Abstract
Background
Multiple micronutrient deficiencies are common among women in low-income countries and may adversely affect pregnancy outcomes.
Objective
This meta-analysis reports the effects on newborn size and duration of gestation of multiple micronutrient supplementation mainly compared with iron plus folic acid during pregnancy in recent randomized, controlled trials.
Methods
Original data from 12 randomized, controlled trials in Bangladesh, Burkina Faso, China, Guinea-Bissau, Indonesia, Mexico, Nepal, Niger, Pakistan, and Zimbabwe, all providing approximately 1 recommended dietary allowance (RDA) of multiple micronutrients to presumed HIV-negative women, were included. Outcomes included birthweight, other birth measurements, gestation, and incidence of low birthweight (LBW) (< 2,500 g), small-for-gestational age birth (SGA, birthweight below the within-each-population 10th percentile), large-for-gestational age birth (LGA, birthweight above the within-each-population 90th percentile), and preterm delivery (< 37 weeks).
Results
Compared with control supplementation (mainly with iron–folic acid), multiple micronutrient supplementation was associated with an increase in mean birthweight (pooled estimate: +22.4 g [95% CI, 8.3 to 36.4 g]; p = .002), a reduction in the prevalence of LBW (pooled OR = 0.89 [95% CI, 0.81 to 0.97]; p = .01) and SGA birth (pooled OR = 0.90 [95% CI, 0.82 to 0.99]; p = .03), and an increase in the prevalence of LGA birth (pooled OR = 1.13 [95% CI, 1.00 to 1.28]; p = .04). In most studies, the effects on birthweight were greater in mothers with higher body mass index (BMI). In the pooled analysis, the positive effect of multiple micronutrients on birthweight increased by 7.6 g (95% CI, 1.9 to 13.3 g) per unit increase in maternal BMI (p for interaction = .009). The intervention effect relative to the control group was + 39.0 g (95% CI, +22.0 to +56.1 g) in mothers with BMI of 20 kg/m2 or higher compared with –6.0 g (95% CI, –8.8 to +16.8 g) in mothers with BMI under 20 kg/m2. There were no significant effects of multiple micronutrient supplementation on birth length or head circumference nor on the duration of gestation (pooled effect: +0.17 day [95% CI, –0.35 to +0.70 day]; p = .51) or the incidence of preterm birth (pooled OR = 1.00 [95% CI, 0.93 to 1.09]; p = .92).
Conclusions
Compared with iron–folic acid supplementation alone, maternal supplementation with multiple micronutrients during pregnancy in low-income countries resulted in a small increase in birthweight and a reduction in the prevalence of LBW of about 10%. The effect was greater among women with higher BMI.
Keywords: Birth outcomes, birthweight, iron–folic acid, maternal body mass index, meta-analysis, multiple micronutrients, pregnancy, preterm delivery
Introduction
Low birthweight (LBW), resulting from restricted fetal growth, preterm birth, or both, is a persistent problem in disadvantaged populations of low-income countries and is associated with increased infant morbidity and mortality, childhood stunting and cognitive impairment, and an increased risk of adult chronic disease [1–4]. Short maternal height (resulting from undernutrition when the mother was herself a fetus and child) and low maternal body weight (resulting from undernutrition before and during pregnancy) are well-established causes of LBW [5, 6]. Randomized, controlled trials have shown that protein and energy supplementation during pregnancy has a positive effect on birthweight, assessed at 37.62 g (–0.21 to 75.45 g) in the latest meta-analysis [7]. The small size of this effect may be due to limiting micronutrient deficiencies.
Mothers in low-income countries frequently have inadequate micronutrient intakes [8]. Iron–folic acid supplements for pregnant women have been routinely recommended for use in most countries for several decades, but there is no consistent evidence of improvement in birthweight or other birth outcomes. Given the requirement for a whole range of micronutrients in metabolic pathways, repletion of only one or two micronutrients in a woman who has multiple deficiencies is likely to be ineffective. Since distribution systems are already in place to deliver iron–folic acid tablets to pregnant women, multiple micronutrient supplements may be a relatively cost-effective way of improving pregnancy outcomes in undernourished populations [8]. A Cochrane Collaboration systematic review of multiple micronutrient supplementation trials in pregnancy, published in 2006, concluded that compared with iron-folate supplementation, there were reductions in LBW and small-for-gestational age (SGA) births, but these were not statistically significant [9]. This paper is a review and meta-analysis of birth outcomes from 12 recently conducted randomized, controlled trials in 10 low-income countries in Asia (Bangladesh [10], China [11], Indonesia [12, 13], Nepal [14, 15], and Pakistan [16]), Africa (Burkina Faso [17], Guinea-Bissau [18], Niger [19], and Zimbabwe [20]), and Central and South America (Mexico [21]).
Methods
Details of the 12 randomized, controlled trials and the exact composition of the multiple micronutrients and control tablets used in each study are provided in the accompanying paper by Margetts et al. [22]. In brief, the investigators recruited mainly HIV-negative women, started supplementation in pregnancy rather than preconceptionally, and used a multiple micronutrient formulation that delivered approximately 1 RDA (recommended dietary allowance) daily. Nine studies used the United Nations International Multiple Micronutrient Preparation (UNIMMAP) of UNICEF/United Nations University/World Health Organization, while the studies in Mexico, Nepal (Sarlahi), and Zimbabwe used slightly different preparations. The UNIMMAP tablets contain 30 mg of iron, whereas the control tablets in most of the studies using the UNIMMAP supplement provided 60 mg of iron [22]. In trials with more than one control group, we compared outcomes in the group receiving multiple micronutrients with outcomes in the control group providing iron–folic acid in the doses closest to those in the multiple micronutrient supplement. Six trials were cluster randomized (China, Indonesia [Indramayu], Indonesia [Lombok], Nepal [Sarlahi], Pakistan, and Niger).
Outcomes included birthweight, other birth measurements (length, head circumference, and mid-upper-arm circumference, where available), duration of gestation, and incidence of LBW (< 2,500 g), small-for-gestational-age (SGA) birth (birthweight below the within-each-study-population 10th percentile for gestational age), large-for-gestational age (LGA) birth (birthweight above the within-each-study-population 90th percentile), and preterm delivery (gestation < 37 weeks). We used within-population definitions of SGA and LGA rather than using an external reference, because commonly used external references are derived from populations in high-income countries and would have produced very high rates of SGA and negligible rates of LGA; we were interested in the effect of the supplement on extremes of birthweight within each population.
Statistical methods
We initially excluded mothers who were known to be HIV positive, known to be carrying multiple pregnancies, or were assigned to intervention groups other than the selected control group or the group receiving multiple micronutrients. Only one pregnancy per woman (the earliest) was included. For the analyses reported in this paper on birth outcomes, the analysis was further restricted to live births occurring after at least 28 weeks of gestation, babies measured within 72 hours after delivery, and babies whose gestational age at delivery was recorded as at least 28 weeks (196 days) and less than 45 weeks (315 days). For the outcomes SGA, LGA, gestational age, and preterm delivery, the sample was further restricted to exclude babies with implausible combinations of birthweight and gestational age (see below). Random-effects meta-regression models were used, adjusted for cluster design where appropriate, to derive treatment effects in each trial, pooled values, and forest plots. Effect size estimates were also derived after adjustment for the infant’s sex and maternal age, weight (at recruitment), parity, and education. Heterogeneity between studies was tested with the use of the I-square statistic with a significance level set at < .10. Interaction tests were used to explore differences in supplementation effects according to maternal age, parity, height, and BMI and the gestational age at which supplementation started. All were treated as continuous variables, except for parity, which was categorized in two ways, into two groups (nulliparous versus multiparous women) and into three groups (parity of 0, 1, or ≥ 2). Both linear and quadratic interactions were tested. Maternal BMI was measured at recruitment, which occurred at various gestational ages and was therefore adjusted to a gestational age of 105 days (15 weeks) using linear regression analysis, within each study.
Exclusion of implausible gestational age data
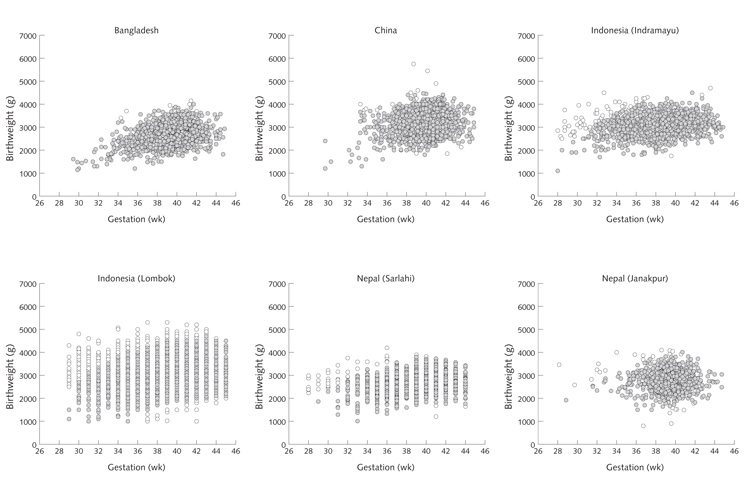
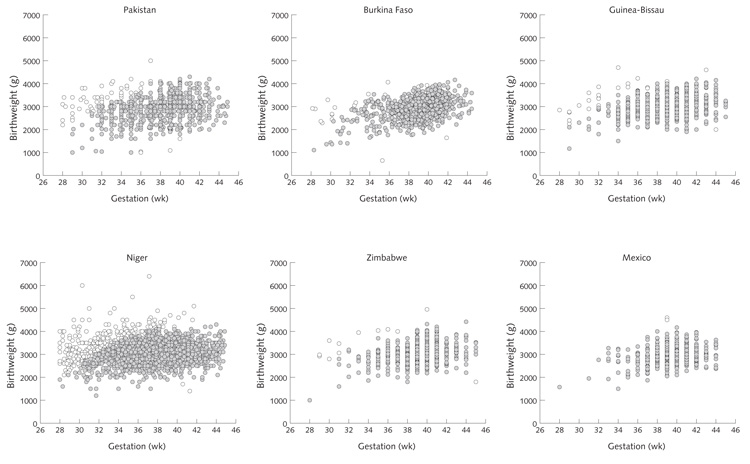
Gestational ages based on the date of the last menstrual period are often underestimated in population studies [23–27]. Many combinations of birthweight and gestational age were implausible in the data from the 12 trials (fig. 1), suggesting frequent underestimation of gestational age. This phenomenon was particularly marked in Pakistan and Niger, leading to an implausibly high incidence of preterm delivery (29% and 41%, respectively). To overcome this problem, a “generic” fetal growth curve was constructed using sex-specific 10th, 50th, and 90th percentile values for birthweight at gestational ages of 27 to 45 weeks from an external reference, based on more than 2 million births in the United States [28]. From this curve, mean (± SD) birthweight values at different gestational ages were derived. This curve was then applied to data from each of the 12 trials, scaled down according to the full-term birthweight (38 to 40 weeks) in each location. Mean (± SD) values for birthweight at all gestational ages were derived for each study. Babies whose birthweight was more than 3 SD above or below the gestation-specific mean were excluded from analyses of outcomes involving gestational age (SGA, LGA, gestational length, and preterm deliveries). Data excluded in this way are indicated by open circles in figure 1.
FIG. 1.
Scatterplots of birthweight according to gestational age, for each of the 12 trials, showing (open circles) data removed because of implausible combinations of birthweight and gestational age (all based on the “restricted sample”)
Results
The numbers of mothers included in the analysis of birth outcomes ranged from 583 in Mexico to 13,270 in Indonesia (Lombok). The mean birthweight (babies in both trial groups) ranged from 2,649 g in Bangladesh to 3,198 g in China, and mean gestation ranged from 263 days (37.6 weeks) in Niger to 279 days (39.9 weeks) in China. Additional details on maternal anthropometry and other characteristics are given in table 2 of the paper by Margetts et al. in this issue [22].
Birthweight
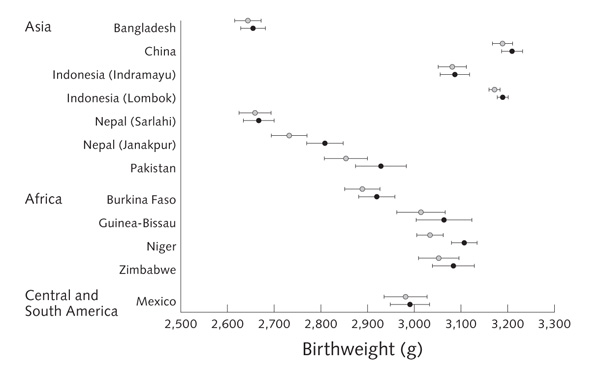
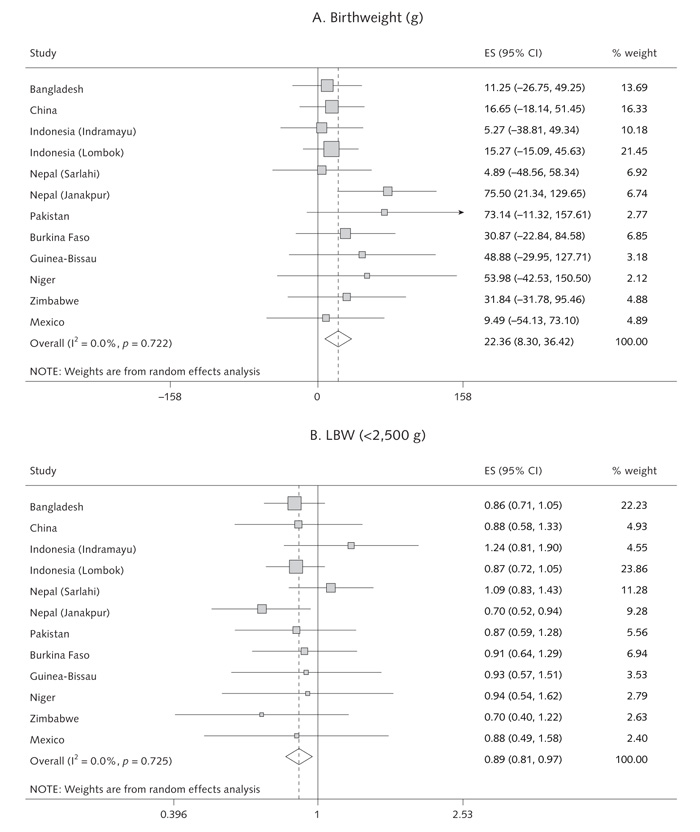
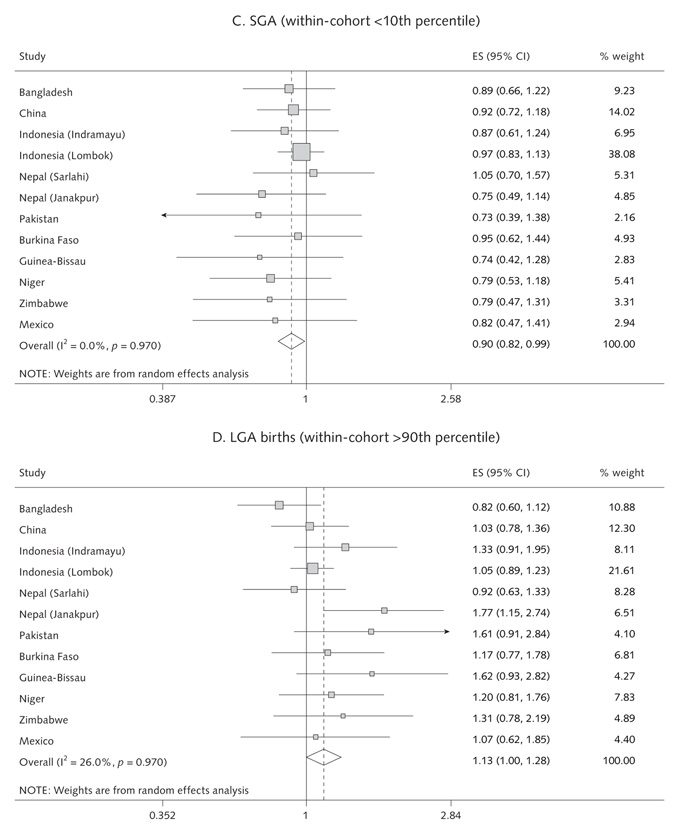
Results for birthweight and prevalence of LBW, SGA, and LGA births are shown in table 1 and figures 2 and 3. Compared with the control group, multiple micronutrient supplementation was associated with an increase in mean birthweight (pooled estimate: + 22.4 g [95% CI, 8.3 to 36.4 g]; p = .002; range across studies: + 4.9 to + 75.5 g). There were reductions in the incidence of LBW (pooled OR = 0.89 [95% CI, 0.81 to 0.97]; p = .01; range, 0.70 to 1.24) and SGA birth (pooled OR = 0.90 [95% CI, 0.82 to 0.99]; p = .03; range, 0.73 to 1.05). There was an increase in LGA births (pooled OR = 1.13 [95% CI, 1.00 to 1.28]; p = .04). These results were not significantly altered when adjusted for the infant’s sex and the mother’s age, weight, parity, and education. The size of the effect on birthweight was unrelated to the mean birthweight in each population (fig. 2) (p-value for meta-regression slope = .75). There was no significant heterogeneity between studies.
TABLE 1.
Birth outcomes in multiple micronutrient and control groupsa
| Study | Mean ± SD birthweight (g) |
LBW (< 2,500 g) (%) |
SGA (within-cohort < 10th centile) (%) |
LGA (within-cohort > 90th centile) (%) |
Mean ± SD gestation (days) |
Preterm birth (< 37 wk) (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MMN | Control | MMN | Control | MMN | Control | MMN | Control | MMN | Control | MMN | Control | |
|
| ||||||||||||
| Bangladesh [10] | 2,654 ± 399 n = 904 |
2,643 ± 427 n = 914 |
32.3 n = 904 |
35.7 n = 914 |
9.5 n = 901 |
10.5 n = 910 |
9.1 n = 901 |
10.9 n = 910 |
275 ± 14 n = 901 |
274 ± 15 n = 910 |
9.8 n = 901 |
12.7 n = 910 |
| China [11] | 3,208 ± 428 n = 1,364 |
3,189 ± 415 n = 1,418 |
3.3 n = 1,364 |
3.7 n = 1,418 |
9.6 n = 1,352 |
10.4 n = 1,415 |
10.2 n = 1,352 |
9.8 n = 1,415 |
279 ± 12 n = 1,352 |
279 ± 12 n = 1,415 |
4.1 n = 1,352 |
4.7 n = 1,415 |
| Indonesia (Indramayu) [12] |
3,086 ± 436 n = 726 |
3,081 ± 416 n = 709 |
6.9 n = 726 |
5.6 n = 709 |
9.4 n = 692 |
10.7 n = 676 |
11.1 n = 692 |
8.7 n = 676 |
269 ± 20 n = 692 |
270 ± 19 n = 676 |
27.9 n = 692 |
25.7 n = 676 |
| Indonesia (Lombok) [13] |
3,188 ± 485 n = 6,791 |
3,171 ± 490 n = 6,479 |
4.6 n = 6,791 |
5.3 n = 6,479 |
9.9 n = 6,492 |
10.2 n = 6,177 |
10.1 n = 6,492 |
9.7 n = 6,177 |
273 ± 19 n = 6,492 |
273 ± 19 n = 6,177 |
21.7 n = 6,492 |
21.0 n = 6,177 |
| Nepal (Sarlahi) [14] | 2,667 ± 432 n = 676 |
2,659 ± 429 n = 611 |
34.5 n = 676 |
33.2 n = 611 |
10.2 n = 648 |
9.8 n = 589 |
9.6 n = 648 |
10.4 n = 589 |
272 ± 18 n = 648 |
70 ± 19 n = 589 |
17.9 n = 648 |
21.1 n = 589 |
| Nepal (Janakpur) [15] |
2,809 ± 446 n = 500 |
2,733 ± 426 n = 496 |
19.2 n = 500 |
25.4 n = 496 |
8.8 n = 480 |
11.4 n = 483 |
12.5 n = 480 |
7.5 n = 483 |
275 ± 13 n = 480 |
276 ± 12 n = 483 |
8.1 n = 480 |
7.5 n = 483 |
| Pakistan [16] | 2,928 ± 568 n = 413 |
2,853 ± 521 n = 488 |
18.9 n = 413 |
21.1 n = 488 |
8.6 n = 372 |
11.4 n = 456 |
12.1 n = 372 |
8.1 n = 456 |
266 ± 20 n = 372 |
266 ± 21 n = 456 |
29.6 n = 372 |
29.4 n = 456 |
| Burkina Faso [17] | 2,919 ± 442 n = 493 |
2,889 ± 418 n = 494 |
14.6 n = 493 |
15.8 n = 494 |
9.8 n = 481 |
10.2 n = 488 |
10.6 n = 481 |
9.2 n = 488 |
273 ± 16 n = 481 |
274 ± 16 n = 488 |
13.9 n = 481 |
13.1 n = 488 |
| Guinea-Bissau [18] | 3,063 ± 524 n = 294 |
3,014 ± 460 n = 306 |
11.9 n = 294 |
12.7 n = 306 |
8.6 n = 279 |
11.3 n = 291 |
12.2 n = 279 |
7.9 n = 291 |
274 ± 21 n = 279 |
274 ± 20 n = 291 |
23.2 n = 279 |
22.3 n = 291 |
| Niger [19] | 3,106 ± 492 n = 1,214 |
3,033 ± 466 n = 1,107 |
6.9 n = 1,214 |
7.9 n = 1,107 |
9.1 n = 1,039 |
11.1 n = 937 |
10.9 n = 1,039 |
9.0 n = 937 |
263 ± 21 n = 1,039 |
264 ± 21 n = 937 |
41.9 n = 1,039 |
40.9 n = 937 |
| Zimbabwe [20] | 3,084 ± 430 n = 341 |
3,052 ± 426 n = 355 |
6.5 n = 341 |
9.0 n = 355 |
8.9 n = 325 |
11.1 n = 334 |
11.1 n = 325 |
8.6 n = 334 |
276 ± 15 n = 325 |
274 ± 17 n = 334 |
11.1 n = 325 |
14.7 n = 334 |
| Mexico [21] | 2,990 ± 380 n = 297 |
2,981 ± 403 n = 286 |
7.7 n = 297 |
8.7 n = 286 |
9.1 n = 296 |
11.0 n = 283 |
10.1 n = 296 |
9.5 n = 283 |
277 ± 14 n = 296 |
277 ± 15 n = 283 |
7.1 n = 296 |
6.4 n = 283 |
|
|
||||||||||||
| Pooled effect | + 22.4 g 95% CI, 8.3 to 36.4 g p = .002 |
OR = 0.89 95% CI, 0.81 to 0.97 p = .01 |
OR = 0.90 95% CI, 0.82 to 0.99 p = .03 |
OR = 1.13 95% CI, 1.00 to 1.28 p = .04 |
0.17 days 95% CI, − 0.35 to +0.70 p = .51 |
OR = 1.00 95% CI, 0.93 to 1.09 p = .9 |
||||||
| Pooled adjusted effect |
+ 22.5 g 95% CI, 8.1 to 36.9 g p = .002 |
OR = 0.88 95% CI, 0.80 to 0.97 p = .01 |
OR = 0.90 95% CI, 0.82 to 0.99 p = .04 |
OR = 1.15 95% CI, 0.99 to 1.34 p = .06 |
0.11 days 95% CI, − 0.45 to + 0.68 p = .70 |
OR = 1.02 95% CI, 0.94 to 1.11 p = .7 |
||||||
CI, confidence interval; LBW, low-birthweight; LGA, large-for-gestational age; MMN, multiple micronutrients; OR, odds ratio; SGA, small-for-gestational age
The following were excluded: women known to be HIV-positive, women known to have a multiple pregnancy, fetal losses before 28 weeks, stillbirths, infants with gestational age at delivery < 189 or > 314 days, and babies measured < 72 hours after birth. Only one pregnancy (the earliest) was included for each mother. Information on the age at which the baby was measured was not available for Burkina Faso, Zimbabwe, and Mexico. For outcomes including gestation (SGA, LGA, length of gestation, and preterm birth), women were excluded from the analysis if the gestational age was unlikely given the birthweight (see Statistical Methods). The pooled estimates are derived from random-effects meta-analysis, with adjustment for cluster design in six of the studies. Adjusted effects are further adjusted for maternal age and weight at recruitment, parity, and education and the sex of the baby.
FIG. 2.
Mean (95% CI) birthweight for multiple micronutrient (filled circles) and control (open circles) groups in each center
FIG. 3.
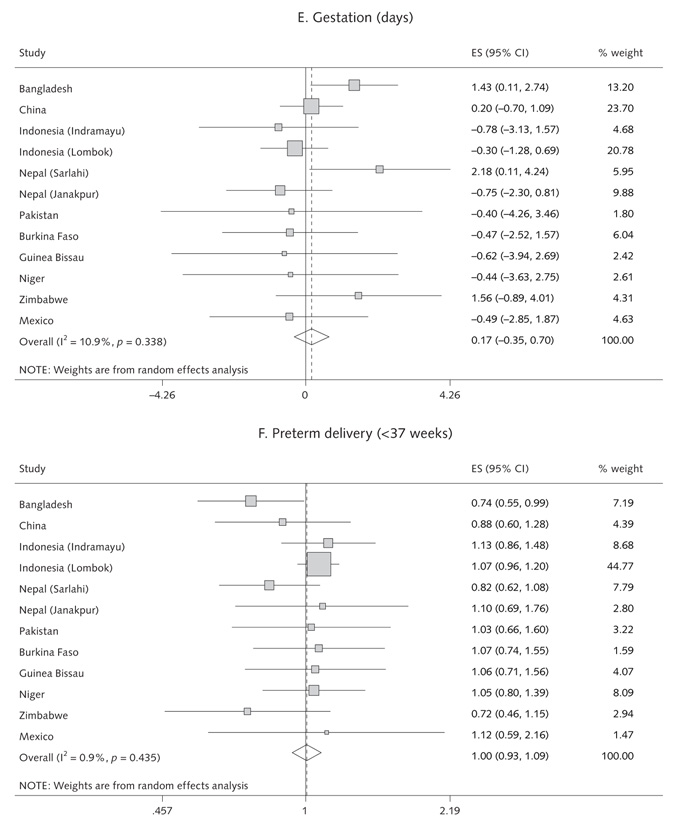
Random-effects model forest plots for effects of MMN supplementation compared with controls on (a) birthweight, (b) low birthweight, (c) SGA births, (d) LGA births, (e) gestation and (f) pre-term delivery
Interactions with maternal characteristics
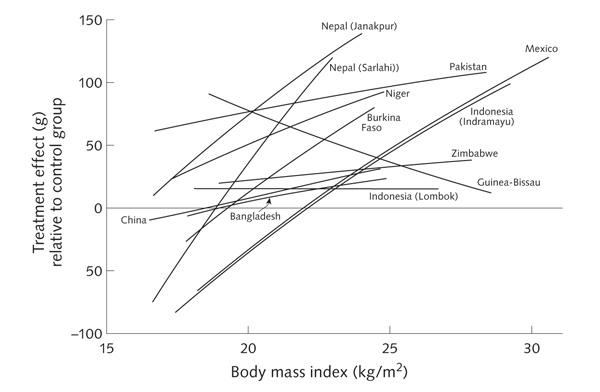
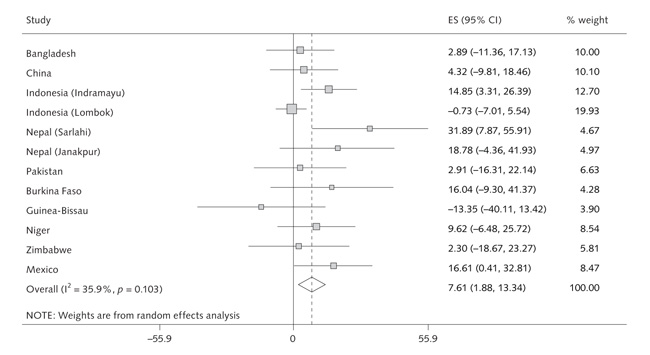
We examined whether the effects of multiple micronutrients on birthweight varied according to maternal age, parity, BMI, and height. The Indonesia (Indramayu), Nepal (Sarlahi), and Mexico studies showed significant linear interactions with maternal BMI, with a larger effect of multiple micronutrient supplementation on birthweight as maternal BMI increased (figs. 4 and 5). Meta-analysis (fig. 5) showed an overall difference in effect on birthweight between the multiple micronutrient and control groups of 7.6 g (95% CI, 1.9 to 13.3 g) per kilogram per square meter increase in maternal BMI (p for interaction = .009). There was moderate heterogeneity among studies (I2 = 36%, p = .10) (fig. 5). In some studies, the intervention effect relative to the control group was negative in women with low BMI (fig. 4). In a pooled analysis of all studies, the intervention effect relative to the control group was +39.0 g (95% CI, +22.0 to +56.1 g) in mothers with BMI of 20 kg/m2 or higher, compared with –6.0 g (95% CI, –28.8 to +16.8 g) in mothers with BMI less than 20 kg/m2, a difference that was highly statistically significant (p < .001) and did not change after adjustment for maternal age and education.
FIG. 4.
Effect on birthweight of MMN supplementation relative to the control group according to maternal BMI. The lines are truncated to the 5th and 95th percentiles for BMI for each dataset
FIG. 5.
Random effects model showing the interaction between maternal BMI and supplement effect. The effect size indicates the change in birthweight (g) in the MMN group relative to the control groups per unit increase in maternal BMI
The Guinea-Bissau and Niger studies showed significant interactions with maternal height, with larger effects on birthweight in taller mothers. However, this effect was inconsistent between studies, and there was no significant overall effect (the difference in the effect on birthweight between multiple micronutrient supplementation and iron–folic acid supplementation was + 1.4 g per centimeter increase in maternal height; p for interaction = .18). The Burkina Faso study showed a positive effect of the intervention with multiple micronutrients on birthweight in multiparous mothers, but no effect in nulliparous mothers. The opposite was true in the Nepal (Janakpur) and Pakistan studies. In a pooled analysis, there was no significant interaction with parity, regardless of how the data were categorized (p for interaction = 0.7 using parity as a binary variable, nulliparous versus multiparous). There was no significant interaction between supplement type and maternal age.
Other birth measurements
Birth length was available in all 12 studies except Indonesia (Lombok). There were no significant effects of micronutrient supplementation on birth length in any individual study (range, –0.2 to +0.7 cm), and there was no significant effect in the meta-analysis (pooled estimate, +0.06 cm; p = .20). Head circumference was measured in 10 studies (not in Bangladesh or Mexico). The mean effect of multiple micronutrient supplementation ranged from –0.4 to +0.3 cm. The meta-analysis showed no overall effect (pooled estimate, +0.03 cm; p = .47). Mid-upper-arm circumference was measured in three studies (Pakistan, Burkina Faso, and Guinea-Bissau). The effect of multiple micronutrient supplementation ranged from +0.1 to +0.8 cm. The meta-analysis showed no overall effect (pooled estimate, +0.6 cm; p = .16).
Duration of gestation and preterm births
The intervention with multiple micronutrients was not associated with an increase in gestation (pooled effect, +0.17 days [95% CI, –0.35 to +0.70]; p = .51; range, –0.76 to +2.2 days) or a reduction in preterm births (pooled OR = 1.00 [95% CI, 0.93 to 1.09]; p = .92; range, 0.72 to 1.13) (table 1 and fig. 3).
Duration of supplementation
There was no evidence that starting supplements earlier in pregnancy was associated with greater effects (data not shown).
Fully adjusted analyses
The findings for all outcomes were little changed if we adjusted for maternal weight, age, parity, and education and infant’s sex (see pooled adjusted estimates in table 1). The findings were similar if the raw data were used and if subjects with implausible gestational ages were retained, and there were no changes in the main findings if the Mexico trial, in which the control group received iron alone, was excluded.
Discussion
We had the privilege of access to the raw data from 12 recent, high-quality, randomized, controlled trials, all carried out with similar protocols in low-income countries. All used a multiple micronutrient supplement providing approximately 1 RDA of an extensive range of vitamins and minerals (nine used an identical multiple micronutrient supplement, UNIMMAP [United Nations International Multiple Micronutrient Preparation]). Overall, multiple micronutrient supplementation led to a significant, although small, increase (22.4 g) in birthweight, reductions in LBW and SGA births (11% and 10%, respectively), and an increase (13%) in LGA births, compared with iron–folic acid (nine trials), iron–folic acid and vitamin A (one trial), iron alone (one trial), or placebo (one trial). There were no significant increases in other birth measurements (length, head circumference, and mid-upper-arm circumference), although data on mid-upper-arm circumference were limited. There was a consistent lack of effect on the duration of gestation or the incidence of preterm delivery. The effect of multiple micronutrient supplementation on birthweight therefore appears to be due mainly to an increase in size for gestational age (indicating more rapid fetal growth) and to increased soft tissue rather than skeletal growth and to be manifested by an upward shift in the entire birthweight distribution. The effect on birthweight of multiple micronutrient supplementation was most strongly positive in mothers with higher BMI. In mothers with low BMI, the effect of multiple micronutrients relative to the control group was close to zero in most studies and was negative in some studies (fig. 4).
The increase in birthweight and reduction in LBW and SGA births was consistent with the results of an earlier meta-analysis [9] and with other, more recent trials [29, 30]. There was no increase in other birth measurements. The Burkina Faso trial reported a 2.9-mm increase in birth length [17], but there was no significant effect in the subset of pregnancies included in this meta-analysis, and none of the other original trials reported an increase in birth length. There was no reduction in preterm births. This conclusion seems robust, because although gestational age was clearly inaccurately estimated in some studies (fig. 1), there was no effect of multiple micronutrient supplementation on gestation or incidence of preterm delivery, even in the studies in which very few babies were excluded because of implausible associations between birthweight and gestation.
An important question is whether the small overall increase in birthweight translates into functional benefits for the children. Based on the relationship of birthweight to infant mortality [28], an increase of 22.4 g would be expected to result in a negligible reduction in infant mortality. Only 1 of the 12 trials (Lombok, Indonesia) [13] was powered to examine this outcome, and this trial showed a reduction in infant mortality. However, a meta-analysis in this issue by Ronsmans et al. of infant mortality in the 12 trials [31] showed no reduction in stillbirths, perinatal mortality, or early and late neonatal mortality. There are now some published studies of other functional outcomes in the children born in these trials. The Nepal (Sarlahi) group found no benefits of multiple micronutrient supplementation on morbidity in infancy [32]. However, the Nepal (Janakpur) study group recently reported that at 2.5 years, children of mothers who had taken multiple micronutrient supplements were on average 200 g heavier than control children, had larger head, chest, and mid-upper-arm circumferences and triceps skinfold thickness, and lower systolic blood pressure [33]. More such follow-up studies are needed in order to weigh up the potential public health benefits of multiple micronutrient supplementation. Effects on the well-being of the mother also need to be included in the assessment of benefits; the paper by Allen and Peerson in this issue [34] showed that multiple micronutrient supplementation improved maternal hemoglobin and micronutrient status.
We found that multiple micronutrient supplementation was associated with an increase in the incidence of LGA babies (defined as above the 90th within-population percentile) as well as a reduction in the incidence of SGA babies. In debates about nutritional interventions in malnourished women, concern has been expressed about the possibility of inducing cephalopelvic disproportion and thus increasing the number of obstructed deliveries [35]. Against this is the argument that interventions would have their maximal effect at the lower end of the birthweight range and would prevent LBW but not produce an increase in the number of large babies [36]. Our analysis showed that multiple micronutrient supplementation produced an upward shift of the whole birthweight distribution. The Nepal (Sarlahi) study group has carried out a detailed analysis of the birthweight distribution in the different arms of their trial [37]. Multiple micronutrient supplementation increased birthweight across the whole distribution, in contrast to the other interventions (folic acid, iron–folic acid, and iron–folic acid–zinc), which specifically reduced the number of babies in the lower tail of the distribution. The authors suggested that reporting the change in mean birthweight is not a sufficient description of the effects of an intervention. They argued that interventions acting mainly at the lower end of the distribution may be preferable to those that produce a rightward shift of the whole distribution, and that the latter may even be harmful and could explain the (nonstatistically significant) increases in birth asphyxia and neonatal mortality in the group receiving multiple micronutrients in their study [37–39]. Head size is the neonatal measurement most likely to influence the risk of cephalopelvic disproportion. In our analysis, there was no significant effect of multiple micronutrient supplementation on newborn head circumference. However, the duration of labor and the incidence of assisted or operative delivery were not included in our analysis, and it would be important to consider these outcomes in further work. Data on these outcomes were not included in most of the publications from these 12 trials.
The positive effect of multiple micronutrient supplementation on birthweight was greatest in heavier women (fig. 4). The corollary of this was that the mean effect on birthweight among women with low BMI was around zero for most studies. The trials were not designed to examine interactions with maternal size, and these post hoc analyses must therefore be treated with caution [40, 41]. However, it is reasonable to speculate that micronutrients are not optimally utilized in the presence of maternal energy deficiency and may conceivably place additional strain on energy-deficient mothers because of the need to metabolize them.
We suggest that better evidence of functional benefits for mothers and children is required before multiple micronutrients can be recommended on a large scale in place of iron–folic acid. The data on infant mortality presented by Ronsmans et al. in this issue [31] suggest that further research and monitoring in studies with larger sample sizes are required. We included in our meta-analysis only studies that provided approximately 1 RDA of multiple micronutrients. The Guinea-Bissau trial included in this review had a 2× RDA group, and the increase in birthweight was larger than in the 1× RDA group [18]. Another study in Tanzania, using supplements containing twice the RDA of micronutrients, in HIV-positive mothers, also showed a large effect on birthweight and a 44% reduction in LBW [42]. However, a 2× RDA supplement for HIV-negative women in the same setting [43] did not suggest any greater benefit. There is evidence from animal studies that improvements in maternal nutrition require more than one generation to produce improvements in fetal growth [44]. In the Guatemala INCAP trial, maternal supplementation with Atole (a high-energy, highprotein, multiple micronutrient supplement) had no significant effect on birthweight compared with Fresco (a supplement containing the same micronutrients but less energy and no protein), but the children grew taller [45]. This should produce improved birth outcomes in the next generation [46]. Such long-term follow-up will be needed to establish the full effects of nutritional interventions in mothers. It is important that the trials included in our analysis be utilized to their full potential in this way.
There is already good evidence that micronutrient supplementation before conception has benefits for fetal development. The best example is periconceptional folic acid supplementation, which reduces the incidence of neural tube defects [47]. Supplementation of women only after they know that they are pregnant is therefore clearly a less than optimal intervention. Furthermore, there is growing evidence that maternal undernutrition in the periconceptional period increases the risk of preterm delivery [48, 49] and reduces fetal growth, even if nutrition improves later in pregnancy. Future research should include studies of maternal nutrient repletion before as well as during pregnancy.
In conclusion, supplementation of pregnant women with 1 RDA of multiple micronutrients increases birthweight and substantially reduces the rates of LBW and SGA births. The effect on birthweight is greater in women with higher BMI and is very small in energydeficient mothers. There appears to be an upward shift of the whole birthweight distribution, resulting in an increase in LGA births. Further research is needed to assess the effects of these changes in fetal growth on future health and capacity.
Acknowledgments
Support for this paper came from UNICEF and the United Nations System Standing Committee on Nutrition (SCN).
Footnotes
The Maternal Micronutrient Supplementation Study Group (MMSSG) is composed of Pierre Adou, Víctor M. Aguayo, Lindsay H. Allen, Zulfiqar Ahmed Bhutta, Parul Christian, Shaonong Dang, Gwenola Desplats, Michael Dibley, Shams El Arifeen, Caroline Fall, David Fisher, Henrik Friis, Exnevia Gomo, Batool Azra Haider, Adi Hidayat, Abbas Jahari, Pernille Kaestel, Patrick Kolsteren, Kusharisupeni, Aissa Mamadoultaibou, Dharma Sharna Mandandhar, Barrie Margetts, Clive Osmond, David Osrin, Lars Ake Persson, Usha Ramakrishnan, Dominique Roberfroid, Carine Ronsmans, Anuraj H. Shankar, Subarkah, Sunawang, Budi Utomo, Anjana Vaidya, Hong Yan, Noel Zagre, and Lingxia Zeng.
Contributor Information
Caroline H. D. Fall, MRC Epidemiology Resource Centre, University of Southampton, Southampton, UK
David J. Fisher, MRC Epidemiology Resource Centre, University of Southampton, Southampton, UK
Clive Osmond, MRC Epidemiology Resource Centre, University of Southampton, Southampton, UK.
Barrie M. Margetts, Institute of Human Nutrition, University of Southampton, Southampton, UK.
References
- 1.De Onis M, Blossner M, Villar J. Levels and patterns of intra-uterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52(suppl 1):S5–15. [PubMed] [Google Scholar]
- 2.Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and children. Eur J Clin Nutr. 1998;52(suppl 1):S34–42. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hoffman HJ, Cliver SP. Neurodevelopmental outcome of small-for-gestational-age infants. Eur J Clin Nutr. 1998;52(suppl 1):S54–8. [PubMed] [Google Scholar]
- 4.Barker DJP. Mothers, babies and health in later life. Churchill Livingstone; Edinburgh, UK: 1998. [Google Scholar]
- 5.Kramer MS. Determinants of low birthweight: methodological assessment and metaanalysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Maternal anthropometry and pregnancy outcomes: a WHO collaborative study. Bull World Health Organ. 1995;73(suppl):1–98. [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003;4:CD000032. doi: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- 8.Huffman SL, Baker J, Schumann J, Zehner ER. The case for promoting multiple vitamin and mineral supplements for women of reproductive age in developing countries. Food Nutr Bull. 1999;20:379–94. [Google Scholar]
- 9.Haider BA, Bhutta ZA. Multiple micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2006;4:CD004905. doi: 10.1002/14651858.CD004905.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Tofail F, Persson LA, El Arifeen S, Hamadani JD, Mehrin F, Ridout D, Ekstrom EC, Huda SN, Grantham-McGregor SM. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am J Clin Nutr. 2008;87:704–11. doi: 10.1093/ajcn/87.3.704. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, Dibley M, Cheng Y, Dang S, Chang S, Kong L, Yan H. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double-blind cluster randomised controlled trial. Br Med J. 2008;337:a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunawang, Utomo B, Hidayat A, Kusharisupeni, Subarkah Preventing low birthweight through maternal multiple micronutrient supplementation: a clusterrandomized, controlled trial in Indramayu, West Java. Food Nutr Bull. 2009;30:S488–95. doi: 10.1177/15648265090304S403. [DOI] [PubMed] [Google Scholar]
- 13.Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group. Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, Muadz H, Soesbandoro SD, Tijong R, Fachry A, Shankar AV, Atmarita, Prihatini S, Sofia G. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: A double-blind cluster-randomised trial. Lancet. 2008;371:215–27. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 14.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Adhikari RK, Sommer A, West KP., Jr Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: Double blind randomised community trial. Br Med J. 2003;326:571–6. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, Filteau S, Tomkins A, Costello AM. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: Double-blind, randomised controlled trial. Lancet. 2005;365:955–62. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 16.Bhutta Z, Rizvi A, Raza F, Hotwani S, Zaidi S, Moazzam, Hossain S, Soofi S, Bhutta S, Maternal Micronutrient Supplementation Study Group A comparative evaluation of multiple micronutrient and iron–folic acid supplementation during pregnancy in Pakistan: Impact on pregnancy outcomes. Food Nutr Bull. 2009;30:S496–505. doi: 10.1177/15648265090304S404. [DOI] [PubMed] [Google Scholar]
- 17.Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten J, Kolsteren P, MISAME Study Group. for the MISAME Study Group Effects of maternal multiple micronutrient supplementation on fetal growth: A double-blind, randomised controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–40. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 18.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal micronutrient supplements on birth weight and perinatal mortality: A randomised controlled trial in Guineau Bissau. Eur J Clin Nutr. 2005;59:1081–9. doi: 10.1038/sj.ejcn.1602215. [DOI] [PubMed] [Google Scholar]
- 19.Zagre NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: A cluster randomised, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull. 2007;28:317–27. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- 20.Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Kaestel P, Michaelsen KF. Effect of multimicronutrient supplementation on gestational length and birth size: A randomised, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr. 2004;80:178–84. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan U, Gonzalez-Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron-only supplementation: A randomised controlled trial in a semirural community in Mexico. Am J Clin Nutr. 2003;77:720–5. doi: 10.1093/ajcn/77.3.720. [DOI] [PubMed] [Google Scholar]
- 22.Margetts BM, Fall CHD, Ronsmans C, Allen LH, Fisher DJ, Maternal Micronutrient Supplementation Study Group (MMSSG) Multiple micronutrient supplementation during pregnancy in low-income countries: review of methods and characteristics of studies included in the meta-analyses. Food Nutr Bull. 2009;30:S517–26. doi: 10.1177/15648265090304S406. [DOI] [PubMed] [Google Scholar]
- 23.Gruenwald P. Growth of the human fetus. I. Normal growth and its variation. Am J Obstet Gynecol. 1966;94:1112–9. doi: 10.1016/0002-9378(66)90774-5. [DOI] [PubMed] [Google Scholar]
- 24.Milner RD, Richards B. An analysis of birth weight for gestational age of infants born in England and Wales, 1967–1971. J Obstet Gynaecol Br Commonw. 1974;81:956–67. doi: 10.1111/j.1471-0528.1974.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 25.Naeye RL, Dixon JB. Distortions in fetal growth standards. Pediatr Res. 1978;12:987–91. doi: 10.1203/00006450-197810000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Babson SG, Behrman RE, Lessel R. Fetal growth: liveborn birth weights for gestational age of white middle class infants. Pediatrics. 1970;45:937–44. [PubMed] [Google Scholar]
- 27.David RJ. The quality and completeness of birthweight and gestational age data in computerized birth files. Am J Public Health. 1980;70:964–73. doi: 10.2105/ajph.70.9.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–32. [PubMed] [Google Scholar]
- 29.Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, Tapia JL, Bastias G, Bacallao J, Rojas I. Effects of a dairy product fortified with multiple micronutrients and omega-s fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutr. 2008;11:30–40. doi: 10.1017/S1368980007000110. [DOI] [PubMed] [Google Scholar]
- 30.Gupta P, Ray M, Dua T, Radhakrishnan G, Kumar R, Sachdev HPS. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring. Arch Pediatr Adolesc Med. 2007;161:58–64. doi: 10.1001/archpedi.161.1.58. [DOI] [PubMed] [Google Scholar]
- 31.Ronsmans C, Fisher DJ, Osmond C, Margetts BM, Fall CHD, Maternal Micronutrient Supplementation Study Group (MMSSG) Multiple micronutrient supplementation during pregnancy in low-income countries: a metaanalysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr Bull. 2009;30:S547–55. doi: 10.1177/15648265090304S409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christian PS, Darmstadt GL, Wu L, Khatry SK, LeClerq SC, Katz J, West KP, Jr, Adhikari RK. The impact of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomized, controlled, community trial. Arch Dis Child Fetal Neonatal Ed. 2007 Aug 3; doi: 10.1136/adc.2006.114009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of a double blind randomised controlled trial. Lancet. 2008;371:492–9. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen LH, Peerson JM. Maternal Micronutrient Supplementation Study Group (MMSSG) Impact of multiple micronutrient versus iron–folic acid supplements on maternal anemia and micronutrient status in pregnancy. Food Nutr Bull. 2009;30:S527–32. doi: 10.1177/15648265090304S407. [DOI] [PubMed] [Google Scholar]
- 35.Garner P, Kramer MS, Chalmers I. Might efforts to increase birthweight in undernourished women do more harm than good? Lancet. 1992;340:1021–3. doi: 10.1016/0140-6736(92)93022-f. [DOI] [PubMed] [Google Scholar]
- 36.Prentice AM, Ceesay SM, Whitehead RG. Maternal supplementation and birthweight. Lancet. 1993;341:52–3. doi: 10.1016/0140-6736(93)92528-2. [DOI] [PubMed] [Google Scholar]
- 37.Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birth weight distribution in rural Nepal. J Nutr. 2006;136:1389–94. doi: 10.1093/jn/136.5.1389. [DOI] [PubMed] [Google Scholar]
- 38.Christian P, West KP, Khatry SK, LeClerq SC, Pradhan EK, Katz J, Shrestha SR, Sommer A. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 39.Christian P, Osrin D, Manandhar DS, Khatry SK, de L Costello AM, West KP., Jr Antenatal micronutrient supplements in Nepal. Lancet. 2005;366:711–2. doi: 10.1016/S0140-6736(05)67166-8. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher J. Sub-group analyses: how to avoid being misled. Br Med J. 2007;335:96–7. doi: 10.1136/bmj.39265.596262.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 42.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, Willett W, Hunter DJ. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–82. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 43.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, Spiegelman D. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–31. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 44.Stewart RJC, Sheppard H, Preece R, Waterlow JC. The effect of rehabilitation at different stages of development of rats marginally malnourished for ten to twelve generations. Br J Nutr. 1980;43:403–12. doi: 10.1079/bjn19800108. [DOI] [PubMed] [Google Scholar]
- 45.Webb AL, Conlisk AJ, Barnhart HX, Martorell R, Grajeda R, Stein AD. Maternal and childhood nutrition and later blood pressure levels in young Guatemalan adults. Int J Epidemiol. 2005;34:898–904. doi: 10.1093/ije/dyi097. [DOI] [PubMed] [Google Scholar]
- 46.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HPS, the Maternal and Child Undernutrition Study Group Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;3:CD001056. doi: 10.1002/14651858.CD001056. [DOI] [PubMed] [Google Scholar]
- 48.Oliver MH, Jaqueiry AL, Bloomfield FH, Harding JE. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc Reprod Fertil Suppl. 2007;64:397–410. doi: 10.5661/rdr-vi-397. [DOI] [PubMed] [Google Scholar]
- 49.Rayco-Solon P, Fulford AJ, Prentice AM. Maternal preconceptional weight and gestation length. Am J Obstet Gynecol. 2005;192:1133–6. doi: 10.1016/j.ajog.2004.10.636. [DOI] [PubMed] [Google Scholar]