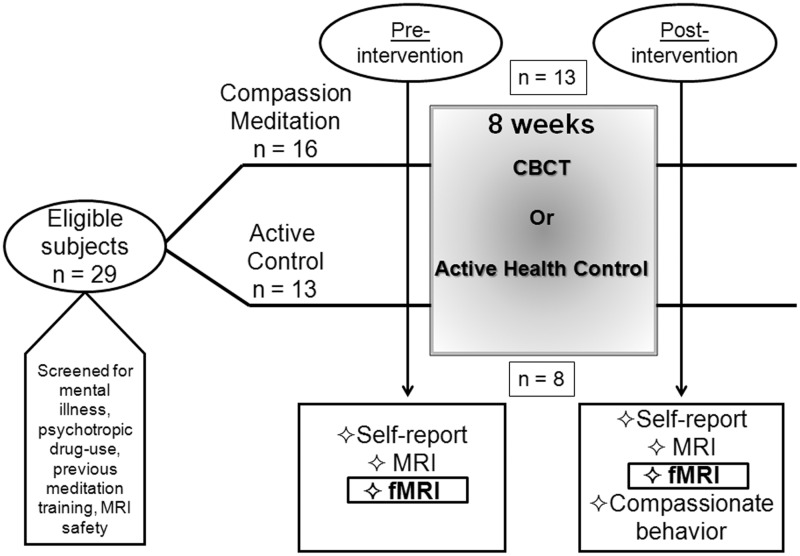
Following approval from the Emory University Institutional Review Board, 29 (16 male; 16 CBCT and 13 controls) participants from the Atlanta area were recruited using a combination of flyers and electronic notifications posted at several local universities, as well as electronic advertisements. Participants were between the ages of 25 and 55 years (M = 31.0, s.d. = 6.02), were screened and excluded for (self-reported) use of any psychotropic medication (i.e. antidepressants, anxiolytics, psychostimulants or mood stabilizers) within 1 year of screening, as well as for regular use of any medications that might influence activity of the autonomic nervous system, HPA axis or inflammatory pathways. Subjects’ were also excluded for any serious ongoing medical or psychiatric condition that might influence the results of the study, including post-traumatic stress disorder (PTSD), chronic pain or other pain disorders, major depression, anxiety disorders or a history of schizophrenia or bipolar disorder, as well as for substance abuse occurring within 1 year of study entry. Current and past psychiatric history was obtained using the Structured Clinical Interview for DSM-IV (SCID) administered by trained raters, and depressive and anxiety symptom severity were assessed using standardized instruments (Beck et al., 1961, 1988). Subjects were screened for MRI safety and handedness (only right-handed participants were included in the study), and all participants had normal or corrected-to-normal vision. For the post-intervention assessment, subjects underwent a scanning protocol identical to the one employed at baseline. As a result of subject attrition, 21 (12 males, 13 CBCT and 8 controls) subjects were scanned the second time (M = 31.9, s.d. = 6.70). The CBCT group that received a Time 2 scan comprised 7 females, 6 males, and the control group comprised 2 females and 6 males. See Figure 1 for a schematic of the study design.
Fig. 1.
Schematic of entire study design. FMRI assessments are the focus of the current study.
Image acquisition
All MRI images were acquired on a Siemens 3 T Trio scanner. Functional images were acquired using an echo planar (EPI) sequence with the following parameters: TR = 2000 ms, TE = 28 ms, matrix = 64 × 64, FOV = 192 mm, slice thickness = 3 mm, gap = 0.45 mm, 34 axial slices. The maximum total task duration was 10 min 40 s. A 4.5-min T1-weighted MPRAGE scan (TR = 2600 ms, TE = 3.02 ms, matrix = 256 × 256, FOV = 256 mm, slice thickness = 1 mm, gap = 0 mm) was also acquired for anatomical localization of fMRI activations.
fMRI image preprocessing and analysis
Image preprocessing was conducted using Brain Voyager QX (version 2.0.8) software (Brain Innovation, Maastricht, The Netherlands). The first six volumes of each run were discarded in order to allow the tissue magnetization to equilibrate. Preprocessing involved slice scan-time correction, 3D motion correction and temporal filtering by linear trend removal and high-pass filtering of frequencies below two cycles per run length. Next, images were normalized into Talairach space (Talairach and Tournoux, 1988) and spatially smoothed with an 8-mm full-width-at-half-maximum (FWHM) Gaussian kernel. A separate general linear model (GLM) was defined for each subject that examined the neural response to the two task conditions: gender and emotion. For each subject, the contrast in parameter estimates (i.e. emotion–gender) was calculated at every voxel in the brain.
To isolate neural systems related to empathic accuracy at Time 1, a one-sample t-test was used to identify voxels in which the average contrast (emotion–gender) for the whole group (n = 29 subjects) differed significantly from 0 (i.e. a random-effect analysis). The resulting map of the t-statistic was thresholded at P < 0.001, with a spatial extent threshold of 10 contiguous voxels. To identify neural activations related to empathic accuracy, we conducted a whole-brain analysis using RMET scores as a covariate at a threshold of P < 0.001.
Statistical analysis
We interrogated the effects of meditation training on empathic accuracy scores and on neural activity specifically in brain regions previously implicated in the RMET: bilateral IFG, posterior STS, dmPFC and the temporal poles. The Time 1 whole-brain activation map revealed activations in bilateral IFG, dmPFC, bilateral temporal poles and in the left posterior STS. The right posterior STS did not reach significance at P < 0.001. Functional regions of interest (ROIs) were defined in these regions from the Time 1 activation map using the following method. For each region, the peak voxel was identified and a 15-mm isotropic cube was centered on that voxel (Figure 2). Functional activations that spanned multiple anatomical regions were partitioned by identifying the local maxima within the larger activation. Prior to performing outcome analyses, independent samples t-tests were used to ensure that the CBCT and control groups did not differ significantly from one another at Time 1 in terms of accuracy scores or brain activity during the task.
Fig. 2.

Activation map (emotion–gender) was thresholded at P < 0.001. Location of functional ROIs in left IFG and posterior STS indicated by arrow.
Next, accuracy scores as well as beta-contrast values from the functional ROIs were entered into mixed-design analyses of variance (ANOVAs) in order to investigate changes related to meditation training. If there was a significant difference at Time 1 between the groups, we performed stricter univariate analyses controlling for Time 1 beta contrast values. Χ2 tests were performed to determine whether more participants randomized to the CBCT condition experienced increases in RMET scores and task-related neural activity compared to the control group. Those neural regions that showed a group × time interaction effect were then entered into linear regression analyses to test whether changes in brain activity were related to changes in empathic accuracy. In other words, controlling for Time 1 brain activity and Time 1 RMET scores, we investigated whether the residual change in brain activity accounted for a significant amount of the variance in Time 2 RMET scores.
CBCT protocol
The CBCT protocol used here was designed by one of us (L.T.N.). Although secular in presentation, CBCT derives from the 11th century Tibetan Buddhist lojong tradition. In its operationalization, however, CBCT employs several important modifications to traditional lojong teachings. First, all discussions of soteriological or existential themes (e.g. the attainment of Buddhahood, Karma) are omitted. Second, participants receive instructive sessions for concentrative (i.e. shamatha) and mindful-awareness (i.e. vipassana) practices at the beginning of the course. While not specifically included in traditional lojong curricula, these foundational meditation practices were an assumed prerequisite for commencing lojong training in a traditional Buddhist context (The Dalai Lama, 2001). As such, the course content of CBCT proceeds according to the following schedule (see Supplemental Data for a more complete description of the weekly schedule).
-
Week 1
: Developing Attention and Stability of Mind
-
Week 2
: Cultivating Insight into the Nature of Mental Experience
-
Week 3
: Cultivating Self-Compassion
-
Week 4
: Developing Equanimity
-
Week 5
: Developing Appreciation and Gratitude for Others
-
Week 6
: Developing Affection and Empathy
-
Week 7
: Realizing Wishing and Aspirational Compassion
-
Week 8
: Realizing Active Compassion for Others
In the current study, CBCT courses were taught by two experienced meditators who had undergone extensive training with Lobsang Tenzin Negi, Ph.D., creator of the CBCT protocol. Both teachers were present for all classes. Study participants were asked to attend 2 h of class time per week for 8 weeks. Class sessions combined a didactic teaching and discussion section with ∼20 min of meditation per hour class time. Participants were provided with a meditation compact disc to guide ‘at-home’ practice sessions that reflected in-class material, were asked to practice for 20 min each day and to keep track of practice time daily using a practice time log.
Health discussion control group protocol
Participants randomized to the control condition attended a 2-h discussion group per week. Classes were designed and taught by graduate students from the Emory University Rollins School of Public Health. Topics included history of medicine, nutrition, sleep, nature, interpreting health information, mental health, health through the lifespan, exercise, stress, infectious disease, sexual health and complementary and alternative medicine. The health discussion group was designed to control for non-specific effects of the meditation class, including education and social engagement with a collective group. Subjects randomized to the control condition were not asked to do any at-home work that would have controlled for the practice time expectation for subjects randomized to CBCT. Importantly, all study participants were blind to group assignment at the Time 1 assessments, and all experimenters were blind throughout the entire data collection, data entry and fMRI preprocessing phases.
RESULTS
Complete data were collected from 21 participants (13 meditators). The rates of drop out in the control (5 of 13) and meditation group (3 of 16) were not significantly different [χ2(1, N = 21) = 1.40, P = 0.24] and drop outs were not significantly different in terms of RMET scores [t(27) = −0.68, P = 0.50], age [t(27) = 1.31, P = 0.20], gender [t(27) = 0.33, P = 0.74] or education [t(27) = 0.43, P = 0.67] (reasons for drop out are detailed in Supplementary Data). Neither was there a significant difference in class attendance between the two groups (control: M = 7.13, s.d. = 3.83; meditation: M = 7.38, s.d. = 3.97).
Empathic accuracy
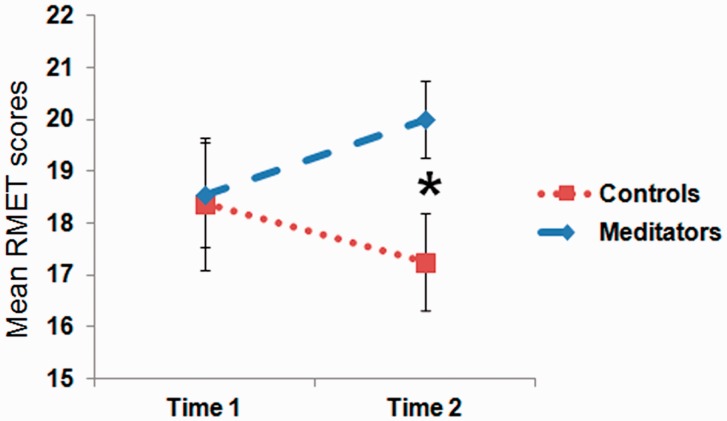
The reaction times for the gender vs emotion recognition tasks were significantly different at both Time 1 and 2 (RT: emotion Time 1: M = 4.68 s; s.d. = 0.81 s; gender Time 1: M = 2.05 s; s.d. = 0.48 s; emotion Time 2: M = 4.27 s; s.d. = 0.73 s; gender Time 2: M = 1.86 s; s.d. = 0.50 s) suggesting that the emotion task was more difficult [Time 1: t(20) = 16.7; P < 0.001; Time 2: t(20) = 15.2; P < 0.001]. The groups were not significantly different at Time 1 in terms of RMET emotion scores (i.e. correct answers) (control: M = 18.4, s.d. = 3.42; meditation: M = 18.5, s.d. = 3.76; t(19) = − 0.10, P = 0.92) or emotion reaction times [control: M = 4.88 s, s.d. = 0.43 s; meditation: M = 4.56 s, s.d. = 0.97 s; t(19) = 0.86, P = 0.40]. The main effect of group was not significant [F(19) = 1.25, P = 0.28]. Neither was there a main effect of time on scores [F(19) = 0.00, P = 0.995]. A mixed-design ANOVA revealed a trend for a group by time interaction for RMET scores [F(19) = 3.83, P = 0.065], such that at Time 2, the meditation group scored significantly higher in RMET accuracy than the control group [Time 2 scores: control: M = 17.3, s.d. = 2.05; meditation: M = 20.0, s.d. = 2.94; t(19) = 2.31, P = 0.03] (Figure 3). The effect size for the interaction effect was large (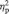 = 0.17). A greater proportion of meditators showed an increase in accuracy across the training period compared with controls, χ2(1, N = 21) = 4.86, P = 0.03. Of 13 meditators, 8 had increased accuracy from Time 1 to Time 2 compared with only 2 of 8 control subjects. There was no effect of meditation on reaction times [Time 2: control: M = 4.58 s, s.d. = 0.63 s; meditation: M = 4.06 s, s.d. = 0.76 s; t(19) = 1.62, P = 0.12]. There was no correlation between practice time and changes in empathic accuracy in the CBCT group (M = 315.9 min, s.d. = 228.9 min).
= 0.17). A greater proportion of meditators showed an increase in accuracy across the training period compared with controls, χ2(1, N = 21) = 4.86, P = 0.03. Of 13 meditators, 8 had increased accuracy from Time 1 to Time 2 compared with only 2 of 8 control subjects. There was no effect of meditation on reaction times [Time 2: control: M = 4.58 s, s.d. = 0.63 s; meditation: M = 4.06 s, s.d. = 0.76 s; t(19) = 1.62, P = 0.12]. There was no correlation between practice time and changes in empathic accuracy in the CBCT group (M = 315.9 min, s.d. = 228.9 min).
Fig. 3.
Repeated measures of empathic accuracy scores broken up according to group. At Time 2, the meditation group scored significantly higher than the control group [t(19) = 2.31, P = 0.03].
fMRI
Across all subjects at the baseline evaluation (Time 1), the contrast between the emotion and gender tasks revealed a main effect in brain regions previously identified as important for inferring the emotions of others based on their facial expression. In particular, bilateral temporal poles and IFG, right anterior STS, left posterior STS, the dmPFC, including the anterior paracingulate cortex, and the left amygdala were more active during the emotion task than the gender task (Table 1). In a whole-brain analysis (P < 0.001), there were no regions identified that significantly covaried with empathic accuracy scores.
Table 1.
Main effect of RMET (emotion–gender)
| Brain regions | Brodmann’s area | x | y | z | Voxels | Peak t |
|---|---|---|---|---|---|---|
| Supplementary motor | 6 | −6 | 14 | 46 | 14 625 | 8.51 |
| dmPFC | 8 | −9 | 50 | 37 | ↓ | 7.09 |
| dACC | 32 | 12 | 14 | 34 | 315 | 4.97 |
| L IFG | 45 | −45 | 29 | 4 | 72 831 | 14.09 |
| L post. STS | 22 | −51 | −31 | 4 | ↓ | 11.91 |
| L amygdala | −30 | −4 | −17 | ↓ | 5.32 | |
| L caudate | −12 | −28 | 22 | 2378 | 6.38 | |
| R temporal pole | 20 | 42 | 17 | −20 | 2184 | 7.04 |
| R caudate | 15 | 14 | 13 | 496 | 5.01 | |
| R caudate | 21 | −37 | 19 | 308 | 4.79 | |
| R IFG | 45 | 45 | 26 | −2 | 44 | 3.97 |
| R ant. STS | 21 | 51 | −7 | −11 | 168 | 4.16 |
| Cerebellum | 15 | −67 | −29 | 6657 | 7.52 | |
| Cerebellum | −3 | −49 | −23 | 991 | 4.46 | |
| Brainstem | −6 | −16 | −11 | 173 | 4.02 | |
| Thalamus | −3 | −19 | 13 | 312 | 4.62 | |
| Occipital lobe | 18 | −24 | −85 | −2 | 719 | 4.31 |
For regions that were subdivided into smaller ROIs using the local maxima, an ‘↓’ is entered for number of voxels, which means that the size of the entire activation is listed above. L, left; R, right; dACC, dorsal anterior cingulate cortex; L post. STS, left posterior STS; R ant. STS, right anterior STS.
Of the regions differentially activated by the emotion vs the gender task, the dmPFC, bilateral IFG and temporal poles, and left posterior STS were further investigated in ROI analyses. At Time 1, there was not a significant difference between the groups in terms of activity in the dmPFC, right or left IFG, left temporal pole or left STS ROIs. However, there was a significant difference between the groups at Time 1 in activity in the right temporal pole [t(19) = 2.82, P = 0.01]. The main effect of group was not significant for any of the regions tested. There was not a significant main effect of time for the right or left IFG, dmPFC, or right or left temporal poles. There was a significant main effect of time in the left STS [F(19) = 5.07, P < 0.05). A mixed-design ANOVA revealed a significant interaction (group by time) effect for brain activity in the left IFG [F(19) = 7.05, P < 0.02], dmPFC [F(19) = 6.16, P < 0.05] and a strong trend in the same direction in the left STS [F(19) = 4.20, P = 0.06]. The effect size for the interaction effect was large for all three regions [left IFG: 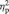 = 0.27; dmPFC:
= 0.27; dmPFC:  = 0.25; left STS:
= 0.25; left STS: 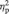 = 0.18). Since there was a significant Time 1 difference between the groups in terms of right temporal pole activity, we conducted a stricter univariate analysis controlling for Time 1 beta values in the right temporal pole, which revealed that there was not a significant group difference in the right temporal pole at Time 2 [F(19) = 2.50, P = 0.132]. At Time 2, there was not a significant group difference in any functional region tested [dmPFC: t(19) = 0.64, P = 0.53; left IFG: t(19) = 1.38, P = 0.18; left STS: t(19) = 1.67, P = 0.11]. When analyzed separately according to group assignment, there was not a significant increase in activity for those in the meditation group in any of the regions tested [left IFG: t(12) = 0.50, P = 0.62; dmPFC: t(12) = 1.13, P = 0.28; left STS: t(12) = 0.17, P = 0.87]. However, for the control group, there was a significant decrease from Time 1 to Time 2 in all regions tested [left IFG: t(7) = − 3.07, P < 0.05; dmPFC: t(7) = 3.33, P < 0.05; left STS: t(7) = 2.67, P < 0.05]. No effect of practice time was observed for changes in brain activity in the CBCT group.
= 0.18). Since there was a significant Time 1 difference between the groups in terms of right temporal pole activity, we conducted a stricter univariate analysis controlling for Time 1 beta values in the right temporal pole, which revealed that there was not a significant group difference in the right temporal pole at Time 2 [F(19) = 2.50, P = 0.132]. At Time 2, there was not a significant group difference in any functional region tested [dmPFC: t(19) = 0.64, P = 0.53; left IFG: t(19) = 1.38, P = 0.18; left STS: t(19) = 1.67, P = 0.11]. When analyzed separately according to group assignment, there was not a significant increase in activity for those in the meditation group in any of the regions tested [left IFG: t(12) = 0.50, P = 0.62; dmPFC: t(12) = 1.13, P = 0.28; left STS: t(12) = 0.17, P = 0.87]. However, for the control group, there was a significant decrease from Time 1 to Time 2 in all regions tested [left IFG: t(7) = − 3.07, P < 0.05; dmPFC: t(7) = 3.33, P < 0.05; left STS: t(7) = 2.67, P < 0.05]. No effect of practice time was observed for changes in brain activity in the CBCT group.
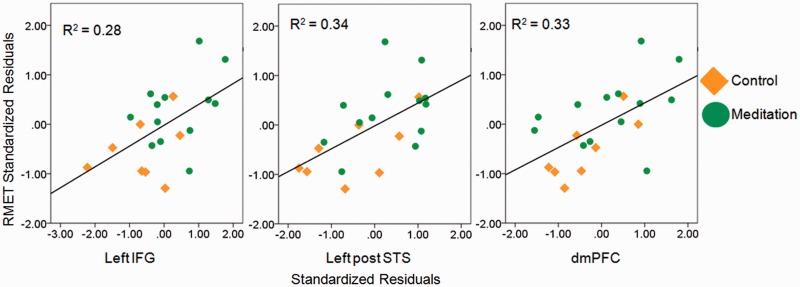
We next assessed whether changes in brain activity were related to changes in accuracy using a series of hierarchical regression analyses. After controlling for Time 1 RMET scores and brain activity, the residual change in activity in the left IFG [R2 = 0.22, F(1,17) = 7.07, P < 0.05], dmPFC [R2 = 0.24, F(1,17) = 8.25, P < 0.01] and left STS [R2 = 0.27, F(1,17) = 9.55, P < 0.01] accounted for a significant amount of the variance in Time 2 RMET scores in the study population as a whole (Figure 4).
Fig. 4.
Correlation of standardized residual change in RMET scores with standardized residual change in brain activity in the left IFG [r(19) = 0.55, P = 0.01], left posterior STS (left post STS) [r(19) = 0.59, P = 0.01] and dmPFC [r(19) = 0.56, P = 0.01].
DISCUSSION
The current study tested the hypothesis that, when compared to an active control condition, 8 weeks of CBCT would enhance empathic accuracy and related brain activity. In support of this hypothesis, participants trained in CBCT were more likely than participants in the control group to increase their scores on the RMET. While the majority of participants in the control group showed reductions in accuracy from the pre- to post-intervention assessment, the majority of those randomized to CBCT increased their accuracy across the study period. Two previous studies have found that experimental administration of oxytocin enhances accuracy on the RMET (Domes et al., 2007; Guastella et al., 2010); however, to our knowledge this is the first study to show that a behavioral intervention is capable of inducing similar effects.
The RMET used in this study elicited fMRI results comparable to those found in previous studies, engaging neural regions such as bilateral temporal poles and IFG, left STS, right anterior STS, the dmPFC and anterior paracingulate cortex. In addition, when compared to the control group, CBCT attenuated the decline in activation in the dmPFC and left IFG and even reversed it in many cases. Moreover, the changes in task accuracy were significantly accounted for by changes in activity in these brain regions. With respect to the IFG, this finding is in line with previous studies by Baron-Cohen and colleagues who found that autistic individuals (Baron-Cohen et al., 1999), who consistently score lower on the RMET, as well as parents of autistic children (Baron-Cohen et al., 2006) had reduced left IFG activity during the RMET. Similarly, Baron-Cohen and colleagues found that females consistently scored higher on the task (Baron-Cohen et al., 2001) and also had greater left IFG activation (Baron-Cohen et al., 2006). Current findings, therefore, lend further support to the notion that IFG activity is crucial for empathic accuracy.
While it is possible that left IFG activity is related to non-specific aspects of the RMET such as language processing, activity in this region may directly relate to the processing of facial expressions (Carr et al., 2003). Putative mirror activity has often been associated with BA44, thought to be the human homologue of the monkey area F5 where mirror neurons were first identified (Gallese et al., 1996; Rizzolatti et al., 1996). However, Dapretto et al. (2006) report activation of BA45 in children as they imitate others’ emotional facial expressions and a recent meta-analysis found that observation of the face consistently engages BA45 bilaterally (Caspers et al., 2010). The peak left IFG coordinate found in this meta-analysis lies within the ROI used in the present study. Related to findings presented here, other studies have shown that motivation modulates the putative mirror system. For example, Cheng and colleagues (2007) report enhanced activity in left IFG in hungry, compared to satiated, participants while they viewed others grabbing food. The peak left IFG coordinate reported by their group also falls within the ROI used here. While we cannot definitively say that the enhanced IFG activity identified in this study is related to increased simulation or mirroring processes, to the best of our knowledge this is the first report of a behavioral intervention enhancing BOLD activity in the IFG during an emotion recognition task.
In addition to changes in left IFG, the CBCT group, compared to the control group, had more activity in the dmPFC, a region consistently implicated in mentalizing (Lieberman, 2007). Importantly, the activation identified here is more dorsal than a region of the PFC previously found to be modulated by perceived similarity, which argues against the interpretation that CBCT enhanced feelings of similarity for practitioners to unknown others (Mitchell et al., 2006). Rather, activity in this more dorsal region appears to be important for mentalizing about dissimilar others, and the fact that changes in activity in meditators were different than in controls suggests that CBCT may increase one’s inclination to think about the mental states of others even if they are felt to be dissimilar.
The CBCT group also showed a strong trend for increased activity in the left STS, and this difference in activity significantly predicted changes in RMET scores. This finding is consistent with a recent study that found reduced posterior STS activity when participants viewed eye stimuli from a racial outgroup, and the magnitude of this reduction was correlated with the extent that participants suffered from out-group deficits in empathic accuracy (Adams et al., 2010). The peak voxel reported by this group falls within the ROI tested in the current study. While all RMET stimuli used in the current study featured white individuals, the participant pool was relatively heterogenous with 7 of 13 participants randomized to meditation self-reporting that they were non-white. Thus, it remains possible that some participants experienced an out-group bias in accuracy and neural activity during the RMET, a bias that may have been affected by meditation training. For this reason, we tested whether ethnicity moderated the effects of CBCT on RMET scores and brain activity in the posterior STS in order to investigate the hypothesis that meditation was ameliorating an out-group bias. However, the moderation effect was non-significant, perhaps as a result of being underpowered to test this hypothesis. This would be a fruitful question for future research, particularly given that CBCT explicitly requires trainees to analyze and re-orient their cognitive biases about individuals they previously considered to be unlike themselves.
Given that CBCT may enhance empathic accuracy and that this enhancement is related to increased activity in neural regions important for the task, it is intriguing to speculate on how a behavioral practice might promote such changes. Flury and Ickes (2006) reviewed the rich literature on empathic accuracy and concluded that several situational attributes affect empathic accuracy, including the perceiver’s level of empathic resonance, attentiveness to the target, and motivation. Since CBCT effects may be accounted for by one or more of these factors, each will be considered in turn.
First, CBCT might have increased the empathic resonance that practitioners experienced while observing targets. While we cannot rule this out, in a related study in the same participant population, our group did not find an enhancement of neural activity in regions known to be important for simulating the affective pain response during empathy for pain task (EFP task) (Mascaro et al., manuscript in review), which reduces the likelihood that empathic resonance was enhanced by CBCT. Second, CBCT may prime or enhance willingness to attend to the facial expressions of others by reminding people to pay attention to those around them. Given the extensive emphasis within CBCT on imagining the mental and motivational states of a variety of others, this explanation has face validity. Future work might use eye tracking in order to investigate whether meditation affects practitioners’ attention to faces. Third, CBCT may enhance motivation. Social psychologists have long highlighted the difference between ‘capacity’ and ‘tendency’ (Davis and Franzoi, 1991; Davis and Kraus, 1997; Klein and Hodges, 2001). While individuals may be capable of being empathically accurate, they may tend to underperform. Perhaps CBCT increases motivation, causing practitioners’ ‘tendency’ to catch up with their ‘capacity’ for empathic accuracy. Consistent with this possibility, more meditation practitioners increase their scores than would be expected by chance, but the majority of the participants randomized to the control group experienced a decrease in empathic accuracy. Brain activity in the IFG and dmPFC mirrored this drop in score. This supports the idea that control participants experienced a lack of motivation while completing the task the second time. In contrast, meditation practitioners enhanced their performance perhaps due to less decline and even increases in motivation in some cases. Future studies might definitively address this possibility, for example, by rewarding participants for their performance on the task. If CBCT enhances accuracy above and beyond the levels exhibited by motivated control participants, we would be in a better position to conclude that the training enhances practitioners’ capacity, not just their tendency, to accurately read others’ mental states.
Finally, we would like to offer a speculative and admittedly post-hoc hypothesis that arises from the emerging pattern of CBCT results. That is, the results of the current study and two other investigations of CBCT (Mascaro et al., manuscript in review) (Pace et al., 2009), combined with the mounting literature regarding the effects of oxytocin administration, are consistent with the hypothesis that CBCT might operate, in part, by enhancing some aspect of the oxytocin (OT) system. Multiple studies now show that performance on the RMET is influenced by the OT system, either by administration of OT (Domes et al., 2007; Guastella et al., 2010) or by polymorphisms in the OT receptor (Rodrigues et al., 2009). In addition, OT administration impacts responses to psychosocial stress (TSST) in ways similar to changes observed with CBCT (Pace et al., 2009). Interestingly, the fact that another study shows that OT administration has no effect on neural responses to an empathy for pain task (Singer et al., 2008) is consistent with and provides additional support for the idea that rather than general effects, CBCT may have specific effects on the OT system, which could mediate changes in specific aspects of social cognition. While central OT is notoriously difficult to study (Churchland and Winkielman, 2012), future studies should explicitly test the hypothesis that meditation-induced changes in social cognition are due to changes in the OT system.
Several limitations of the present study are worth noting. First, the sample size for the discussion control group was relatively small and future studies will be needed to determine whether the findings discussed here generalize to larger samples. Relatedly, there were no neural regions active for the main contrast that covaried with empathic accuracy. This is not entirely surprising given that no other RMET imaging studies, to the best of our knowledge, have reported a relationship between neural activity and task accuracy in an across-subjects analysis (Baron-Cohen et al., 1999, 2006; Adams et al., 2010). While it may be the case that this type of analysis was not conducted in previous studies, we wonder if it is rather that a large sample size would be required for such an analysis.
Moreover, the current study does not allow a definitive determination of whether the increase in IFG activity observed in the CBCT group was related to enhanced face processing. It remains possible that these findings can be explained by the meditation group increasing activity related to linguistic processing, which also recruits the IFG. However, the fact that meditators, compared to control participants, had increased activity in dmPFC and trended toward increased activation in the STS supports the interpretation that the enhancement of neural activity was related to increased mental state attribution and processing of the eye stimuli. Moreover, the focus of the temporal activation lay directly in the STS rather than in more ventral middle temporal regions often implicated in semantic processing (Dronkers et al., 2004; Turken and Dronkers, 2011), suggesting limited importance of linguistic processing for this empathic accuracy task. While the STS is also involved in linguistic processing, it is primarily recruited for processing syntactically complex sentences (Vigneau et al., 2006), which our study did not involve. Related to this, the leftward dominance of the activation pattern, while different than the patterns found in other studies using the RMET (Adams et al., 2010), may be due to a combination of face and linguistic processing demands that were more intensive in the variant of the RMET used in this study. In the future it will be important to validate these results using other, more dynamic and ecologically valid tests of empathic accuracy that do not rely on linguistic processing (Zaki et al., 2009). This is particularly important given the difference in task difficulty between the emotion and gender tasks, which may have introduced to the contrast of interest non-specific brain activity related to attention or reading comprehension. For all of these reasons, future studies should also assess CBCT practitioners’ tendency to relate empathically toward others in everyday life.
In addition, although use of the word ‘meditation’ was limited during study recruitment in order to circumvent confounds such as self-selection bias and placebo effects, it is possible that results were affected by the fact that randomization to the meditation group produced expectancy biases compared to those randomized to the health education group. However, we believe that the fact that those randomized to meditation did not self-report higher levels of empathy nor did they have increased brain activity during other empathy tasks conducted during the same sessions (Mascaro et al., manuscript in review), along with the fact that those in the meditation group did not increase the time spent on each item reduces the likelihood that expectancy biases account for the RMET findings reported here. Similarly, while it is possible that participants randomized to the control group were less motivated to participate in the study, it is important to note that class attendance for the two groups did not differ and there was not a significant difference between the number of control and meditation subjects who dropped out of the study, suggesting minimal differences between the two groups in motivation to participate in the classes.
Finally, while it is tantalizing to speculate on the potential for using CBCT in clinical populations that suffer from empathy deficits, caution is warranted, as it is clear from our larger study (Mascaro et al., manuscript in review) that pre-existing differences in brain function predict differential engagement with meditation. Thus, it is possible that CBCT would resonate less or work differently in populations typified by abnormal social cognitive functioning. Nonetheless, an important next step will be to evaluate the effects of CBCT on diverse populations that may particularly benefit from enhanced empathic accuracy such as those suffering from high-functioning autism, caregivers and healthcare clinicians.
FUNDING
This work was supported by an Emory University Neuroscience Initiative Seed Grant Program, by the National Institutes of Health (NIH)–the National Center for Complementary and Alternative Medicine (NCCAM) [AT004698 to C.R]; the Center for Behavioral Neuroscience and a Ruth L. Kirschstein National Research Service Award (NRSA) for Individual Predoctoral Fellows through NCCAM [1F31AT004878-01 to J.S.M.].
Conflict of Interest
J.S.M., J.K.R. and L.T.N. reported no financial interests or potential conflicts of interest. C.R. reported no financial interests or potential conflicts of interest with the current study. In the previous 12 months, he has served as a consultant for Bristol-Myers Squibb and Pamlab, and has created and delivered promotional disease-state material for Pamlab.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
ACKNOWLEDGEMENTS
We thank Nathan Mascaro for statistical advice. We also thank Brooke Dodson-Lavelle and Brendan Ozawa-de Silva for training participants in the CBCT protocol, and Teri Sivilli for help recruiting and screening study participants.
REFERENCES
- Adams RB, Jr, Rule NO, Franklin RG, Jr, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience. 2010;22(1):97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: The MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Ring H, Chitnis X, et al. fMRI of parents of children with Asperger syndrome: a pilot study. Brain and Cognition. 2006;61(1):122–30. doi: 10.1016/j.bandc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11(6):1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–51. [PubMed] [Google Scholar]
- Batson CD. Altruism and Prosocial behavior. In: Gilbert D, Fiske S, Lindsey G, editors. The Handbook of Social Psychology. Boston: McGraw-Hill; 1998. pp. 282–316. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Acadmy of Sciences USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Meltzoff AN, Decety J. Motivation modulates the activity of the human mirror-neuron system. Cerebral Cortex. 2007;17(8):1979–1986. doi: 10.1093/cercor/bhl107. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Hormones and Behavior. 2012;61(3):392–9. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Franzoi SL. Stability and change in adolescent self-consciousness and empathy. Journal of Research in Personality. 1991;25(1):70–87. [Google Scholar]
- Davis MH, Kraus LA. Personality and empathic accuracy. In: Ickes W, editor. Empathic Accuracy. New York: The Guilford Press; 1997. p. 352. [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mind-reading" in humans. Biological Psychiatry. 2007;61(6):731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Flury J, Ickes W. Emotional intelligence and empathic accuracy in friendships and dating relationships. In: Ciarrochi J, Forgas JP, Mayer JD, editors. Emotional Intelligence in Everyday Life. 2nd edn. New York: Psychology Press; 2006. pp. 140–65. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gleason KA, Jensen-Campbell LA, Ickes W. The role of empathic accuracy in adolescents' peer relations and adjustment. Personality and Social Psychology Bulletin. 2009;35(8):997–1011. doi: 10.1177/0146167209336605. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67(7):692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- The Dalai Lama. The world of Tibetan Buddhism: An Overview of Its Philosophy and Practice. Boston: Wisdom Publications; 1995. [Google Scholar]
- The Dalai Lama. An Open Heart. New York: Little Brown and Company; 2001. [Google Scholar]
- Hill EL, Frith U. Understanding autism: insights from mind and brain. Philosophical Transactions: Biological Sciences. 2003;358(1430):281–9. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson CA, Seppala EM, Gross JJ. Loving-kindness meditation increases social connectedness. Emotion. 2008;8(5):720–4. doi: 10.1037/a0013237. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Ickes W. Empathic accuracy: its links to clinical, cognitive, developmental, social, and physiological psychology. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge: The MIT Press; 2009. pp. 57–70. [Google Scholar]
- Keuken MC, Hardie A, Dorn BT, et al. The role of the left inferior frontal gyrus in social perception: an rTMS study. Brain Research. 2011;1383:196–205. doi: 10.1016/j.brainres.2011.01.073. [DOI] [PubMed] [Google Scholar]
- Klein KJK, Hodges SD. Gender differences, motivation, and empathic accuracy: when it pays to understand. Personality and Social Psychology Bulletin. 2001;27(6):720–30. [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. 2012 doi: 10.1093/cercor/bhs142. Functional neural plasticity and associated changes in positive affect after compassion training. Cerebral Cortex, epub ahead of print available June 1, 2012. Retrieved from http://cercor.oxfordjournals.org/content/early/2012/05/31/cercor.bhs142.short. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Klimecki O, Singer T. Short-term compassion training increases prosocial behavior in a newly developed prosocial game. Plos One. 2011;6(3):e17798. doi: 10.1371/journal.pone.0017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58(1):259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS ONE. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage. 2009;47(3):1038–46. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Mahzarin RB. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mascaro J. doi: 10.1016/j.neuroimage.2012.12.021. Pre-existing Brain Function Predicts Subsequent Practice of Mindfulness and Compassion Meditation. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3(2):131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences USA. 2009;106(50):21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, et al. Exploring the social drain in schizophrenia: left prefrontal underactivation during mental state attribution. American Journal of Psychiatry. 2000;157(12):2040–2. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19(8):1354–72. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Oriña MM, Ickes W. When accuracy hurts, and when it helps: a test of the empathic accuracy model in marital interactions. Journal of Personality and Social Psychology. 2003;85(5):881–93. doi: 10.1037/0022-3514.85.5.881. [DOI] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8(6):781–91. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences USA. 2010;107(33):14817–22. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5(1):1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior. Journal of Personality and Social Psychology. 2007;92(1):56–66. doi: 10.1037/0022-3514.92.1.56. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wallace BA. Intersubjectivity in Indo-Tibetan buddhism. Journal of Consciousness Studies. 2001;8(5–7):209–30. [Google Scholar]
- Yirmiya N, Erel O, Shaked M, Solomonica-Levi D. Meta-analyses comparing theory of mind abilities of individuals with autism, individuals with mental retardation, and normally developing individuals. Psychological Bulletin. 1998;124(3):283–307. doi: 10.1037/0033-2909.124.3.283. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences USA. 2009;106(27):11382–7. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.