Abstract
Purpose
To study the relationship of height and body mass index (BMI) during childhood with adult bone mineral content (BMC), areal density (aBMD) and apparent density (BMAD, estimated volumetric density).
Methods
Participants were 565 men and women aged 33-39 years from the New Delhi Birth Cohort, India, whose weight and height were recorded at birth and annually during infancy (0-2 years), childhood (2-11 years) and adolescence (11 years-adult). Lumbar spine, femoral neck and forearm BMC and aBMD were measured using dual X-ray absorptiometry; lumbar spine and femoral neck BMAD were calculated.
Results
Birth length, and height and height gain during infancy, childhood and adolescence were positively correlated with adult BMC (p≤0.01 all sites except birth length with femoral neck). Correlations increased with height from birth-6 years, then remained constant for later height measurements. There were no associations with BMAD. BMI at birth, and during childhood and adolescence was also positively correlated with BMC (p<0.01 all sites). BMI at 11 years, and BMI gain in childhood and adolescence, were correlated with aBMD and BMAD (p<0.001 for all); these correlations strengthened with increasing age of BMI measurement. The associations with height and BMI in early life became non-significant after adjustment for adult height and BMI.
Conclusions
Greater skeletal growth and BMI gain in utero and during infancy are associated with higher peak BMC, and greater BMI gain in childhood and adolescence is associated with higher peak aBMD and BMAD. These associations are mediated by the attainment of adult height and BMI respectively.
Keywords: Birth cohort, childhood growth, height, body mass index, bone mineral content, bone mineral density
INTRODUCTION
Osteoporosis is a skeletal disorder characterised by low bone mass and microarchitectural deterioration of bone tissue which predisposes to fracture, typically at the hip, spine and wrist [1,2]. It has been estimated that the remaining lifetime risk of a fracture at one of these three sites approaches 40% among women aged 50 years, and is approximately half this value in men [3]. Furthermore, demographic changes over the next 50 years will alter the geography of fracture incidence such that over 50% of osteoporotic fractures worldwide will occur in Asia [4].
The risk of osteoporotic fracture depends on two factors, the mechanical strength of bone and the forces applied to it. Bone mass (a composite measure including bone size and volumetric mineral density) is an established determinant of bone strength. Adult bone mass depends upon the peak attained during skeletal growth and the subsequent rate of bone loss. Peak bone mass is partly inherited, but currently identified genetic markers only explain a small proportion of the variation in individual bone mass and fracture risk [5]. Environmental and lifestyle factors during adult life have been shown to influence bone loss, for example physical activity, dietary calcium intake, cigarette smoking and alcohol consumption [4,6].
Mathematical models suggest that modifying peak bone mass could have biologically relevant effects on skeletal fragility in old age [7], and recent interest has focussed on the accrual of peak bone mass during the period of growth from conception through to the end of adolescence. Several longitudinal studies have shown that bone mass tracks through childhood and adolescence [8]. Evidence is mounting that factors associated with earlier growth, in utero and in infancy, may have persisting influences on skeletal development [9-11]. Lower birthweight and infant weight are associated with reduced adult spine and hip mineral content and areal bone mineral density [12,13]. Previous studies have not investigated the entire growth trajectory in relation to later bone measurements and attempted to identify specific periods of growth that may be critical to the development of peak bone mass.
The New Delhi Birth Cohort Study has serial weight and height data at annual intervals from birth to the age of 20 years, for a large sample of young Indian men and women [14-15]. We have utilised this cohort to explore associations of size at birth, during infancy (0-2 years), childhood (2-11 years) and adolescence (11 years to adult), and of growth (increase in size) during these periods, to adult bone mineral content (BMC), and areal bone mineral density (aBMD) and bone mineral apparent density (BMAD, an estimate of bone mineral volumetric density). We have used conditional growth analysis to identify independent associations between growth at particular ages and these bone measurements. We aimed to test the hypothesis that larger size at birth, and greater growth in infancy and childhood predicted higher adult BMC, aBMD and BMAD.
METHODS
Original cohort study
During 1969-1972, married women living in a 12 km2 area of Delhi (N=20,755) were recruited and followed up. A total of 9,169 of these women became pregnant, resulting in 8,181 live births. Trained research workers recorded the babies’ weight and length within 72 hours of birth and 6-monthly until 14-21 years. Gaps in funding interrupted measurements in 1972-1973 and 1980-1982. At recruitment, 60% of families had incomes >50 rupees per month (national average 28 rupees) and 15% of parents were illiterate (national average 66%). Nevertheless, 43% of families lived in one room. Hindus were the majority religious group (84%), followed by Sikhs (12%), Christians (2%), Muslims (1%) and Jains (1%).
Current study
In 1998-2002 we retraced 2,584 (32%) of the now adult cohort and 1,526 participated in a study to measure cardiovascular risk factors [14]. The current study was carried out in a second phase of investigations during 2006-2009. Participants were selected for dual X-ray absorptiometry (DXA) scans based on the completeness of their weight and height record in early life. A list was prepared of the 1,526 participants in phase 1, in descending order of the number of available measurements in early life, starting with those with complete measurements at birth, 6 months, 1 year and every year up to the age of 17 years (a maximum of 19 measurements, N=631) and the remainder in descending order of the number of measurements. Subjects were invited for DXA measurements starting from the top of this list and aiming for a total of 500. A total of 565 had bone measurements (men 338; women 227). Of these, 375 (66%) had 19 childhood weight and height measurements, a further 135 (24%) had 18 measurements and the remainder had a minimum of 15 measurements.
Participants were visited at home by a social worker who explained the study, obtained consent, and administered a questionnaire. Education was recorded as one of seven categories from ‘no schooling’ (category 1) through to ‘professional degree’ (eg. Masters degree, PhD, medical qualification) and occupation as one of six categories, ranging from ‘unemployed’ (category 1) and ‘unskilled manual labor’ (category 2) to ‘professional’. Housewives were categorized according to their husband’s occupation. Information on material possessions was recorded as an indicator of economic status. Subjects were given a score of one for each of the following household items: electricity, fan, cycle, radio, motorized 2-wheeler vehicle, gas stove, television, cable television, electric mixer, electric air cooler, washing machine, car, air conditioner, computer, television antenna, telephone and mobile phone. Alcohol consumption was recorded as the frequency of intake and measure-size of spirits, beer and wine per week. These data were converted into units of alcohol (1 unit = 25 ml of spirits, 282 ml of beer or 125 ml of wine), and categorized as none, <7 units, 7-14 units, and >14 units per week. Tobacco consumption was recorded as whether subjects smoked (cigarettes, beedis, cigars, or hookah), chewed (raw tobacco or with pan) or inhaled (snuff). Subjects were categorized simply as current tobacco users or non-users. A score was derived as a summary estimate of daily physical activity [15]. Work-related activity was classified on a 6-point scale ranging from ‘almost entirely sedentary’ to ‘heavy physical work’. Additional time spent per day in domestic activities (eg. sweeping, washing clothes, cooking) and leisure activities (eg jogging, swimming, yoga) was recorded. Distances walked and cycled each day, with and without a load, were recorded, and converted into approximate periods of time spent in these activities. These were then multiplied by metabolic constants, derived from the relative energy expenditure of activities [16] and summed to derive a score. For women, obstetric history and age of menarche were obtained by recall. .
Bone measurements
Bone mineral area, content (BMC) and areal density (aBMD) at the femoral neck, lumbar spine (L1-4) and forearm were measured at the All India Institute of Medical Sciences, New Delhi using a Hologic QDR 4500A fan beam DXA machine. The in vivo precision error was 0.62% for femoral neck aBMD, 0.65% for lumbar spine aBMD and 0.77% for forearm aBMD.
We calculated bone mineral apparent density (BMAD) as an estimate of volumetric bone mineral density using the following formulae:
For lumbar spine [17]
[L1 BMC + L2 BMC + L3 BMC + L4 BMC]/[L1 area1.5 + L1 area1.5 + L1 area1.5 + L1 area1.5].
For femoral neck [18]
Femoral neck BMC/(π/4) × 1.512 × femoral neck area2 (where 1.512 is a machine constant).
There is no equivalent formula for the forearm and so we were unable to examine associations of early life growth with forearm volumetric bone density.
None of the participants were taking bisphosphonates; 6 women taking oral contraceptives were included.
Statistical analysis
The distribution of the total exercise score was skewed and was log-transformed for the analysis. We compared the anthropometry in early life between the participants in this study and the remainder of the cohort using Chi square and two-sample t-tests.
We examined associations of height and BMI at exact ages in early life with adult bone outcomes using partial correlations and multiple linear regression. As previously described [14] we used all recorded data from the New Delhi Birth Cohort (not just those for subjects recruited for this study) to derive SD scores for height and BMI for each subject at age six months and at birthdays from age 1 to 21 years. The SD score is the number of standard deviations by which an observation differs from the mean for the cohort. Interpolated values were used if a measurement had been made within 6 months (up to 1 year), 1 year (age of 2 years), 1.5 years (age of 3 years), and 2 years (all older ages). Back transformation provided estimates of the measurements at these exact ages.
We also examined associations of growth (changes in height and BMI) during infancy (birth to 2 years), childhood (2-11 years) and adolescence (11 years to adulthood) with adult bone outcomes. To measure growth during a time interval (for example between the ages of 2 and 11 years) we regressed the value at the end of the interval (age 11 years) on the value at the beginning of the interval (age 2 years) and at earlier time points (birth), and expressed the residual as an SD score. This technique produces variables describing height or BMI changes at specific time points in childhood, independent of and uncorrelated with growth at earlier time points, which we refer to as conditional SD scores. This method is useful for examining associations with growth during specific time periods and overcomes the problem that sequential measurements of height and/or weight in any individual are highly correlated [19,20].
Regression models were initially adjusted for age and sex only, then additionally for adult lifestyle variables (exercise scores, tobacco use, alcohol consumption), socio-economic status (occupation, education, material possessions) and (for women) reproductive history (parity and age at menarche), and finally additionally for adult size (height and BMI). Interaction terms were used to test for differences in associations between the sexes. Analyses were carried out using SPSS version 16.
RESULTS
There were no significant differences in weight, height or BMI in early life between the 565 participants in this study and the remainder of the 1,526 members of the cohort that took part in phase 1 of the adult follow-up from whom they were selected, or the remainder of the original birth cohort for whom these data were available (N=7,528).
Descriptive data for weight, height and BMI in early and adult life, and adult bone measurements and lifestyle variables are shown in Table 1. Relative to the WHO international growth reference [21] the participants were short and thin at birth and during infancy and childhood. When they were re-studied as adults their mean BMI was 26.4 kg/m2 (men) and 27.9 kg/m2 (women); 63% of men and 71% of women were overweight or obese (BMI>25 kg/m2).
Table 1. Characteristics of the study cohort.
| Measurement | Men (N=338) | Women (N=227) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean | SD | N | Mean | SD | |
| ANTHROPOMETRY | ||||||
| Birth | ||||||
| Weight (g) | 337 | 2856 | 467 | 225 | 2795 | 399 |
| WHO weight SD score | 337 | -1.11 | 1.05 | 225 | -1.04 | 0.97 |
| Length (cm) | 337 | 48.5 | 2.2 | 225 | 48.2 | 2.1 |
| WHO length SD score | 337 | -0.71 | 1.15 | 225 | -0.49 | 1.11 |
| BMI (kg/m2) | 337 | 12.1 | 1.3 | 225 | 12.0 | 1.2 |
| WHO BMI SD score | 337 | -1.20 | 1.19 | 225 | -1.23 | 1.10 |
| At 2 years | ||||||
| Weight (kg) | 337 | 10.3 | 1.3 | 226 | 9.9 | 1.2 |
| WHO weight SD score | 337 | -1.52 | 1.09 | 226 | -1.26 | 0.99 |
| Height (cm) | 338 | 81.0 | 3.5 | 226 | 80.0 | 3.4 |
| WHO height SD score | 338 | -2.22 | 1.14 | 226 | -1.97 | 1.06 |
| BMI (kg/m2) | 337 | 15.7 | 1.2 | 226 | 15.5 | 1.2 |
| WHO BMI SD score | 337 | -0.07 | 1.00 | 226 | 0.02 | 0.89 |
| At 11 years | ||||||
| Weight (kg) | 332 | 27.9 | 4.1 | 221 | 28.5 | 5.5 |
| WHO weight SD score | 332 | -1.06 | 0.93 | 221 | -1.12 | 1.16 |
| Height (cm) | 333 | 135.7 | 5.3 | 221 | 135.4 | 6.7 |
| WHO height SD score | 333 | -1.11 | 0.79 | 221 | -1.45 | 1.01 |
| BMI (kg/m2) | 332 | 15.1 | 1.5 | 221 | 15.4 | 1.8 |
| WHO BMI SD score | 332 | -1.27 | 1.02 | 221 | -1.12 | 1.07 |
| Adult | ||||||
| Age (years) | 338 | 36.2 | 0.9 | 227 | 36.2 | 1.0 |
| Weight (kg) | 338 | 76.5 | 12.2 | 227 | 67.8 | 14.0 |
| Height (cm) | 338 | 170.2 | 6.3 | 227 | 155.9 | 5.2 |
| BMI (kg/m2) | 338 | 26.4 | 3.7 | 227 | 27.9 | 5.4 |
| BONE MINERAL MEASUREMENTS | ||||||
| Left femoral neck | ||||||
| Area (cm2) | 338 | 5.3 | 0.4 | 227 | 4.7 | 0.3 |
| BMC (g) | 338 | 4.3 | 0.7 | 227 | 3.6 | 0.6 |
| aBMD (g/cm2) | 338 | 0.81 | 0.12 | 227 | 0.78 | 0.12 |
| BMAD (g/cm3) | 338 | 0.29 | 0.05 | 227 | 0.32 | 0.05 |
| T-score | 338 | -0.88 | 0.92 | 227 | -0.64 | 1.05 |
| Lumbar spine | ||||||
| Area (cm2) | 338 | 60.0 | 5.6 | 227 | 51.0 | 4.6 |
| BMC (g) | 338 | 59.3 | 10.7 | 227 | 50.5 | 9.1 |
| aBMD (g/cm2) | 338 | 0.98 | 0.12 | 227 | 0.99 | 0.12 |
| BMAD (g/cm3) | 338 | 0.25 | 0.03 | 227 | 0.27 | 0.03 |
| T-score | 338 | -0.97 | 1.10 | 227 | -0.56 | 1.08 |
| Left forearma | ||||||
| Area (cm2) | 337 | 21.9 | 2.20 | 227 | 17.7 | 1.80 |
| BMC (g) | 337 | 13.2 | 1.9 | 227 | 9.4 | 1.3 |
| aBMD (g/cm2) | 337 | 0.60 | 0.05 | 227 | 0.53 | 0.04 |
| T-score | 337 | -1.33 | 1.02 | 227 | -0.69 | 0.78 |
| LIFESTYLE FACTORS | ||||||
| Exercise | ||||||
| Total scoreb | 338 | 852 | 1.4 | 227 | 705 | 1.8 |
| Any leisure exercise (%) | 338 | 28.4 | - | 227 | 41.0 | - |
| Tobacco use (%) | ||||||
| In past, not now | 338 | 3.6 | - | 227 | 0.0 | - |
| Current | 338 | 26.3 | - | 227 | 0.0 | - |
| Alcohol intake (%) | ||||||
| Current | 338 | 46.2 | - | 227 | 2.2 | - |
| Employment status (%) | ||||||
| Housewife | 337 | - | - | 226 | 64.2 | - |
| Unemployed | 337 | 1.2 | - | 226 | 0.4 | - |
| Unskilled or semi-skilled manual | 337 | 5.3 | - | 226 | 0.4 | - |
| Skilled manual | 337 | 35.0 | - | 226 | 26.1 | - |
| Non-manual, business or professional | 337 | 58.5 | - | 226 | 8.8 | - |
| Educational status (%) | ||||||
| Middle school or less | 338 | 8.6 | - | 227 | 5.3 | - |
| High school or diploma | 338 | 34.6 | - | 227 | 30.4 | - |
| Graduate | 338 | 43.8 | - | 227 | 48.9 | - |
| Postgraduate or professional | 338 | 13.0 | - | 227 | 15.4 | - |
| No. of household possessions | 338 | 13.3 | 2.3 | 227 | 13.2 | 2.0 |
| Parity | - | - | - | 222 | 2.7 | 1.5 |
| Age at menarche (years) | - | - | - | 223 | 13.6 | 1.2 |
Forearm not measured in one male participant
Geometric mean and standard deviation.
Twenty six percent of men were smokers, and 46% reported consuming alcohol, while no women smoked and only 2% drank alcohol. Most participants were graduates (Bachelors degree level or above); most men were in non-manual employment, and most women were housewives. Among the women, 19% were nulliparous, 19% had had one pregnancy, and 63% had had two or more pregnancies. The mean reported age at menarche was 13.6 years.
Adult size in relation to bone measurements
Adult height was positively correlated with bone area, BMC and (less strongly) with aBMD at all sites, but not with BMAD (Table 2). Adult BMI was positively correlated BMC, aBMD and BMAD, but not with bone area, at all sites.
Table 2. Partial correlations (adjusted for sex) of adult anthropometric and lifestyle variables with bone mineral outcomes.
| Left femoral neck |
Lumbar spine |
Left forearm |
Adult Height |
Adult BMI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | BMC | aBMD | BMAD | Area | BMC | aBMD | BMAD | Area | BMC | aBMD | |||
| ANTHROPOMETRY | |||||||||||||
| Age | 0.02 | 0.05 | 0.05 | 0.032 | 0.02 | 0.05 | 0.06 | 0.06 | 0.06 | 0.02 | −0.05 | ||
| Weight | 0.21*** | 0.55*** | 0.51*** | 0.37*** | 0.21*** | 0.39*** | 0.41*** | 0.37*** | 0.29*** | 0.42*** | 0.38*** | ||
| Height | 0.44*** | 0.39*** | 0.23*** | 0.02 | 0.67*** | 0.56*** | 0.32*** | 0.07 | 0.54*** | 0.53*** | 0.27*** | ||
| BMI | 0.03 | 0.42*** | 0.45*** | 0.39*** | −0.07 | 0.17*** | 0.30*** | 0.36*** | 0.08 | 0.21*** | 0.29*** | ||
| LIFESTYLE FACTORS | |||||||||||||
| Total exercise score | −0.04 | 0.02 | 0.03 | 0.05 | 0.02 | −0.01 | −0.02 | −0.03 | 0.02 | 0.01 | .01 | ||
| Any leisure exercise | 0.03 | 0.10* | 0.08* | 0.06 | 0.09* | 0.08* | 0.06 | 0.03 | 0.08 | 0.10* | 0.08 | ||
| Tobacco use | 0.05 | 0.04 | 0.01 | −0.01 | 0.02 | 0.02 | 0.01 | −0.00 | 0.08* | 0.07 | 0.01 | ||
| Any alcohol intake | 0.09* | 0.07 | 0.03 | −0.02 | 0.07 | 0.05 | 0.02 | −0.01 | 0.17*** | 0.14** | 0.03 | ||
| Employment status | −0.09* | 0.02 | 0.06 | 0.10* | 0.03 | 0.06 | 0.07 | 0.07 | −0.01 | 0.03 | 0.07 | ||
| Education | −0.04 | 0.01 | 0.03 | 0.04 | 0.01 | 0.02 | 0.03 | 0.02 | −0.01 | 0.03 | 0.05 | ||
| Household possessions | 0.04 | 0.10* | 0.14*** | 0.14** | 0.02 | 0.09* | 0.13** | 0.13** | 0.07 | 0.12** | 0.14** | ||
| Paritya | −0.09 | 0.08 | 0.13 | 0.16* | 0.11 | 0.11 | 0.10 | 0.07 | −0.04 | 0.05 | 0.14* | ||
| Age at menarchea | 0.08 | −0.16* | −0.20** | −0.20** | −0.05 | −0.12 | −0.13* | −0.13 | −0.13 | −0.14* | −0.11 | ||
p≤0.05;
p≤0.01;
p≤0.001.
Women only, therefore not adjusted for sex.
Adult lifestyle factors in relation to bone measurements
There were no correlations between bone measurements and total exercise score, but lumbar spine bone area, BMC at all sites, and femoral neck aBMD were higher in participants who took part in leisure exercise (Table 2). Higher socio-economic status as indicated by material wealth (greater number of household possessions) was associated with higher BMC, aBMD and BMAD. There were no associations of the bone measurements with tobacco use, while alcohol consumption was associated with higher forearm bone area and BMC. Women of higher parity had higher femoral neck and forearm BMAD. Earlier menarche was associated with higher femoral neck BMC, aBMD and BMAD, lumbar spine aBMD and forearm BMC. The positive associations of leisure exercise, household possessions and alcohol consumption with bone measurements were not statistically significant after adjusting for adult height and BMI.
Size and growth in early-life in relation to bone measurements – height
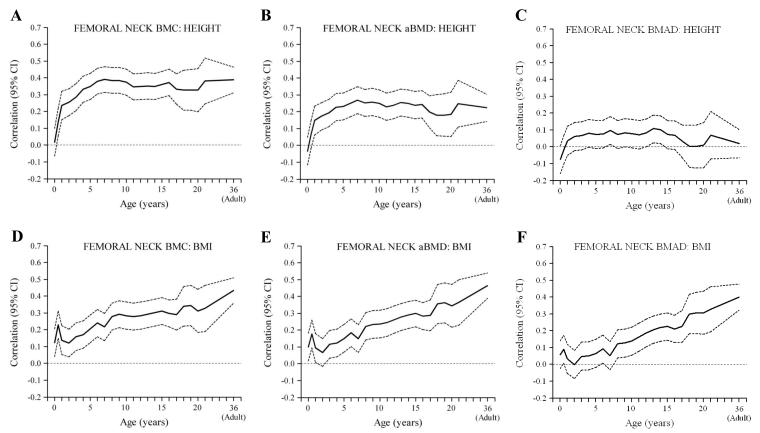
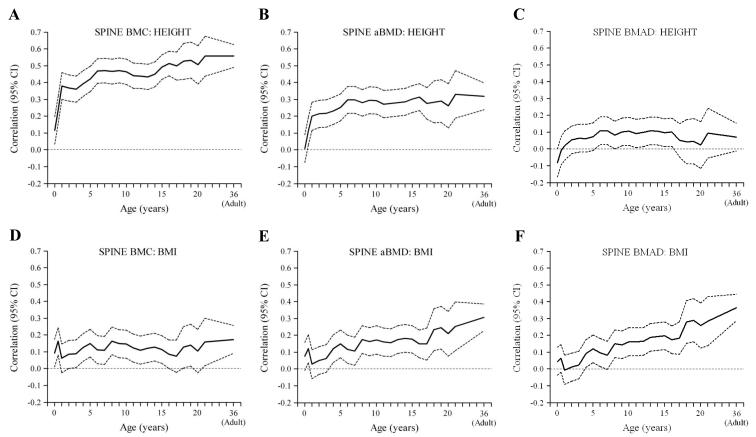
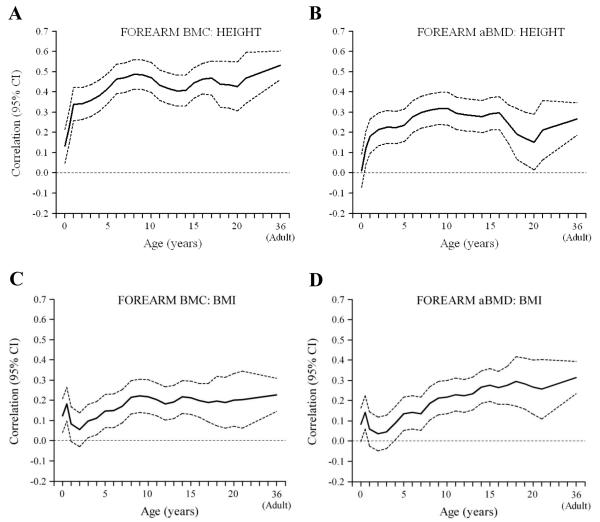
Birth length was positively correlated with lumbar spine and lumbar spine and forearm BMC, but not with aBMD or BMAD at any site (Figures 1 and 2a-c, Figure 3a,b). Length or height at all subsequent ages was, like adult height, positively correlated with bone area and BMC at all sites, less strongly with aBMD and only minimally with BMAD (Figures 1-3). The associations between early height and BMC increased in strength markedly between birth and approximately the age of 6 years, and remained constant thereafter. The positive associations of height at birth, and during infancy, childhood and adolescence with adult BMC were little changed after adjusting for adult lifestyle, socio-economic and reproductive variables and BMI. They were attenuated, however, and mainly non-significant, after adjusting for adult height.
Figure 1. Correlations between height (A-C) and BMI (D-F) in earlier life, and adult bone outcomes (femoral neck) (sexes combined).
Figure 2. Correlations between height (A-C) and BMI (D-F) in earlier life, and adult bone outcomes (lumbar spine) (sexes combined).
Figure 3. Correlations between height (A&B) and BMI (C&D) in earlier life, and adult bone outcomes (forearm) (sexes combined).
An analysis of growth (conditional changes in height) (Table 3) showed that an increase in height SD scores from birth to 2 years, 2-11 years and 11 years to adulthood were all positively associated with BMC at all sites, with the birth-2 year change showing the strongest associations. In contrast, there were non-significant or only weakly significant associations between height gain at any age and BMAD. Results for aBMD were intermediate between those for BMC and BMAD. All these associations were similar after adjusting for adult lifestyle, socio-economic and (in women) reproductive variables.
Table 3. Multiple linear regression using conditional height and BMI SD-scores in earlier life to predict adult BMC, aBMD and BMAD.
| Bone Mineral (SD) | Left femoral neck | Lumbar spine | Left forearm | Adult Height | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Area | BMC | aBMD | BMAD | Area | BMC | aBMD | BMAD | Area | BMC | aBMD | ||
| Length at birth (SD) | ||||||||||||
| B | 0.11 | 0.02 | -0.03 | -0.07 | 0.23 | 0.12 | 0.01 | -0.08 | 0.18 | 0.14 | 0.01 | 0.30 |
| 95% CI | 0.03, 0.19 | -0.06, 0.10 | -0.11, 0.05 | -0.16, 0.01 | 0.16, 0.29 | 0.05, 0.19 | -0.07, 0.09 | -0.16, 0.01 | 0.11, 0.25 | 0.06, 0.21 | -0.07, 0.09 | 0.30, 0.30 |
| p | 0.005 | 0.6 | 0.5 | 0.09 | <0.001 | 0.001 | 0.8 | 0.07 | <0.001 | <0.001 | 0.8 | <0.001 |
| Height change birth - 2 years (SD)a | ||||||||||||
| B | 0.20 | 0.26 | 0.20 | 0.09 | 0.37 | 0.34 | 0.22 | 0.09 | 0.27 | 0.31 | 0.22 | 0.53 |
| 95% CI | 0.12, 0.27 | 0.18, 0.34 | 0.11, 0.28 | 0.01, 0.18 | 0.31, 0.44 | 0.27, 0.41 | 0.14, 0.30 | 0.01, 0.17 | 0.19, 0.34 | 0.24, 0.38 | 0.14, 0.30 | 0.53, 0.54 |
| p | <0.001 | <0.001 | <0.001 | 0.03 | <0.001 | <0.001 | <0.001 | 0.04 | <0.001 | <0.001 | <0.001 | <0.001 |
| Height change 2-11 years (SD)a | ||||||||||||
| B | 0.23 | 0.25 | 0.17 | 0.05 | 0.28 | 0.27 | 0.18 | 0.08 | 0.22 | 0.28 | 0.21 | 0.41 |
| 95% CI | 0.15, 0.30 | 0.18, 0.33 | 0.09, 0.25 | -0.03, 0.14 | 0.21, 0.34 | 0.20, 0.34 | 0.10, 0.26 | -0.00, 0.17 | 0.15, 0.29 | 0.21, 0.35 | 0.13, 0.29 | 0.41, 0.42 |
| p | <0.001 | <0.001 | <0.001 | 0.2 | <0.001 | <0.001 | <0.001 | 0.06 | <0.001 | <0.001 | <0.001 | <0.001 |
| Height change 11-adult (SD)a | ||||||||||||
| B | 0.30 | 0.21 | 0.09 | -0.05 | 0.43 | 0.34 | 0.18 | 0.02 | 0.38 | 0.32 | 0.10 | 0.68 |
| 95% CI | 0.23, 0.38 | 0.13, 0.29 | 0.01, 0.17 | -0.13, 0.04 | 0.37, 0.49 | 0.27, 0.41 | 0.10, 0.26 | -0.06, 0.10 | 0.30, 0.45 | 0.25, 0.39 | 0.02, 0.18 | 0.67, 0.68 |
| p | <0.001 | <0.001 | 0.03 | 0.3 | <0.001 | <0.001 | <0.001 | 0.6 | <0.001 | <0.001 | 0.02 | <0.001 |
|
Adult BMI |
||||||||||||
| BMI at birth (SD) | ||||||||||||
| B | 0.08 | 0.12 | 0.09 | 0.05 | 0.08 | 0.09 | 0.07 | 0.05 | 0.11 | 0.12 | 0.09 | 0.13 |
| 95% CI | 0.00, 0.17 | 0.04, 0.19 | 0.02, 0.17 | -0.03, 0.13 | 0.00, 0.16 | 0.01, 0.17 | -0.01, 0.15 | -0.03, 0.13 | 0.03, 0.19 | 0.04, 0.20 | 0.01, 0.17 | 0.12, 0.13 |
| p | 0.05 | 0.002 | 0.01 | 0.2 | 0.06 | 0.03 | 0.07 | 0.2 | 0.01 | 0.003 | 0.04 | <0.001 |
| BMI change birth - 2 years (SD)a | ||||||||||||
| B | 0.13 | 0.11 | 0.06 | -0.01 | 0.09 | 0.07 | 0.04 | 0.00 | 0.03 | 0.04 | 0.02 | 0.23 |
| 95% CI | 0.05, 0.22 | 0.03, 0.18 | -0.02, 0.13 | -0.08, 0.07 | 0.01, 0.18 | -0.01, 0.16 | -0.04, 0.12 | -0.08, 0.08 | -0.05, 0.12 | -0.04, 0.12 | -0.06, 0.10 | 0.23, 0.24 |
| p | 0.002 | 0.006 | 0.1 | 0.9 | 0.03 | 0.09 | 0.4 | 1.0 | 0.4 | 0.4 | 0.6 | <0.001 |
| BMI change 2-11 years (SD)a | ||||||||||||
| B | 0.07 | 0.25 | 0.24 | 0.18 | -0.03 | 0.09 | 0.15 | 0.18 | 0.08 | 0.19 | 0.23 | 0.50 |
| 95% CI | -0.02, 0.15 | 0.17, 0.33 | 0.17, 0.32 | 0.11, 0.26 | -0.11, 0.06 | 0.01, 0.17 | 0.07, 0.23 | 0.10, 0.26 | 0.00, 0.17 | 0.11, 0.28 | 0.15, 0.31 | 0.50, 0.51 |
| p | 0.1 | <0.001 | <0.001 | <0.001 | 0.5 | 0.03 | <0.001 | <0.001 | 0.05 | <0.001 | <0.001 | <0.001 |
| BMI change 11-adult (SD)a | ||||||||||||
| B | -0.06 | 0.33 | 0.39 | 0.38 | -0.11 | 0.13 | 0.26 | 0.33 | 0.00 | 0.13 | 0.22 | 0.82 |
| 95% CI | -0.15, 0.02 | 0.25, 0.41 | 0.32, 0.47 | 0.30, 0.46 | -0.19, -0.02 | 0.04, 0.21 | 0.18, 0.34 | 0.26, 0.41 | -0.08, 0.09 | 0.05, 0.21 | 0.14, 0.30 | 0.82, 0.83 |
| p | 0.1 | <0.001 | <0.001 | <0.001 | 0.01 | 0.004 | <0.001 | <0.001 | 0.9 | 0.002 | <0.001 | <0.001 |
height and BMI changes are calculated as conditional measures (see Statistical Methods). The continuous outcome variables were standardised so that B (regression coefficient) values indicate the SD change in the outcome per SD change in the predictor. All analyses are adjusted for age and sex.
Size and growth in early-life in relation to bone measurements – BMI
Birth weight, and BMI at birth and during infancy were positively related to BMC at all sites, but not to aBMD or BMAD (Figures 1 and 2 d-f, Figure 3c,d). BMI during childhood and adolescence was positively related to BMC, aBMD and BMAD at all sites. Correlations between BMI and BMAD strengthened as the age of the earlier BMI measurement increased, becoming statistically significant between the ages of 4 years (forearm and lumbar spine) and 8 years (femoral neck).
The positive correlations of BMI at birth, and during infancy, childhood and adolescence with BMC were almost unchanged after adjusting for adult lifestyle, socio-economic and reproductive variables. They were attenuated and non-significant after adjusting for adult BMI, with the exception of the correlations with BMI at birth, which were no longer significant after further adjustment for adult height. The correlations of BMI in childhood and adolescence with aBMD and BMAD, were almost unchanged after adjusting for adult lifestyle, socio-economic and reproductive variables and adult height, but were no longer statistically significant after adjusting for adult BMI.
An analysis of BMI growth (changes in BMI in early life) (Table 3) showed that an increase in BMI SD scores from birth to 2 years, 2-11 years and 11 years to adulthood were all positively associated with femoral neck BMC, with the 11 year-to-adult change showing the strongest association. There were similar but weaker associations with spine and forearm BMC. Both aBMD and BMAD were unrelated to BMI gain from birth to 2 years, but were positively related to BMI gain from 2-11 years and 11 years to adulthood. All these associations were similar after adjusting for adult lifestyle, socio-economic and (in women) reproductive variables.
Interaction tests showed no evidence of differences in the associations described between men and women.
DISCUSSION
We measured femoral neck, lumbar spine and forearm bone area, BMC and aBMD, and spine and femoral neck BMAD, in a sample of 565 young adults from a birth cohort in New Delhi. At the age these subjects were studied, they would have attained peak bone mass and were unlikely to have started to lose bone. Length at birth, and height and height gain during infancy, childhood and adolescence were positively correlated with adult bone area and BMC at all three sites. The strongest associations for BMC were with height gain in infancy and early childhood (between birth and six years). There were no associations between earlier length or height and adult BMAD. Birth weight and BMI at birth, and BMI and BMI gain throughout infancy, childhood and adolescence were positively correlated with adult femoral neck BMC. BMI and BMI gain from mid-childhood onwards were correlated with femoral neck and spine BMAD; these associations strengthened with increasing age of BMI measurement. The significant associations of height and BMI in early life with adult bone measurements were attenuated and became non-statistically significant after adjusting for adult height and BMI, suggesting that the effects of early growth on bone measurements are explained by effects of early growth on adult size.
The mean values for aBMD were lower in Delhi than those observed in population-based studies of young European men and women [22]. We found positive associations of BMC, aBMD and BMAD at all sites with material wealth as indicated by household possessions, and positive associations between leisure-time physical activity and BMC and aBMD, but these were largely explained by adult height and BMI. Among women, earlier age at menarche was associated with higher femoral neck and lumbar spine aBMD. This has been shown previously, though not consistently in all studies [6]. We also found that higher parity was associated with higher femoral neck and forearm aBMD. Previous studies have generally shown no associations between parity and aBMD [6]. Although pregnancy and lactation are associated with bone loss, the deficit appears to be effectively restored afterwards. Consistent with previous studies, tobacco use was negatively related to femoral neck aBMD, though this was not statistically significant in our data.
The marked attenuation of correlations of height at birth and during infancy, childhood and adolescence with adult BMC after adjusting for adult height suggests that they mainly reflect the size of the skeletal envelope and attained adult height. The associations strengthened during early childhood (for approximately the first six years of life) and then reached a plateau (Figures 1-3). This is consistent with the observation that correlations between height in early life and adult height increase steeply between birth and early childhood, and remain virtually unchanged after that age [23]. Infancy and early childhood are critical periods for the attainment of height and therefore adult bone mass. Larger bone size is associated with higher areal density, but not necessarily volumetric density. Height and height growth at birth and during infancy, childhood and adolescence were, like adult height, only weakly related to our estimates of volumetric density, suggesting different determinants of bone size and mineralisation, although height gain from birth to 2 years remained significantly positively related to femoral neck and lumbar spine BMAD in the conditional growth models (Table 3).
Consistent with a number of other studies, in different populations [13], higher weight and BMI at birth were associated with higher adult BMC, but were not related to aBMD or BMAD. Higher BMI throughout infancy, childhood and adolescence were also associated with higher adult BMC, and these associations strengthened with increasing age of earlier BMI measurement (Figures 1-3). In contrast to height and height growth, BMI and BMI gain were associated with higher BMAD in the lumbar spine and femoral neck, effects that strengthened with increasing age (Figures 1 and 2), becoming statistically significant for BMI from age 4-8 years onwards, and BMI gain between 2-11 years and 11years-adulthood (Table 3). These associations were not statistically significant after adjusting for adult BMI, which was itself related to BMAD. These findings suggest that weight-bearing could be a major influence on volumetric bone density, and that the associations between childhood BMI and adult BMAD reflect the strong contribution of childhood and adolescent BMI to the attainment of adult BMI [15]. Another possible explanation is the conjoint effect of neurally-mediated factors such as leptin, on both adipocyte and osteoblast differentiation and function [24]. Finally, it could represent the influence of estrogen release from adipose tissue. The association between childhood BMI gain and adult BMAD could have clinical significance. We have recently shown in a Finnish cohort that poor BMI gain from 1-12 years of age was associated with an increased risk of hip fracture [25]. This study was records-based, and adult size was not available for most of the subjects.
It could be argued that height, weight and BMI in early life give no more information about bone mass and density than that obtained by knowing adult height, weight and BMI. However, this depends upon whether you have an adult or a child standing before you. If adult height and weight are known, size and growth in early life give no additional information about adult bone mass or density. However, if you are considering a child, knowing their early height and weight trajectory gives a more informed prediction of future bone mass and density. Since both bone size and density influence fracture risk [26], our data suggest that factors influencing growth and development in early life have implications for later fracture risk. In developing countries like India, underweight and stunting in childhood are common, and interventions in early life aimed at preventing under-nutrition and promoting infant length gain, which enhance survival and prevent stunting, could also have longer-term beneficial effects on bone health. Although greater BMI and BMI gain during childhood were associated with higher volumetric bone density in our study, we would not advocate promoting excessive childhood weight gain. We already know from this and other cohorts that excessive childhood BMI gain (upward crossing of BMI centiles) is associated with an increased risk of adult cardio-metabolic disease [27]. Furthermore, adult obesity does not protect against fracture risk, and may even increase risk [28].
The main strength of our study was the completeness of the longitudinal weight and height data, recorded by trained researchers, from birth to 21 years. Our study has some limitations. The individuals studied were selected because they were born in New Delhi, continued to live there in adulthood, and were willing to take part in this research. Only 7% of the original births in the cohort were re-traced and participated, and they could be unrepresentative of the original cohort, although we know that they were very similar in terms of early life anthropometry. Their families were affluent and well educated compared with national averages, and are therefore unrepresentative of the general Indian population. Differences between the cohort and the overall Indian population, missing data due to losses to follow up, and sample selection would be particularly important if our prime objective was to report population means for bone measurements. However, because we were analysing ‘internal’ (within-cohort) associations between early size/growth and adult outcomes, our results are less vulnerable to bias. DXA is the gold standard method for measurement of bone mass and density, but has some limitations. Increased adiposity is associated with greater measurement error, and sometimes over-estimation, of areal bone density by DXA, due to variability in the detection of the interface between soft tissue and bone [29,30]. Thus, participants with higher adult BMI may have an artefactual increase in areal BMD. However, we would not expect this to bias the pattern of correlations observed between early BMI and adult bone parameters. Another limitation is that so-called areal ‘density’ is not a true measurement of bone density and retains a correlation with bone size. We were able to overcome this to a large extent by using standard formulae to calculate bone volume, and volumetric bone density, but were unable to do this for forearm measurements.
In conclusion, we have demonstrated associations of birthweight and postnatal growth with bone mass in young adulthood in both sexes. Measures of bone size (area and BMC) were positively related to height and weight at birth and height growth during infancy, while measures of bone density were positively related to BMI and BMI gain during childhood and adolescence. Taller adults have larger bones, and thus higher bone mineral content, and heavier adults have denser bones; and taller height and larger BMI are attained through larger size and faster growth in early life. To our knowledge, this is the largest cohort in South Asia with recorded birthweight and postnatal growth to have been studied in this way. Our study supports previous observations that growth in utero, during infancy and early childhood is associated with skeletal size, mineral content and density in adulthood.
Mini abstract: Growth in early life may predict adult bone health. Our data showed that greater height and BMI gain in utero and infancy are associated with higher peak bone mass, and greater BMI gain in childhood/adolescence with higher peak bone density. These associations are mediated by attained adult height and BMI.
Acknowledgements
We thank the participants, and field and laboratory staff. We acknowledge Dr Shanti Ghosh and IM Moriyama who initiated the cohort study with Dr Bhargava, Vinod Kapani for technical input, Rajeshwari Verma and Bhaskar Singh for maintaining liaison with the cohort, Dileep Gupta, data manager and statistician at the Sitaram Bhartiya Institute for Science and Research, and Sushil Chugh and Jose Augustine, DXA technicians at the All India Institute of Medical Sciences. The original study was funded by the Indian Council of Medical Research and National Institutes of Health (USA). The current study was funded by the British Heart Foundation, the Wellcome Trust UK, the Medical Research Council UK, the NIHR Nutrition and Metabolism Biomedical Research Unit, University of Southampton and the NIHR Musculoskeletal Biomedical Research Unit, University of Oxford.
Funding source: British Heart Foundation; Wellcome Trust, UK; Medical Research Council, UK.
Footnotes
Conflict of interest: None of the authors has a conflict of interest
References
- 1.Consensus Development Conference Prophylaxis and treatment of osteoporosis. Osteoporosis Int. 1991;1:114–117. [PubMed] [Google Scholar]
- 2.Cooper C. Epidemiology of osteoporosis. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5th ed. American Society for Bone and Mineral Research; Lippincott-Raven, Philadelphia, USA: 2003. [Google Scholar]
- 3.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly; a worldwide projection. Osteoporosis Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Ralston SH. Do genetic markers aid in risk assessment? Osteoporosis Int. 1998;8:S37–S42. [PubMed] [Google Scholar]
- 6.Waugh EJ, Lam M-A, Hawker GA, McGowan J, Papaioannou A, Cheung AM, Hodsman AB, Leslie WD, Siminoski K, Jamal SA. Risk factors for low bone mass in healthy 40-60 year old women: a systematic review of the literature. Osteoporosis Int. 2009;20:1–21. doi: 10.1007/s00198-008-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporosis Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 8.Fujita Y, Iki M, Ikeda Y, Morita A, Matsukura T, Nishino H, Yamagami T, Kagamimori S, Kagawa Y, Yoneshimi H. Tracking of appendicular bone mineral density for 6 years including the pubertal growth spurt: Japanese Population-based Osteoporosis Kids Cohorts Study. J Bone Mineral Res. 2011;29:208–216. doi: 10.1007/s00774-010-0213-0. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Developmental origins of osteoporotic fracture. Osteoporosis Int. 2006;17:337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winsloe C, Earl S, Dennison EM, Cooper C, Harvey NC. Early life factors in the pathogenesis of osteoporosis. Curr Osteoporos Rep. 2009;4:140–144. doi: 10.1007/s11914-009-0024-1. [DOI] [PubMed] [Google Scholar]
- 12.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 13.Baird J, Kurshid MA, Kim M, Harvey N, Dennison EM, Cooper C. Does birth weight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporosis Int. 2011;22:1323–1334. doi: 10.1007/s00198-010-1344-9. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava SK, Sachdev HPS, Fall CHD, Osmond C, Lakshmy R, Barker DJP, Dey Biswas SK, Ramji S, Prabharkaran D, Reddy KS. Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. New Eng J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev HPS, Fall CHD, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, Reddy KS, Barker DJP, Bhargava SK. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood; the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Energy and protein requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Geneva: 1985. (Technical Report Series No. 724). [PubMed] [Google Scholar]
- 17.Carter D, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Clin Endocrinol Metab. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 18.Lu PW, Cowell CT, LLoyd-Jones SA, Briody JN, Howman-Giles R. Volumetric bone mineral density in normal subjects, aged 5-27 years. J Clin Endocrinol Metab. 1996;81:1586–1590. doi: 10.1210/jcem.81.4.8636372. [DOI] [PubMed] [Google Scholar]
- 19.Gale CR, O’Callaghan FJ, Bredow M, Martyn CN, Avon Longitudinal Study of Parents and Children Study Team The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118:1486–1492. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- 20.Adair LS, Martorell R, Stein AD, Hallal PC, Sachdev HPS, Prabhakaran D, Wills AK, Norris SA, Dahly DL, Lee NR, Victora CG, COHORTS group Size at birth, weight gain in infancy and childhood, and adult blood pressure in five low and middle income country cohorts: When does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Child growth standards. 2006 Available at: http://www.who.int/childgrowth.
- 22.Kaptoge S, da Silva JA, Brixen K, Reid DM, Kroger H, Nielsen TL, Andersen M, Hagen C, Lorenc R, Boonen S, de Vernejoul M-C, Stepan JJ, Adams J, Kaufman J-M, Reeve J. Geographical variation in DXA bone mineral density in young European men and women. Results from the network in Europe on male osteoporosis (NEMO) study. Bone. 2008;43:332–339. doi: 10.1016/j.bone.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Stein AD, Wang M, Martorell R, Norris SA, Adair LS, Bas I, Sachdev HPS, Bhargava SK, Fall CHD, Gigante DP, Victora CG, COHORTS Group Growth patterns in early childhood and final attained stature: data from five birth cohorts from low and middle-income countries. Am J Human Biol. 2010;22:353–359. doi: 10.1002/ajhb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amling M, Pogoda P, Beil FT, Schilling AF, Holzmann T, Priemel M, Blicharski D, Català-Lehnen P, Rueger JM, Ducy P, Karsenty G. Central control of bone mass; brainstorming the skeleton. Adv Exp Med Biol. 2001;496:85–94. doi: 10.1007/978-1-4615-0651-5_9. [DOI] [PubMed] [Google Scholar]
- 25.Javaid MK, Eriksson JG, Kajantie E, Forsen T, Osmond C, Barker DJP, Cooper C. Growth in childhood predicts hip fracture in later life. Osteoporosis Int. 2011;22:69–73. doi: 10.1007/s00198-010-1224-3. [DOI] [PubMed] [Google Scholar]
- 26.Ruyssen-Witrand A, Gossec L, Kolta S, Dougados M, Roux C. Vertebral dimensions as risk factor of vertebral fracture in osteoporotic patients: a systematic literature review. Osteoporosis Int. 2007;18:1271–8. doi: 10.1007/s00198-007-0356-6. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev HPS, Osmond C, Fall CHD, Lakshmy R, Ramji S, Dey Biswas SK, Prabhakaran D, Tandon N, Reddy KS, Barker DJP, Bhargava SK. Predicting adult metabolic syndrome from childhood body mass index; follow-up of the New Delhi Birth Cohort. Arch Dis Child. 2009;94:768–74. doi: 10.1136/adc.2008.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comptson JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Diez-Perez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi J, Rossini M, LaCroix AZ, Roux C, Sambrook PN, Siris ES, GLOW investigators Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hangartner TN, Johnston CC. Influence of fat on bone measurements with dual-energy absorptiometry. Bone Miner. 1990;9:71–81. doi: 10.1016/0169-6009(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 30.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2011 doi: 10.1002/jbmr.506. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]