Abstract
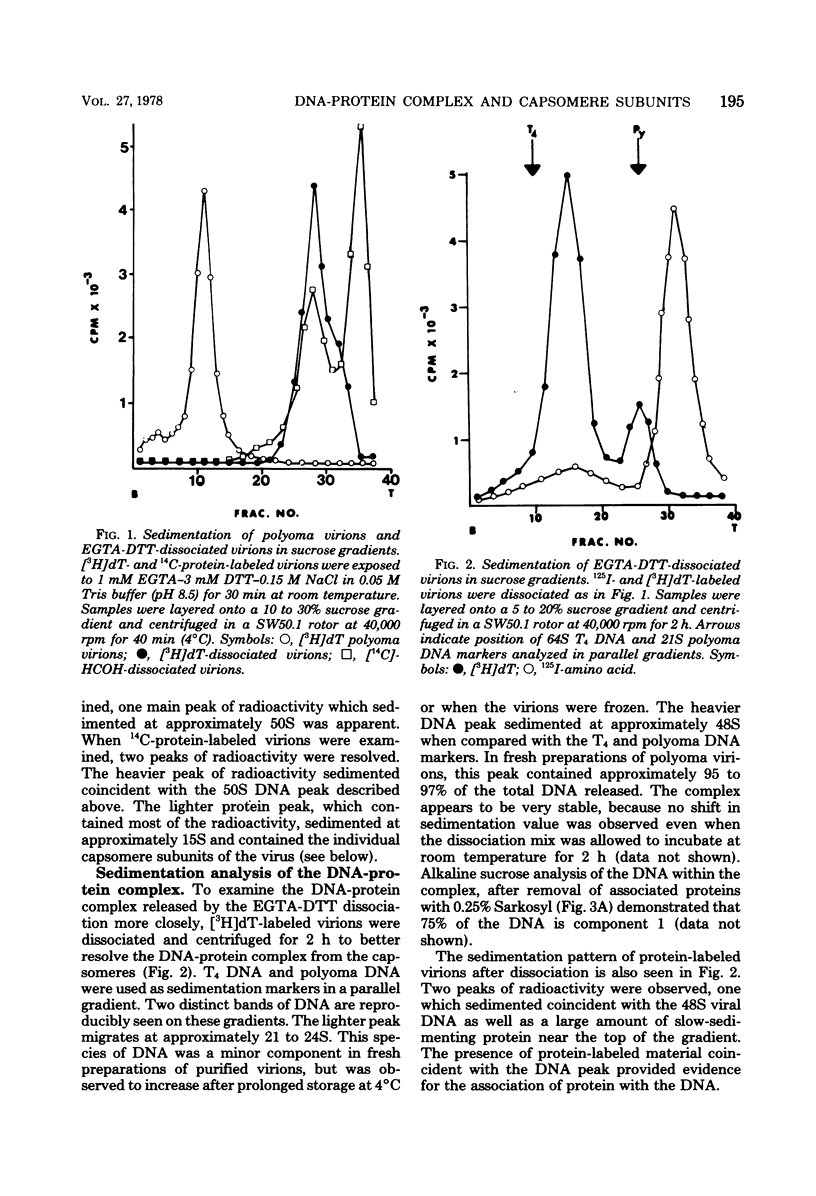
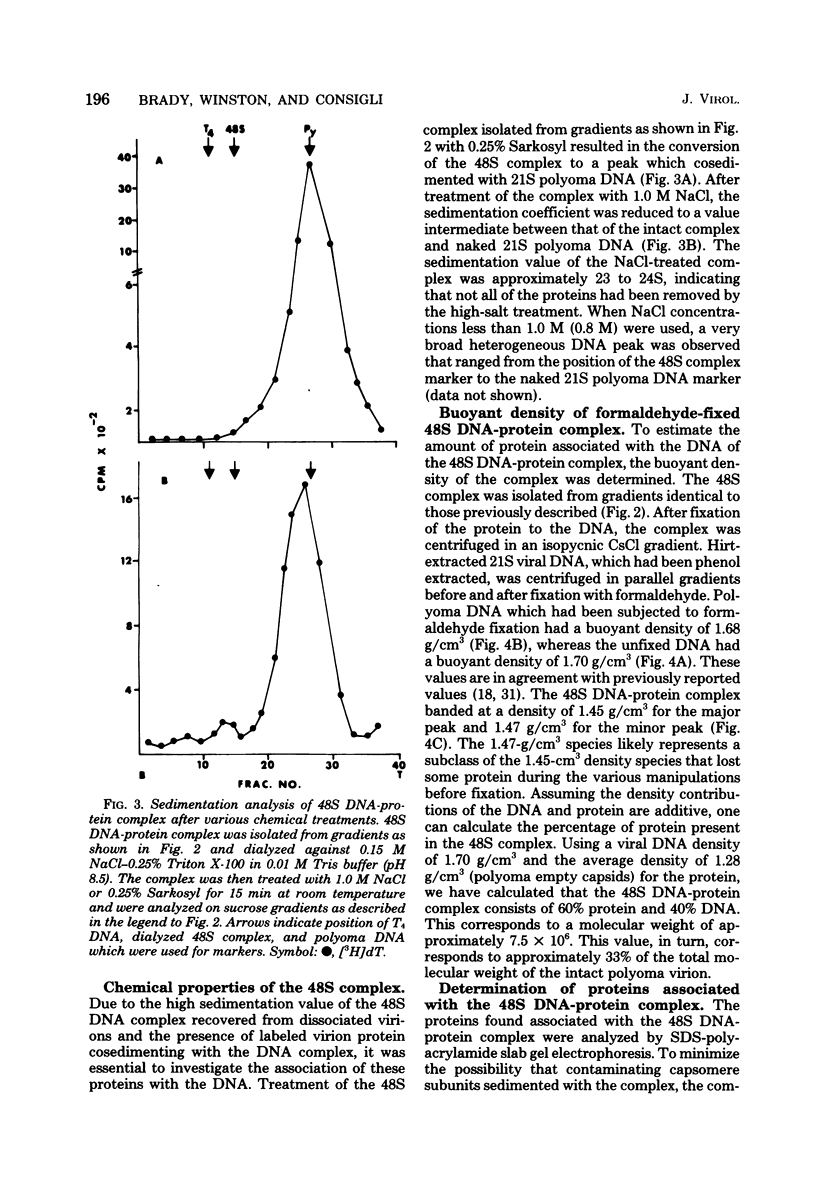
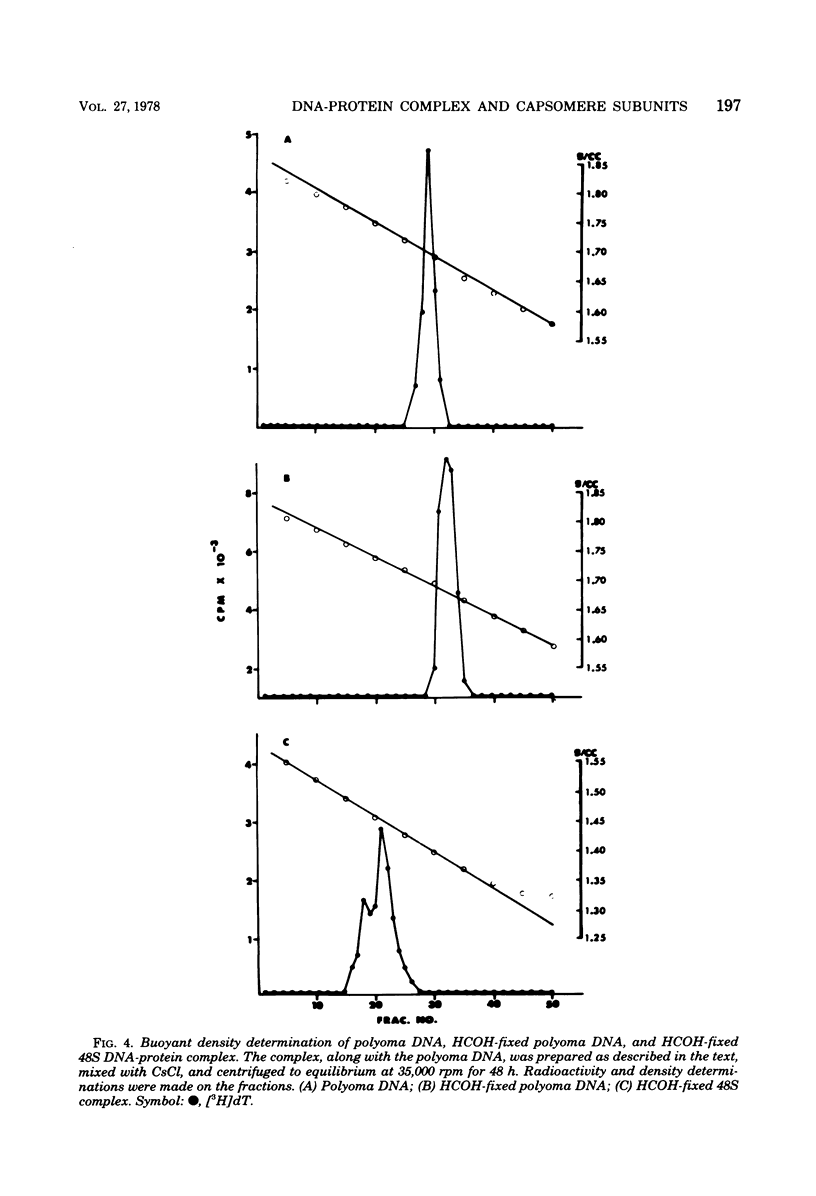
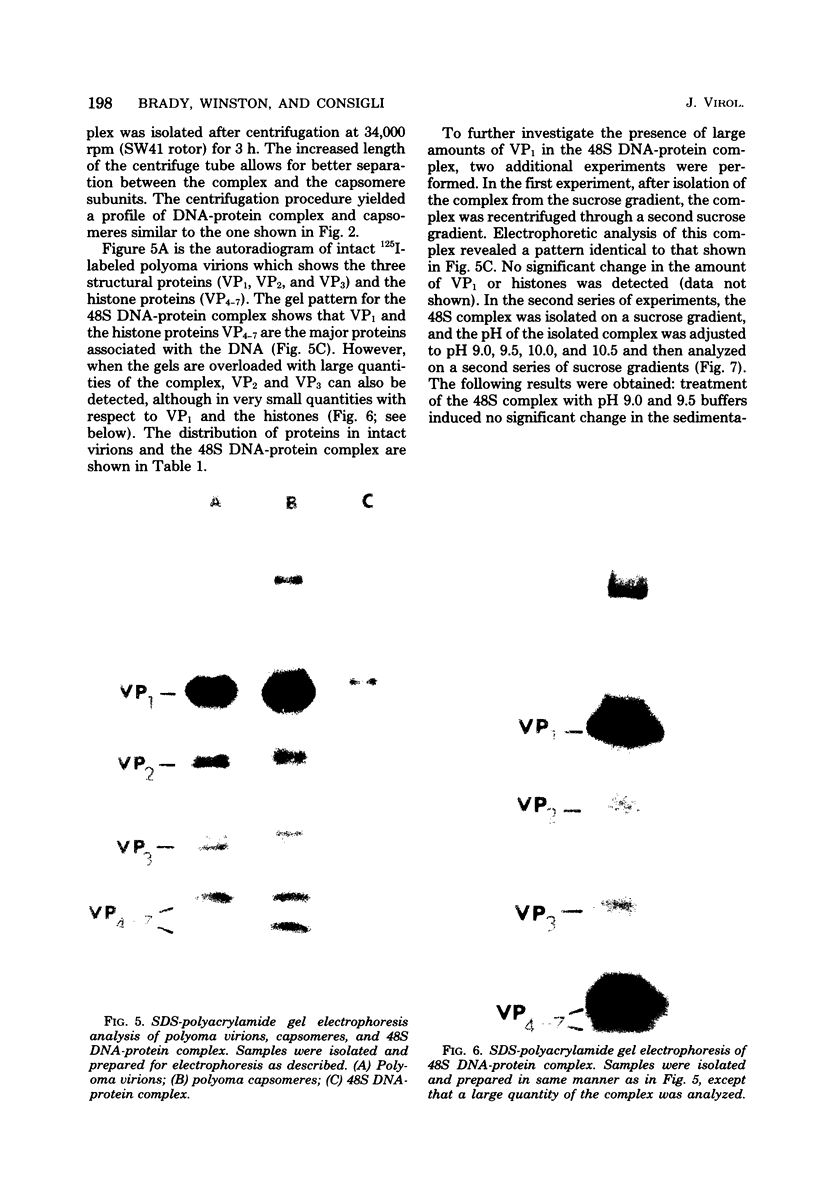
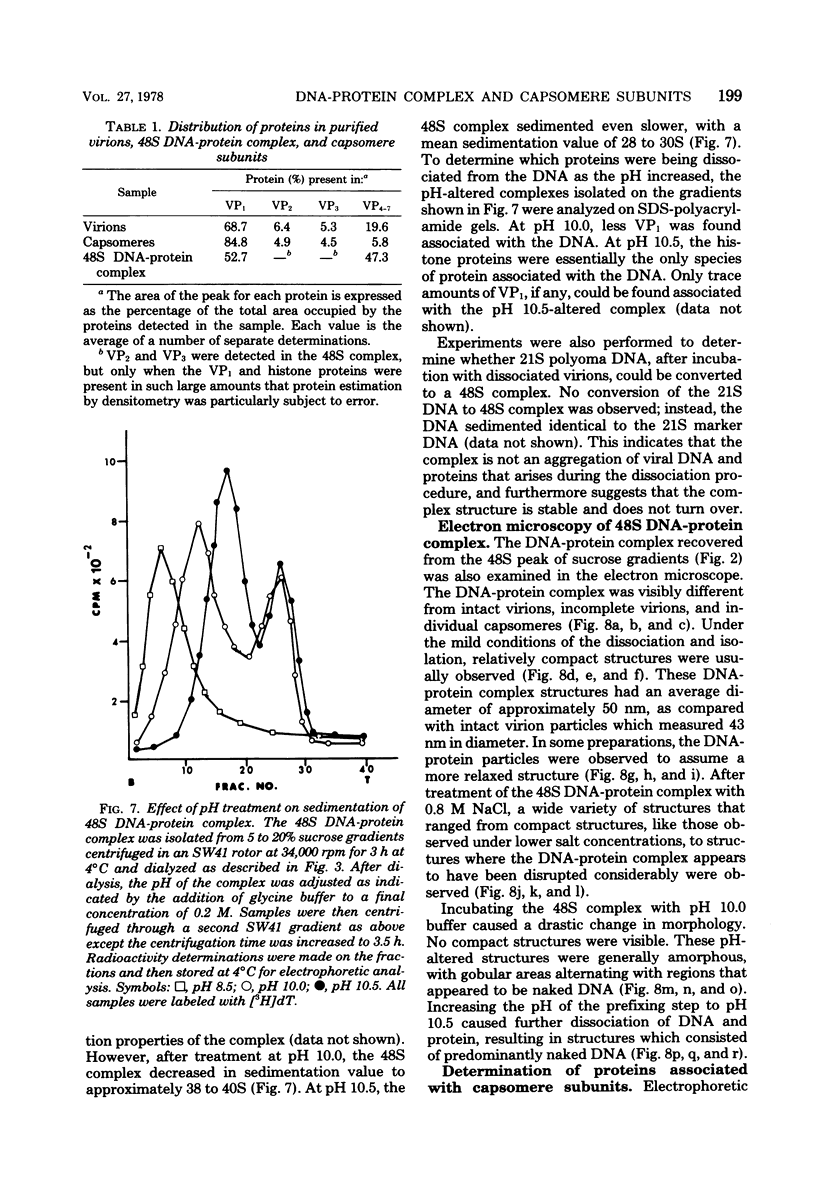
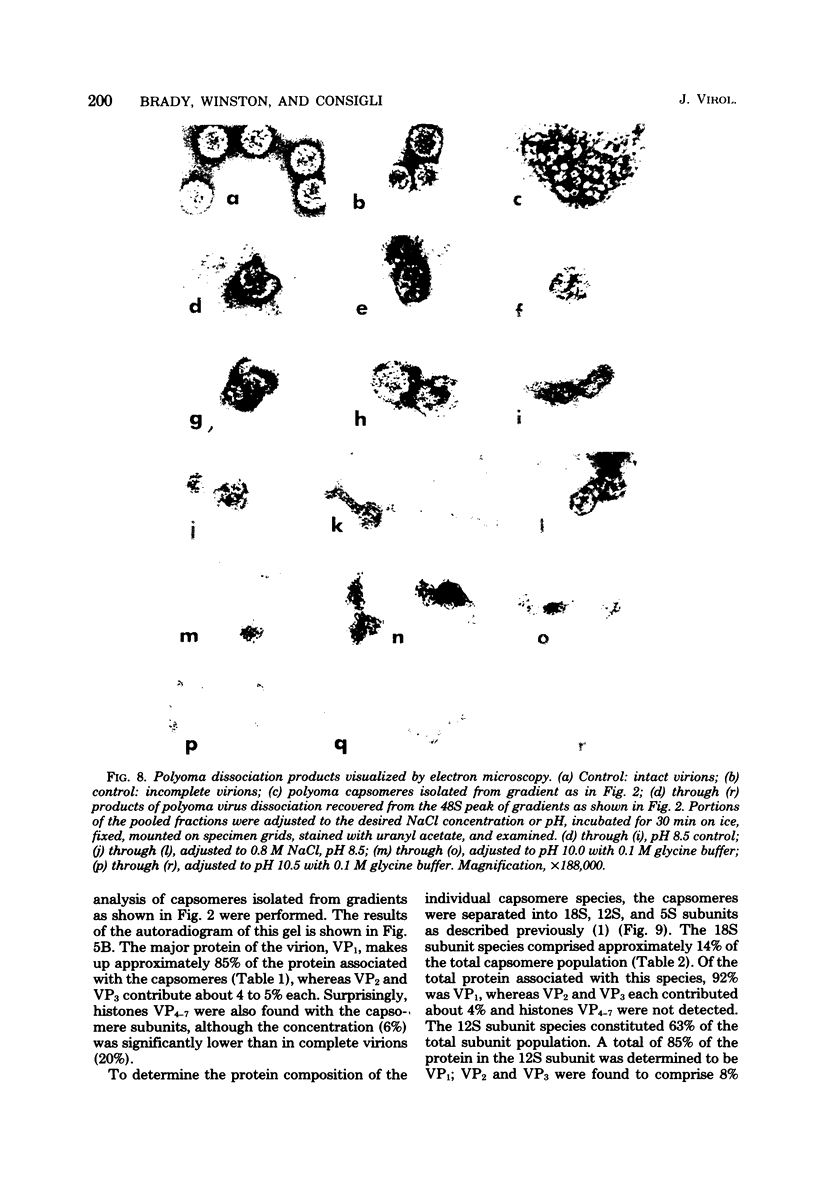
Treatment of polyoma virions with ethyleneglycol-bil-N,N'-tetraacetic acid (EGTA) and dithiothreitol (DTT) at pH 8.5 resulted in the dissociation of the virions into a DNA-protein complex and individual structural capsomere subunits. The sedimentation value of the DNA-protein complex in sucrose gradients was approximately 48S, and it had a density of 1.45 g/cm3 in equilibrium CsCl gradients. Alkaline sucrose analysis of the DNA within this DNA-protein complex demonstrated that approximately 75% of the DNA is component 1. The proteins associated with the DNA were dissociated by treatment with either NaCl or the anionic detergent Sarkosyl. VP1 and the histone proteins VP 4--7 were the major proteins associated with the DNA. Treatment of the DNA-protein complex with alkaline pH resulted in the specific removal of FP1. Electron microscopy of the 48S DNA-protein complex demonstrated that it is a very tightly coiled structure that is slightly larger than the intact virion. Treatment of the complex with either NaCl or with pH 10.5 buffer resulted in the loss of protein and subsequent loosening of the DNA-protein complex such that the DNA could be visualized. The capsomere subunits released as a result of the EGTA-DTT treatment sedimented as 18S, 12S, and 5S subunits in sucrose gradients. Electrophoretic analysis of the isolated capsomeres demonstrated that VP1, VP2, and VP3 were present in each species, although the ratios of the proteins varied. In addition to the structural proteins, histones VP 4--7 were found to be predominantly associated with the 5S capsomere subunit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Zabielski J., Weil R. Plaque assay for polyoma virus on primary mouse kidney cell cultures. Appl Microbiol. 1973 Oct;26(4):627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Walter G. Subunit interactions in polyoma virus structure. Virology. 1977 Apr;77(2):783–796. doi: 10.1016/0042-6822(77)90499-8. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Friedmann T. In vitro reassembly of shell-like particles from disrupted polyoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2574–2578. doi: 10.1073/pnas.68.10.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Decapsidation of polyoma virus: identification of subviral species. Virology. 1975 Nov;68(1):245–255. doi: 10.1016/0042-6822(75)90165-8. [DOI] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Electrophoretic analysis of the structural polypeptides of polyoma virus mutants. Virology. 1975 May;65(1):286–288. doi: 10.1016/0042-6822(75)90032-x. [DOI] [PubMed] [Google Scholar]
- Goldstein D. A., Hall M. R., Meinke W. Properties of nucleoprotein complexes containing replicating polyoma DNA. J Virol. 1973 Oct;12(4):887–900. doi: 10.1128/jvi.12.4.887-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozier J., Renz M., Nehls P. The chromosome fiber: evidence for an ordered superstructure of nucleosomes. Chromosoma. 1977 Jul 18;62(4):301–317. doi: 10.1007/BF00327030. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G. On the nucleoprotein core of simian virus 40. Virology. 1977 Dec;83(2):433–437. doi: 10.1016/0042-6822(77)90190-8. [DOI] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. In vitro radioisotopic labeling of proteins associated with purified polyoma virions. J Virol. 1974 Dec;14(6):1627–1629. doi: 10.1128/jvi.14.6.1627-1629.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancock R. Separation by equilibrium centrifugation in CsC1 gradients of density--labelled and normal deoxyribonucleoprotein from chromatin. J Mol Biol. 1970 Mar 14;48(2):357–360. doi: 10.1016/0022-2836(70)90167-1. [DOI] [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Robbins A. K., Crawford L. V. Phophorylation of polyoma and SV40 virus proteins. J Gen Virol. 1977 Oct;37(1):75–83. doi: 10.1099/0022-1317-37-1-75. [DOI] [PubMed] [Google Scholar]
- Qureshi A. A., Bourgaux P. Polypeptides of a viral DNA-protein complex form polyoma virus-infected cells. Virology. 1976 Oct 15;74(2):377–385. doi: 10.1016/0042-6822(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Weil R. Polyoma viral DNA replicated as a nucleoprotein complex in close association with the host cell chromatin. J Virol. 1974 Mar;13(3):567–576. doi: 10.1128/jvi.13.3.567-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- WEIL R. The denaturation and the renaturation of the DNA of polyoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:480–487. doi: 10.1073/pnas.49.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Deppert W. Intermolecular disulfide bonds: an important structural feature of the polyoma virus capsid. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):255–257. doi: 10.1101/sqb.1974.039.01.033. [DOI] [PubMed] [Google Scholar]