SUMMARY
Anterior-posterior (A-P) patterning of the vertebrate limb is controlled by sonic hedgehog (SHH) signaling, and the precise restriction of Shh expression to the posterior limb bud is essential for its polarizing effect. Fibroblast growth factor (FGF) signaling, a key control of proximal-distal (P-D) limb outgrowth, is known to promote Shh expression in the posterior limb bud. Here we show that conditional knockout of FGF-activated transcription factor genes Etv4 and Etv5 in mouse led to ectopic Shh expression in the anterior limb bud, and a preaxial polydactyly (PPD) skeletal phenotype. These unexpected results suggest that ETV4 and ETV5 act downstream of FGF signaling to inhibit Shh expression in the anterior limb bud. This finding elucidates a novel aspect of the mechanism coordinating limb development along the A-P and P-D axes.
INTRODUCTION
Formation of the tetrapod limb serves as a paradigm for pattern formation in developmental biology. Along the anterior-posterior (A-P, thumb to little finger) axis of the limb, asymmetry in skeletal pattern is executed by signals from the Zone of Polarizing Activity (ZPA), a region in the posterior mesenchyme of the limb bud (McGlinn and Tabin, 2006). The ZPA is defined by its ability to induce posterior limb skeletal elements when transplanted to the anterior limb bud. Cells in the ZPA can be distinguished by their expression of sonic hedgehog (SHH), a secreted signal that is necessary and sufficient for ZPA function. In addition to its role in polarizing the limb bud, SHH signaling is also essential for limb bud growth (Towers et al., 2008; Zhu et al., 2008). Thus precise regulation of the amount and localization of Shh expression is essential for normal limb development.
Several mechanisms are known to control Shh expression in the limb bud. Antagonism between transcription factors HAND2 and GLI3 is essential for establishing the prepattern that instructs Shh expression in the posterior mesenchyme (te Welscher et al., 2002a). Fibroblast Growth Factors (FGFs) expressed in the Apical Ectodermal Ridge (AER and AER-FGFs) are essential for inducing and maintaining Shh (Niswander, 2002). Repressors of Shh expression emerged from studies of mutants that exhibit extra digits on the anterior (thumb) side of the autopod, a phenotype known as preaxial polydactyly (PPD). In many of these, including in Twist1, Alx4 and Gli3 mutants, Shh is ectopically expressed in the anterior limb bud, in addition to its expression in the ZPA (Bourgeois et al., 1998; Dunn et al., 1997; Qu et al., 1997). Thus these PPD genes are required to restrict Shh expression to the ZPA.
In addition to promoting Shh expression, AER-FGFs act as key signals for development of the proximal-distal (P-D, shoulder to digit tip) axis of the limb (Mariani and Martin, 2003). Downstream mediators of FGF activity in either P-D development or Shh regulation remain to be identified. Here we investigate the role of the PEA3 group of ETS-domain containing transcription factors in mouse limb buds. We show that two of the three members of this group, Etv4 (also termed Pea3) and Etv5 (also termed Erm) are expressed under the control of AER-FGF signaling. Conditional inactivation of Etv4 and Etv5 led to ectopic Shh expression and a PPD skeletal phenotype. These observations reveal a novel mechanism by which AER-FGFs influence limb patterning along the A-P axis.
RESULTS
FGF signaling is required to maintain Etv4 and Etv5 expression in mouse limb buds
To identify genes that mediate FGF function in limb development, we searched for transcription factors that are regulated by FGF signaling. By RNA in situ analysis, we found that two PEA3 group genes, Etv4 and Etv5 (Etv4;5), but not the third member Etv1, are expressed in the distal portion of the limb bud mesenchyme adjacent to the AER (Fig. 1A,B and data not shown). To address if their expression is controlled by AER-FGF signaling, we analyzed Etv4;5 expression in FGF pathway mutants. In Msx2cre;Fgf4−/fl;Fgf8−/fl (or Msx2cre;4;8) limb buds where two of the four AER-Fgfs are inactivated (Sun et al., 2002), Etv4;5 expression is reduced (Fig. 1A–D). Similarly, in Prx1cre;Fgfr1co/co; Fgfr2c/c (or Prx1cre;Fgfr) limb buds where the principle FGF receptors (Fgfrs) expressed in the limb bud are inactivated in the entire mesenchyme (Eswarakumar et al., 2002; Logan et al., 2002; Xu et al., 2002), Etv4;5 expression is also downregulated (Fig. 1E–H). To address whether Fgfrs are required cell autonomously for regulating Etv4;5 expression, we analyzed Shhcre/+;Fgfr1co/co;Fgfr2c/c (or Shhcre;Fgfr) limb buds where Fgfrs are inactivated in the posterior limb bud mesenchyme (Verheyden and Sun, 2008). We found that downregulation of Etv4;5 expression is confined to the Fgfr-inactivated domain (Fig. 1I–L), indicating that AER-FGFs maintain Etv4;5 expression without acting through additional secreted signals. These findings raised the possibility that Etv4;5 act directly downstream of AER-FGF signaling.
Figure 1. Inactivation of AER-Fgfs or FGF receptors leads to downregulation of Etv4;5 expression in limb buds.
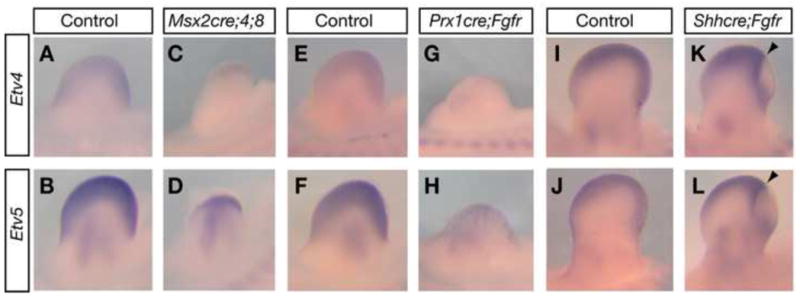
(A–L) Etv4 and Etv5 expression in E10.75 (A–H) or E11.5 (I–L) limb buds. Arrowheads indicate anterior boundary of Fgfr-inactivated domain as identified both by downregulation of Fgfr1 and FGF readout Spry4 expression (data not shown) (Verheyden et al., 2005; Verheyden and Sun, 2008).
Inactivating Etv4;5 function in mouse limb buds
To address whether Etv4;5 mediate FGF function in limb development, we inactivated both genes as their shared expression domain indicates possible redundancy. Inactivation was achieved by generating a conditional allele of Etv5 (Etv5fl) and combining it with an existing null allele of Etv4 (Etv4−) (Livet et al., 2002). In the Etv5fl allele, loxP sites were engineered to flank exons that encode the N-terminal portion of the DNA binding domain (Fig. 2A). Excision of floxed exons led to Etv5−, predicted to encode a protein without the DNA binding domain and the rest of the C-terminal region. Transcriptional assay shows that the remaining protein, if made, harbors no residual transcriptional activity and does not interfere with full-length ETV5 function (Fig. 2B). These results indicate that deletion of the floxed exons abolishes ETV5 activity.
Figure 2. Deletion of exons encoding ETV5-DNA binding domain leads to inactivation of ETV5 transcriptional activity.

(A) Etv5 targeting strategy where loxP sites flank exons 10 and 11. Germline FLP or cre mice were used to generate Etv5fl or Etv5− allele, respectively. The majority of Etv5−/− animals die shortly after birth. This lethality was not reported in an existing mutant of Etv5 (Chen et al., 2005), suggesting that our Etv5− is a more severe loss-of-function allele. (B) Luciferase (Turo-luc) assay. Reporter fold induction is normalized to activity when empty vector is transfected (column 1). Expression of mutant ETV5 alone does not lead to a change (column 2 versus 1, n=3, p=0.498). Expression of mutant ETV5 in the presence of wild-type ETV5 does not lead to downregulation of reporter activity compared to wild-type ETV5 alone (column 4 versus 3, n=3, p=0.0483). (C) qRT-PCR of Etv5 in prospective hindlimb field of E9.5 embryos to detect intact, but not mutated transcripts. The level of transcripts in mutant tissue is 2.5±1.8(SD)% (n=3) of that in Etv5fl/+control (n=3). (D) RT-PCR of Etv5 and β-actin in E10.0 limb buds. Two-tailed student t-test was used and error bars in all figures represent standard deviation (SD).
Double homozygous mutants of Etv4− and Etv5− (in Etv4−/−;Etv5−/−) died prior to limb bud initiation (data not shown). To bypass early lethality, we used the Brachyury (T) cre to inactivate Etv5 in mesoderm-derived cells (Perantoni et al., 2005), and combined this mutant with Etv4− (generating Tcre;Etv4−/−;Etv5−/fl, or Tcre;Etv mutant). Quantitative RT (qRT)-PCR analysis at Embryonic day (E) 9.5 indicates that at the onset of limb development, approximately 2.5% of intact Etv5 transcript was detected in the prospective hindlimb bud region in Tcre;Etv embryos (n=3) (Fig. 2C). This residual expression is diminished by E10.0 in both the forelimb and hindlimb buds (Fig. 2D).
Inactivation of Etv4;5 leads to preaxial polydactyly
To investigate whether Etv4;5 mediate FGF signaling, we first examined the expression of Mkp3 and Spry2, common readouts of FGF activity (Tsang and Dawid, 2004). At ~E11, we detected no difference in their expression in Tcre;Etv mutants compared to normal (Fig. 3A–E), indicating that Etv4;5 are not essential for mediating FGF regulation of these genes in the limb bud.
Figure 3. Inactivation of Etv4;5 leads to no defects along the P-D axis and a PPD phenotype along the A-P axis of hindlimbs.

(A–E) Expression of Mkp3 and Spry2 is normal in Tcre;Etv mutant limb buds as assayed by RNA in situ hybridization in E11 (A–D) or qRT-PCR in E10.75 (37–39 somite stage) hindlimb buds (E)(p=0.49 for Mkp3, n=3; p=0.46 for Spry2, n=3). (F–H) Postnatal day 1 Tcre;Etv hindlimbs are normal along the P-D axis as confirmed by measurements of the three segments (n=6; p=0.24, 0.17 and 0.32 for S, Z and A′, respectively). (I–N) Tcre;Etv hindlimbs exhibit triphalangeal extra digits anteriorly (asterisks), and frequent digit 1 transformation from biphalangeal to triphalangeal (1′, n=8/12). Anterior digits in I, K and M are magnified in J, L and N, respectively. (O,P) Sox9 expression in E12.5 limb buds indicates extra digit (arrow) and enlarged digit 1′ primordium. (Q–T) Lysotracker Red staining. Arrows indicate normal cell death. Abbreviations: autopod (A′), metatarsal (mt), stylopod (S) and zeugopod (Z).
Tcre;Etv embryos die shortly after birth due to internal organ defects. In newborn limbs along the P-D axis, Tcre;Etv embryos exhibit normal skeletal patterning (Fig. 3F–H). There is no upregulation of Etv1 in the mutant limb bud that could compensate for the loss of Etv4;5 function (data not shown). Thus in limb P-D outgrowth, Etv4;5 do not play a major role in mediating FGF function.
Along the A-P axis of the limb, however, all Tcre;Etv embryos exhibit a PPD phenotype in the hindlimbs, but not forelimbs (Fig. 3I–N, n=9/9 embryos, Supplemental Table). Either one or two extra digits were observed, and a majority of them are triphalangeal, representing posterior digit identity. In over 50% of the limbs, digit 1 itself is transformed into a triphalangeal digit (Fig. 3M,N). This transformation likely occurs early in autopod patterning, as suggested by a longer digit 1 condensation outlined by Sox9 expression at E12.5 (Fig. 3O,P). Inactivation of both Etv4 and Etv5 contributes to the PPD phenotype, as Etv4−/− animals exhibit no limb skeletal defects (Laing et al., 2000), and Etv5−/− embryos exhibit a milder PPD defect with partial penetrance (n=12/17, often only one extra digit, Supplemental Table). These data demonstrate that Etv4;5 genes together are essential for establishing proper digit number and anterior digit patterning.
Tcre;Etv mutant limb buds exhibit reduced cell death
To uncover the cellular basis of the PPD phenotype, we examined the cell death pattern in Tcre;Etv mutants. In control limb buds, we detected a previously described pattern in the region termed foyer préaxial primaire (fpp), an area equivalent but not identical to the anterior necrotic zone observed in chick limb buds (Fernandez-Teran et al., 2006; Milaire, 1992). Cell death is reduced in this domain in Tcre;Etv limb buds (Fig. 3Q–T), similar to previous observations in other PPD mutants (Milaire, 1992). This observation offers a cellular mechanism for the presence of extra preaxial digits in Tcre;Etv mutants.
ETV4;5 repress Shh expression
Ectopic SHH activity is often observed in PPD mutants (Bourgeois et al., 1998; Dunn et al., 1997; Qu et al., 1997). In Tcre;Etv embryos starting from the 42 somite stage (~E11), we detected ectopic Shh expression in the anterior mesenchyme of hindlimb, but not forelimb buds (Fig. 4A–D and data not shown). In all mutant limb buds, expression of Shh in the posterior mesenchyme remains normal. SHH regulated genes, including Gli1, Patched1 (Ptch1), Hand2, Hoxd13 and Gremlin1, are all ectopically expressed in E11.5 Tcre;Etv limb buds (Fig. 4E–N), consistent with upregulation of SHH activity. Furthermore, Fgf4 expression in the AER, which is positively regulated by SHH, extends anteriorly following, but not prior to ectopic Shh expression (Fig. 4O–R). These data indicate that Etv4;5 are essential for repressing Shh expression in anterior limb bud mesenchyme.
Figure 4. Inactivation of Etv4;5 leads to ectopic Shh expression in the anterior hindlimb bud.
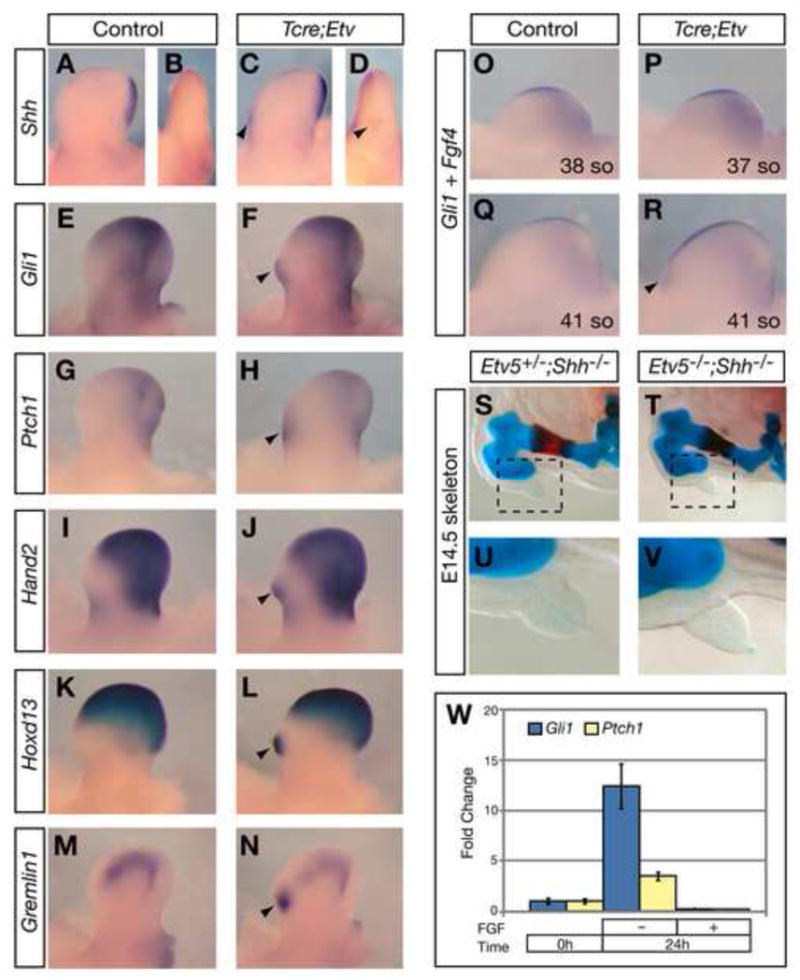
(A–N) Gene expression in E11.5 hindlimb buds. Arrowheads indicate ectopic anterior expression. B, D are anterior views of limb buds in A, C, respectively. (O–R) Non-overlapping Fgf4 (AER) and Gli1 (mesenchyme) expression at indicated somite (so) stages. Arrowhead indicates ectopic Gli1. (S–V) Hindlimb skeleton. Boxed regions in S and T are magnified in U and V. (W) Changes of Gli1 and Ptch1 expression in cultured anterior limb mesenchyme cells as assayed by qRT-PCR and normalized to their levels without culture (n≥3 for each time point and condition).
Previous studies show that ectopic Shh accounts for the extra digit phenotype observed in some, but not all PPD mutants (Litingtung et al., 2002; te Welscher et al., 2002b). To address this trait in our mutants, we introduced a null allele of Shh into the Etv5−/− mutant background, which exhibits PPD in approximately 70% of the embryos (n=12/17, Supplemental Table). All Etv5−/−;Shh−/− mutant embryos obtained (n=6) show a limb skeletal phenotype that is indistinguishable from the phenotype of either Etv5+/−;Shh−/− or Shh−/− littermates (Fig. 4S–V and data not shown) (Chiang et al., 2001; Kraus et al., 2001). This result suggests that the PPD phenotype caused by disruption of Etv4;5 function is dependent on SHH activity.
To investigate the relationship between Etv4;5 and other regulators of Shh transcription, we examined the expression of Hand2, Gli3, Alx4, Twist1, Hoxd11, Hoxd12 and Hoxd13 (Bourgeois et al., 1998; Charite et al., 2000; Dunn et al., 1997; Qu et al., 1997; Zakany et al., 2004). As SHH feeds back to control the expression of most of these genes (Fernandez-Teran et al., 2000; Laufer et al., 1994), we assayed Tcre;Etv mutant hindlimb buds at around 39-somite stage just before ectopic Shh expression is detected. By either RNA in situ analysis or qRT-PCR, no change in the expression of these regulators was observed (Supplemental Fig. 1 and data not shown). The results suggest that Etv4;5 act either downstream of or in parallel with these factors to control Shh expression.
FGF inhibits polarizing activity in the anterior limb bud mesenchyme
As FGFs are known positive regulators of Shh, the finding that FGF-maintained genes Etv4;5 inhibit Shh expression, and hence restrict polarizing activity is unexpected. However, consistent with the general theme of our finding, a previous report shows that anterior mesenchymal cells can be converted to ZPA signaling cells when cultured in the absence of the AER (Anderson et al., 1994). Furthermore, this conversion is prevented if cells are cultured in the presence of FGF2.
We repeated similar experiments and addressed whether the expression of Gli1 and Ptch1, sensitive readouts of SHH signaling, is altered in these cells in accordance with the reported polarizing activity. We found that when anterior mesenchymal cells are placed in culture without FGF, there is an increase in Gli1 and Ptch1 expression (Fig. 4W). This increase is inhibited by the presence of FGF. We speculate that the reason why a similar increase of SHH activity has not been observed in vivo following AER removal is because it is obscured by the massive cell death following this surgical manipulation. Taken together, the results presented in this study are consistent with the overall interpretation that FGF signaling, via its regulation of Etv4;5, inhibits polarizing activity in the anterior limb bud mesenchyme.
DISCUSSION
In this study, we show that in the limb bud, two PEA3 group genes, Etv4;5 are expressed under the control of AER-FGF signaling. This finding is in agreement with similar regulations in other regions of the embryo (Bottcher and Niehrs, 2005), raising the possibility that Etv4;5 genes function as general mediators of FGF function. Consistent with this possibility, in several developing tissues including the kidney, conditional inactivation of both Etv4;5 led to gross phenotypes that resemble those described in Fgf mutants (data not shown). However in the limb, we show the surprising finding that instead of an apparent reduction of the P-D axis, a phenotype displayed by Fgf mutants, Tcre;Etv limbs exhibit an expansion of the A-P axis. A similar A-P phenotype is observed in the accompanying study following the expression of a constitutively repressor form of ETV4 (EtvEnR) (Mao et al.). In EtvEnR limbs, while a slight decrease in P-D length is observed, this reduction resembles only the mild phenotypes exhibited by Fgf or Fgfr mutants (Boulet et al., 2004; Mariani et al., 2008; Sun et al., 2002; Verheyden et al., 2005; Yu and Ornitz, 2008). Thus, combined results from the two studies indicate that Etv4;5 genes are not the principle mediators of FGF function in limb P-D outgrowth. Rather, the A-P axis defects reveal an unexpected role of FGF-regulated genes in PPD, a common birth defect.
We found that cell death is reduced in the Tcre;Etv limb buds, providing a plausible mechanism for the formation of extra digits. Preceding this reduction of cell death, anterior ectopic Shh and expansion of Fgf4 were observed. Either one of these molecular changes was shown to result in decreased cell death (Lu et al., 2006; Sanz-Ezquerro and Tickle, 2000). Several lines of evidence suggest that altered Shh, rather than Fgf4, is the primary cause for the phenotypes in Tcre;Etv mutants. First, in these limbs, posterior fate has been imposed on anterior digits, an outcome associated with overexpression of SHH, but not FGFs (Riddle et al., 1993). Second, our finding that Etv5−/−;Shh−/− limbs are indistinguishable from Shh−/− limbs indicate that the extra anterior digits are dependent on SHH activity. Finally, while overexpression of Fgf4 does not lead to ectopic anterior Shh expression, increase in SHH activity can lead to extension of Fgf4 expression (Laufer et al., 1994; Lu et al., 2006; Niswander et al., 1994). Taken together, these findings support the conclusion that ETV4;5 control digit number and digit identity by inhibiting Shh expression in anterior limb bud mesenchyme.
Evidence suggests that Etv4;5 are likely required at the beginning of limb bud development for Shh inhibition. In Tcre;Etv mutant limb buds, although complete Etv5 inactivation is observed at E10.0 in all limb buds, this is an earlier time point in the course of hindlimb bud development than in forelimb bud development. Thus the restriction of defects to the hindlimbs may be due to distinct recombination timing. Possible differences in inactivation efficiency may also contribute to the phenotype variations between Tcre;Etv and EtvEnR mutants (Mao. et al.). In Tcre;Etv limb buds, although Tcre is active early in limb bud development (Verheyden et al., 2005), loss of Etv4;5 function is contingent upon degradation of wild-type Etv5 transcripts/protein that are produced prior to cre-mediated recombination. However, in EtvEnR mutants, Etv4;5 function is inhibited as soon as the competing mutant protein is made. Thus it is possible that disruption of Etv4;5 function occurred earlier in EtvEnR mutants than in Tcre;Etv mutants. Despite these variations, results from the two studies support a common conclusion that Etv4;5 repress Shh expression in the limb bud.
It remains to be determined whether this repression is achieved through direct binding of ETV4;5 to the Shh enhancer/promoter, or through ETV4;5 control of other Shh regulators. Among factors that are unaltered in Tcre;Etv limb buds are HAND2 and GLI3, proteins that establish the initial A-P polarity of the limb bud (te Welscher et al., 2002a). Further studies will determine if Etv4;5 expression is altered in mutants of these regulators, an indication that ETV4;5 act downstream. Alternatively, ETV4;5 may interact with these factors on the protein level, as has been demonstrated between HAND2 and TWIST1 (Firulli et al., 2005). Parallel protein-level interactions may also explain why ETV4;5 do not inhibit Shh expression in the ZPA, where positive regulators such as HAND2 are present at high levels (Charite et al., 2000), and may override the inhibitory effect of ETV4;5.
For A-P patterning of the limb, it is as important to prevent Shh expression in the anterior limb bud as it is to sustain its expression in the posterior limb bud (McGlinn and Tabin, 2006). Our findings indicate that in addition to the known function in promoting Shh expression, activities downstream of FGF signaling also play a role in inhibiting Shh expression. In this context, ETV4;5 are distinct from other inhibitors of Shh in that they are positively regulated by AER-FGF signaling. It appears that FGF signaling coordinates the positive and inhibitory regulator properties, providing an efficient mechanism to achieve polarization of the limb bud.
EXPERIMENTAL PROCEDURES
Phenotype analyses
Tcre;Etv mutants were generated as described (Supplemental Methods). Whole-mount in situ hybridization, skeletal preparations and cell death analysis (using LysoTracker Red DND-99, Invitrogen) were performed using standard or published protocols (Neubuser et al., 1997; Zucker et al., 1999). To examine the level of intact Etv5 transcripts, cDNA was prepared from lateral plate mesoderm in the hindlimb bud field of embryos at the 25–27 somite stage (~E9.5) and limb buds of embryos at the 29–30 somite stage (~E10). To examine the expression of Mkp3 and Spry2, cDNA was prepared from hindlimb buds of embryos at the 37–39 somite stage (Supplemental Methods). Student t-test was used for statistical analysis, and standard deviation was presented.
Cell culture and luciferase assay
Each well of Hela cells was transfected with a total of 800ng of DNA, including TK-hRL (Promega) as internal control, Turo-luc as ETV4;5 responsive reporter plasmid (Monte et al., 1995), either empty vector alone, pDEST-wtEtv5 and/or pDEST-mutEtv5 (Supplemental Methods). Expression was assayed 36 hours later using the Dual Luciferase kit (Promega).
Limb bud cell culture
Following an established protocol (Anderson et al., 1994), FBS was pretreated with Heparin beads to remove FGF prior to use in medium. Anterior limb bud mesenchyme was dissected from 40 E10.5 limb buds and pooled. Cells were dissociated and aliquoted into 16 wells and cultured in the presence or absence of FGF2 (100ng/ml) (Peprotech). Expression of Gli1 and Ptch1 was assayed by qRT-PCR (Supplemental Methods).
Supplementary Material
Acknowledgments
We thank Drs. A. McMahon, C. Tabin, and their laboratories for sharing unpublished data and helpful comments. We are grateful to Dr. J. Fallon and members of the Sun laboratory, in particular L. Abler, for discussions and reading of the manuscript. We thank Drs. B. Harfe, M. Lewandoski, P. Lonai and C. Tabin for mouse strains. We are grateful to A. Lashua and M. Zhao for technical assistance. J.M.V. was supported by the NIH predoctoral training program in genetics (5T32GM07133). This work was supported by a NIH grant RO1 HD045522 (to X.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson R, Landry M, Reginelli A, Taylor G, Achkar C, Gudas L, Muneoka K. Conversion of anterior limb bud cells to ZPA signaling cells in vitro and in vivo. Dev Biol. 1994;164:241–257. doi: 10.1006/dbio.1994.1195. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Boulet AM, Moon AM, Arenkiel BR, Capecchi MR. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol. 2004;273:361–372. doi: 10.1016/j.ydbio.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bourgeois P, Bolcato-Bellemin AL, Danse JM, Bloch-Zupan A, Yoshiba K, Stoetzel C, Perrin-Schmitt F. The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet. 1998;7:945–957. doi: 10.1093/hmg/7.6.945. [DOI] [PubMed] [Google Scholar]
- Charite J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Monsonego-Ornan E, Pines M, Antonopoulou I, Morriss-Kay GM, Lonai P. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129:3783–3793. doi: 10.1242/dev.129.16.3783. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the Sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran MA, Hinchliffe JR, Ros MA. Birth and death of cells in limb development: a mapping study. Dev Dyn. 2006;235:2521–2537. doi: 10.1002/dvdy.20916. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mechanisms of development. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- Laing MA, Coonrod S, Hinton BT, Downie JW, Tozer R, Rudnicki MA, Hassell JA. Male sexual dysfunction in mice bearing targeted mutant alleles of the PEA3 ets gene. Mol Cell Biol. 2000;20:9337–9345. doi: 10.1128/mcb.20.24.9337-9345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Lu P, Minowada G, Martin GR. Increasing Fgf4 expression in the mouse limb bud causes polysyndactyly and rescues the skeletal defects that result from loss of Fgf8 function. Development. 2006;133:33–42. doi: 10.1242/dev.02172. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- McGlinn E, Tabin CJ. Mechanistic insight into how Shh patterns the vertebrate limb. Curr Opin Genet Dev. 2006;16:426–432. doi: 10.1016/j.gde.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Milaire J. A new interpretation of the necrotic changes occurring in the developing limb bud paddle of mouse embryos based upon recent observations in four different phenotypes. The International journal of developmental biology. 1992;36:169–178. [PubMed] [Google Scholar]
- Monte D, Coutte L, Baert JL, Angeli I, Stehelin D, de Launoit Y. Molecular characterization of the ets-related human transcription factor ER81. Oncogene. 1995;11:771–779. [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Niswander L. Interplay between the molecular signals that control vertebrate limb development. Int J Dev Biol. 2002;46:877–881. [PubMed] [Google Scholar]
- Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Qu S, Niswender KD, Ji Q, van der Meer R, Keeney D, Magnuson MA, Wisdom R. Polydactyly and ectopic ZPA formation in Alx-4 mutant mice. Development. 1997;124:3999–4008. doi: 10.1242/dev.124.20.3999. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. Autoregulation of Shh expression and Shh induction of cell death suggest a mechanism for modulating polarising activity during chick limb development. Development. 2000;127:4811–4823. doi: 10.1242/dev.127.22.4811. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002a;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, Zeller R. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002b;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci STKE. 2004;2004:pe17. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Lewandoski M, Deng C, Harfe BD, Sun X. Conditional inactivation of Fgfr1 in mouse defines its role in limb bud establishment, outgrowth and digit patterning. Development. 2005;132:4235–4245. doi: 10.1242/dev.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyden JM, Sun X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 2008;454:638–641. doi: 10.1038/nature07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Qiao W, Li C, Deng CX. Generation of Fgfr1 conditional knockout mice. Genesis. 2002;32:85–86. doi: 10.1002/gene.10028.abs. [DOI] [PubMed] [Google Scholar]
- Yu K, Ornitz DM. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development. 2008;135:483–491. doi: 10.1242/dev.013268. [DOI] [PubMed] [Google Scholar]
- Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen MT, Bao XZ, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RM, Hunter ES, 3rd, Rogers JM. Apoptosis and morphology in mouse embryos by confocal laser scanning microscopy. Methods. 1999;18:473–480. doi: 10.1006/meth.1999.0815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


