Abstract
Nuclear factor erythroid-2 related factor 2 (Nrf2), a redox-sensitive transcription factor, regulates the expression of antioxidant enzymes and several anti-apoptotic proteins, which confer cytoprotection against oxidative stress and apoptosis. Constitutive activation of Nrf2 in lung cancer cells promotes tumorigenicity and contributes to chemoresistance by upregulation of glutathione, thioredoxin, and the drug efflux pathways involved in detoxification of electrophiles and broad spectrum of drugs. In this study, we show that RNAi-mediated lowering of Nrf2 levels in non-small-cell lung cancer (NSCLC) cell lines (A549 and H460) led to a dramatic increase in endogenous reactive oxygen species (ROS) levels. Similarly, γ-irradiation-induced formation of protein carbonyls were significantly higher in Nrf2-depleted lung cancer cells, suggesting increased lethality of ionizing radiation in the absence of Nrf2. Radiation-induced protein oxidation in Nrf2shRNA cells correlated with reduced survival as measured by clonogenic assay. Radiation-induced cell death was abrogated by pretreatment with antioxidants such as N-acetyl-L-cysteine, glutathione, and vitamin-E, highlighting the importance of antioxidants in conferring protection against radiation injury. Using genetically-modified gain and loss of function models of Nrf2, in mouse embryonic fibroblasts, we establish that constitutive activation of Nrf2 protects against ionizing radiation toxicity and confers radioresistance. Thus, targeting Nrf2 activity in radioresistant tumors could be a promising strategy to circumvent radioresistance. Antioxid. Redox Signal. 13, 1627–1637.
Introduction
Radiotherapy combined with chemotherapy is routinely used for treatment of lung cancers with curative intent in primary lesions, as well as palliative therapy of metastatic disease. However, intrinsic or acquired radioresistance remains a major obstacle affecting the clinical outcome of radiotherapy or combined radiochemotherapy for non-small-cell lung cancer (NSCLC). Another major issue that limits the effectiveness of radiotherapy is radiation-induced toxicity to normal tissues such as the lung and esophagus. The mechanism of radioresistance in NSCLC remains unclear. Studies have shown the potential involvement of either p53 mutations (15), overexpression of prosurvival genes such as XIAP and survivin (44), and activation of the Akt pathway (3). A recent study by Diehn et al. reported that increased expression of free radical scavenging enzymes results in low endogenous ROS levels and contributes to tumor radioresistance (4).
Radiation therapy involves delivery of high-energy radiation to kill cancer cells and shrink tumors. The cellular responses to ionizing radiation (IR) include activation of cell cycle checkpoints that delay the progression of cell growth, as well activation of apoptotic pathways. However, the exact mechanism by which irradiation either activates the checkpoint in surviving cells or induces apoptosis are not clear, although generation of reactive oxygen species (ROS) seems to be a key factor. Radiation induces ROS production within the cells, due to radiolysis of water. These include formation of superoxide, hydroxyl, and nitric oxide radicals. Inadequate removal of ROS results in oxidative stress that leads to damage to biological macromolecules; the products of lipid peroxidation can cause DNA damage leading to cell death (7, 33). To protect against ROS accumulation, cells are equipped with several nonenzymatic and enzymatic antioxidant systems (7, 33). Superoxide catalyzes the dismutation of O2•− into O2 and H2O2, and the peroxide can be destroyed by glutathione peroxidase and catalase. Upregulation of antioxidant enzyme expression or addition of free radical scavengers has been reported to protect cells from the cytotoxic effects of radiation (14, 42).
Nuclear factor erythroid-2–related factor 2 (Nrf2), a cap ‘n’ collar basic leucine zipper transcription factor regulates a transcriptional program that maintains cellular redox homeostasis and protects against toxic xenobiotics. Keap1 is a Nrf2 binding protein that regulates Nrf2-dependent transcription by targeting Nrf2 for proteasomal degradation (8, 11, 45). Keap1 constitutively suppresses Nrf2 activity in the absence of stress. Oxidants, xenobiotics, and electrophiles hamper the Keap1-mediated proteasomal degradation of Nrf2, which results in increased nuclear accumulation and transcriptional induction of target genes. The Nrf2-directed transcriptional program includes a battery of genes, including genes those encode antioxidants (e.g., the glutathione system: γ-glutamyl cysteine synthetase catalytic subunit [GCLc] (29), γ-glutamyl cysteine synthetase modifier subunit [GCLm], glutathione reductase [GSR], glutathione synthetase [GSS], glutathione peroxidase [GPX], and cysteine/glutamate transporter [SLC7A11]); the thioredoxin system: thioredoxin-1 [TXN], thioredoxin reductase [TXNRD1], and peroxiredoxins [PRDX], xenobiotic metabolism enzymes (e.g., NADP[H] quinone oxidoreductase 1 [NQO1], UDP-glucuronosyltransferase), and the members of the glutathione-S-transferase family [GSTs]), (6, 9, 16, 19, 23, 24, 37, 40). Nrf2 also confers protection against apoptotic cell death induced by oxidants and FAS ligand (12, 18, 23). Thus, Nrf2 promotes survival against stress caused by exposure to electrophiles and xenobiotics.
Recently, we and others have reported somatic mutations in Keap1 gene in NSCLC cell lines and tumors (21, 30, 32). Similar mutations in Keap1 gene have been reported in breast cancer and gall bladder cancer (20, 26, 27, 32). Furthermore, we showed that gain of Nrf2 function, resulting from the loss of Keap1 activity, promotes tumor growth and confers chemoresistance in cancer (29). Attenuation of Nrf2 expression by stable expression of short hairpin RNA (shRNA) targeting Nrf2 mRNA in lung cancer cells harboring Keap1 mutations-induced generation of ROS, suppressed tumor growth and resulted in enhanced sensitivity to chemotherapeutic drug-induced cell death in vitro and in vivo (29). In this study, we determined whether gain of Nrf2 function in human lung cancer cells, A549 and H460, confers radioresistance by maintaining cellular redox status. Our results show that inhibition of Nrf2 activity in lung cancer cells lowers the expression of electrophile detoxification enzymes and total cellular thiol levels, leading to increased accumulation of ROS and thus enhance the sensitivity to radiation-induced cytotoxicity.
Materials and Methods
Cell culture and reagents
A549 and H460 cells were purchased from American Type Culture Collection (Manassas, VA) and cultured under recommended conditions. All transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Wild-type, Keap1-/-, Nrf2-/-, and Keap1-/-Nrf2-/- double knockout mouse embryonic fibroblast cell lines were generated as described previously (41). Cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen) containing 10% fetal bovine serum and 1% penicillin streptomycin.
Real time RT-PCR
Total RNA was extracted from lung tissues and or cells using the RNeasy kit (Qiagen, Alameda, CA) and was quantified by UV absorbance spectrophotometry. The reverse transcription reaction was performed by using high capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA). The reverse transcription reaction was performed by using the Multiscribe first strand synthesis system (Applied Biosystems) in a final volume of 20 μl containing 1 μg of total RNA, 100 ng of random hexamers, 1X reverse transcription buffer, 1 mM dNTP, multiscribe reverse transcriptase, and nuclease free water. Quantitative real time RT-PCR analyses of Nrf2, GCLc, GCLm, GSR, G6PD, TXN, and TXNRD1 were performed by using assay-on-demand primers and probe sets from Applied Biosystems. Assays were performed by using the ABI 7000 Taqman system (Applied Biosystems). β-actin was used for normalization.
Western blot analysis
To obtain total protein lysates, cancer cells or tissues were lysed in RIPA buffer containing Halt Protease Inhibitor Cocktail (Pierce, Rockford, IL) and centrifuged at 12,000 g for 15 min at 4°C. For immunoblot analysis, 100 μg of total protein lysate was resolved on 10% SDS-PAGE gels. Proteins were transferred onto PVDF membranes, and the following antibodies were used for immunoblotting: anti-Nrf2, and anti-P53, anti-p21, and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). All the primary antibodies were diluted in PBS-T containing nonfat dry milk (5%) and incubated overnight at 4°C.
Assay for oxidative protein damage
For assessing carbonyl modification of proteins after irradiation, we used an Oxyblot Protein Oxidation Detection Kit (S7150) from Chemicon International (Temecula, CA) as per the manufacturer's instructions. The carbonyl groups in the protein side chains were derivatized to 2,4-dinitrophenylhydrazone by reaction with 2,4-dinitrophenylhydrazine (DNPH) for 15 min in 3% (w/v) SDS. Western blotting: the DNP-derivatized protein samples were separated by polyacrylamide denaturing gel electrophoresis and the proteins were transferred to nitrocellulose membrane (BioRad, Hercules, CA). The membranes were incubated with primary antibody, specific to the DNP moiety of the proteins. This step was followed by incubation with a horseradish peroxidase-antibody conjugate directed against the primary antibody (secondary antibody: goat anti-rabbit IgG). The membranes were then treated with chemiluminescent substrate (GE Healthcare, Piscataway, NJ) and imaged by exposure to light sensitive films (Kodak, Rochester, NY).
Clonogenic assays
Exponentially growing cells were counted, diluted, and seeded in triplicate at 1000 cells per culture dish (100 mm). Cells were incubated for 24 h in a humidified CO2 incubator at 37°C, exposed to high dose rate (0.68 Gy/min) radiation using a Gamma Cell-40 137Cs irradiator (Atomic Energy of Canada, Ltd). To assess clonogenic survival following radiation exposure, cell cultures were incubated in complete growth medium at 37°C for 14 days and then stained with 50% methanol-crystal violet solution. Only colonies with more than 50 cells were counted, and the surviving fraction was calculated and compared with the control. Before radiation exposure, cells were pretreated with antioxidants N-acetyl cysteine (NAC; 10–20 mM), glutathione (GSH) ester (5 mM) (25), and vitamin E analogue Trolox (1 mM) for 1 h, followed by exposure to ionizing radiation. After the exposure, cells were incubated with the same medium containing antioxidants supplements for additional 6 h. The medium was replaced with complete growth medium, and cells were incubated at 37°C for 14 days.
Measurement of ROS levels
To measure endogenous ROS levels, cells were incubated with 10 μM 7’-dichlorodihydrofluorescein diacetate (c-H2DCFDA) (Molecular Probes, Invitrogen, Carlsbad, CA) for 30 min at 37°C, and ROS-mediated oxidation of the fluorescent compound c-H2DCF was measured. Fluorescence of oxidized c-H2DCF was measured at an excitation wavelength of 480 nM and an emission wavelength of 525 nM using a FAC Scan flow cytometer (Becton Dickinson, San Jose, CA).
Cell proliferation assay
Cellular proliferation was analyzed using the colorimetric MTS assay kit (Promega Corp., Madison, WI). Briefly, MEF cells (1000 cells per well) were plated in 96-well plates, and the growth rate was measured.
Statistical analysis
Statistical comparisons were performed by Student's t test. A value of p < 0.05 was considered statistically significant.
Results
Generation of non-small-cell lung cancer cell lines stably expressing Nrf2shRNA
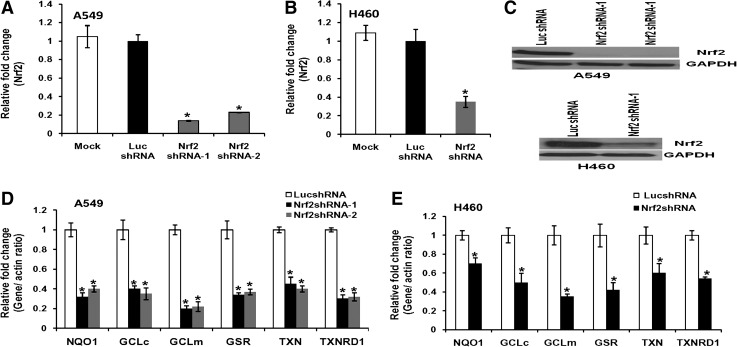
To inhibit the expression of Nrf2 in lung cancer cells, we generated stable A549 and H460 cell lines constitutively expressing the shRNA targeting the 3’ end of the Nrf2 transcript, as described in our previous reports (30, 31). The shRNA targeting luciferase gene was used as control. Stable cell clones with reduced Nrf2 expression were screened. We selected two independent clones of A549 cells expressing Nrf2 shRNA, which demonstrated approximately 85% downregulation of Nrf2 mRNA (Fig. 1A). A single clone expressing Nrf2 shRNA derived from H460 cells demonstrated 70% inhibition of Nrf2 mRNA (Fig. 1B). Detection of Nrf2 protein by immunoblotting showed similar decrease in protein levels (Fig. 1C). The expression of Nrf2 did not change between the control cells transfected with luciferase shRNA and the untransfected cancer cells.
FIG. 1.
Attenuated expression of Nrf2 and its downstream target genes in lung cancer cells constitutively expressing short hairpin RNA against Nrf2 mRNA. (A, B) Real time RT-PCR analysis of Nrf2 expression in A549 and H460 cells stably expressing Nrf2 shRNA. β-actin was used as normalization control. (C) Immunoblot showing reduced levels of Nrf2 protein in A549 and H460 cells stably transfected with shRNAs targeting Nrf2. (D, E) Real time RT-PCR-based analysis of NQO1, GCLc, GCLm, GSR, TXN, and TXNRD1 in PCR in A549 and H460 cells stably expressing Nrf2 shRNA. Cells stably expressing nontargeting luciferase shRNA (Luc-shRNA) were used as baseline control to calculate the fold change. *p ≤ 0.01 relative to the cells expressing luciferase shRNA.
In NSCLC cells, Nrf2 is constitutively activated and upregulates the expression of antioxidants, electrophile and drug detoxification enzymes, and efflux proteins. We measured the expression of genes involved in the glutathione biosynthesis and thioredoxin pathway in Nrf2-depleted A549 and H460 cells constitutively expressing Nrf2shRNA by real time RT-PCR (23, 36, 37). Cells expressing nontargeting luciferase shRNA were used as controls. Lowering of Nrf2 levels led to a decline in the expression of electrophile detoxification genes (Fig. 1D and 1E).
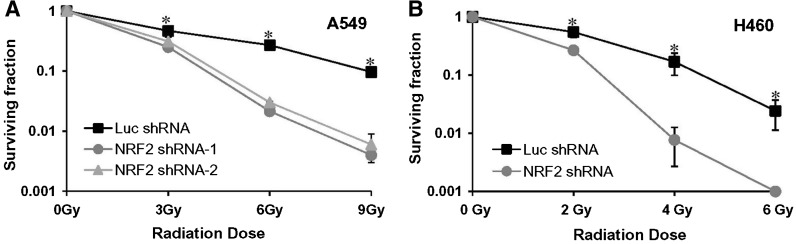
Downregulation of Nrf2 causes radiosensitization
We determined whether inhibition of Nrf2 expression, which causes a decrease in the electrophile detoxification system, could also alter cellular responses to ionizing radiation. A549 and H460 cells stably expressing Nrf2 shRNA and control nontargeting shRNA transfectants were exposed to ionizing radiation, and then assayed for in vitro cell clonogenic survival. As expected, clonogenic survival in all cell lines decreased as the radiation dose increased. The Nrf2 shRNA transfectants showed a markedly increased radiosensitivity that was more pronounced at higher doses compared with cells transfected with the nontargeting control shRNA. Thus, attenuation of Nrf2 activity by shRNA enhanced radiosensitivity in both A549 and H460 cells (Figs. 2A and 2B). At a dose of 6 Gy, the surviving fraction of the A549-Nrf2 knockdown cells was approximately 2%–3%, compared with 27% for the A549 control cells. Similarly, a dose of 4 Gy to H460-Nrf2shRNA cells reduced the survival to approximately 0.7% relative to 20% for the H460-Luc shRNA cells. There was no noticeable difference in radiosensitivity between the control nontargeting shRNA-transfected and the parental lung cancer cell lines (data not shown).
FIG. 2.
Inhibition of Nrf2 activity confers sensitivity to ionizing radiation. (A) Clonogenic survival of A549 cells stably expressing Nrf2 shRNA or control luciferase shRNA and exposed to various doses of ionizing radiation. Surviving fractions were calculated as the plating efficiency of treated cells divided by plating efficiency of untreated cells. (B) Clonogenic survival of H460 cells constitutively expressing Nrf2 shRNA or control luciferase shRNA cells. These cells were treated with different doses of ionizing radiation and surviving fraction was calculated. *p ≤ 0.01 relative to the Luc-shRNA cells.
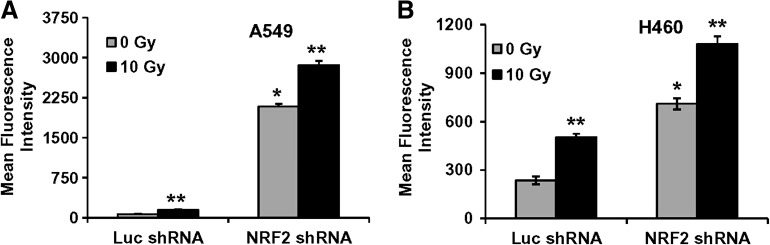
Enhanced production of ROS in cells stably transfected with Nrf2 shRNA
To determine the degree of overall increase in oxidative stress as a result of exposure to iononizing radiation, intracellular ROS levels were monitored using c-H2DCFDA and flow cytometry. Exponentially growing cultures of control lung cancer cells (A549–LucshRNA and H460–Luc shRNA) and lung cancer cells constitutively expressing Nrf2shRNA (A549–Nrf2shRNA and H460–Nrf2shRNA) were irradiated, and ROS levels were measured 24 h post irradiation. Fluorescence of oxidized c-H2DCF was measured by flow cytometry, and the mean fluorescence intensity (MFI) was calculated after correcting for autofluorescence. The level of fluorescence was high in both H460 and A549 cells expressing Nrf2 shRNA (Figs. 3A and 3B). Untreated (0 Gy) A549-Nrf2 shRNA cells demonstrated an increase in ROS level as compared to untreated A549 LucshRNA cells. Similarly, untreated (0 Gy) H460-Nrf2shRNA cells demonstrated a modest (3-fold) increase in ROS levels as compared to control H460-LucshRNA cells. Exposure to ionizing radiation further enhanced the ROS production and increased the MFI in the cells expressing Nrf2 shRNA and control cells. These results suggest that the generation of ROS at a steady state is relatively increased in Nrf2 shRNA transfectants compared to control cells and may be responsible for enhanced radiation sensitivity of Nrf2shRNA expressing A549 and H460 cells.
FIG. 3.
Nrf2-induced radioresistance correlates with lower endogenous levels of ROS in A549 and H460 cells. (A, B) The A549 and H460 stable cells constitutively expressing control Luc-shRNA or Nrf2shRNA were exposed to ionizing radiation and ROS levels were determined 24 h post irradiation. A549 and H460 cells were exposed to a radiation dose of 10 Gy and ROS levels were measured by c-H2DCFDA staining and flow cytometry. *p ≤ 0.01 relative to the cells untreated Luc-shRNA cells; **p < 0.01 relative to the 0 Gy exposed untreated cells.
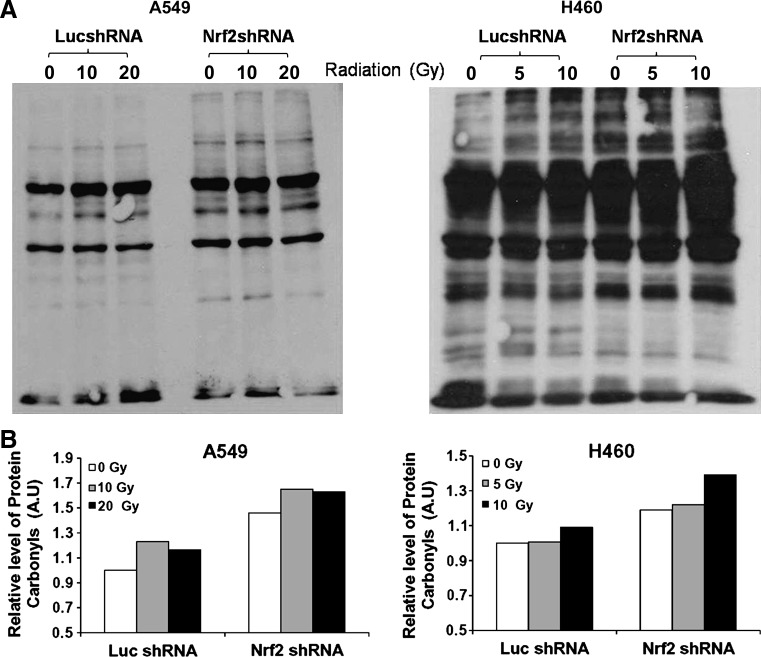
Oxidative stress/ROS may cause direct damage to proteins or the chemical modification of amino acids in proteins (22). This can give rise to protein carbonyls, which may serve as biomarkers for general oxidative stress (22). To determine whether Nrf2 activation in lung cancer cells conferred protection against radiation-induced protein damage, we performed carbonyl content measurements of protein oxidation after cellular exposure to ionizing radiation. In 0 Gy-exposed Nrf2-depleted A549 and H460 cells, we detected higher protein carbonyl content as measured by dinitrophenylhydrazine derivatization of the protein, followed by immunodetection. Exposure to ionizing radiation led to an increase in protein oxidation as measured by total protein carbonyl content in both A549 and H460 cells with Nrf2 deficient A549 and H460 cells having increased protein carbonyls as compared to their respective control cells (A549–LucshRNA and H460–Nrf2shRNA) (Fig. 4).
FIG. 4.
Ionizing radiation induces increased protein oxidation in Nrf2-depleted lung cancer cells. Exponentially growing A549 and H460 cells were exposed to ionizing radiation, and protein carbonyl content was analyzed 24 h post irradiation. A549 cells were exposed to a radiation dose of 0 Gy, 10 Gy, and 20 Gy. H460 cells were exposed to a dose of 0 Gy, 5 Gy, and 10 Gy. (A) Increase in protein carbonyl content in A549 Nrf2shRNA and H460-Nrf2shRNA cells in response to radiation exposure was detected by immunoblotting. (B) Densitometric quantification of relative protein carbonyls in control and radiation exposed A549 and H460 cells. The values in 0 Gy exposed Luc-shRNA cells was arbitrarily set to 1 (arbitrary units [A.U.]). Band intensities were quantified using Image-J software (National Institutes of Health, Bethesda, MD).
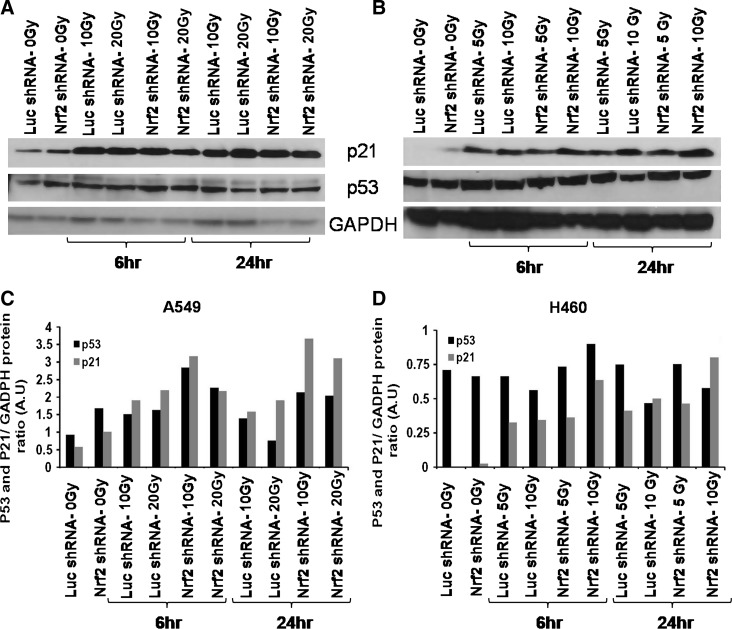
P53/P21 expression after irradiation in Nrf2shRNA transfectants
Ionizing radiation induces double-strand breaks in DNA. One of the key components in damaged DNA recognition and signaling after irradiation is the tumor suppressor protein p53 (34). The cell lines H460 and A549 contain a wild-type p53 sequence and are functional with respect to radiation-induced expression of p53 and p21WAF1/CIP1 proteins. To determine whether Nrf2 suppression had any effect on p53 expression or p53-dependent p21 expression in response to γ-irradiation, we exposed the lung cancer cells constitutively expressing control shRNA or Nrf2shRNA to ionizing radiation and determined the level of p53 and p21 at 6 h and 24 h by immunoblotting. As shown in Figure 5, there was a slight increase in P53 and marked increase in the expression of p21 protein in both H460 and A549 cells. However, the radiation-induced expression of P53 and P21 protein did not differ dramatically between control cells expressing LucshRNA and radiosensitive Nrf2shRNA cells.
FIG. 5.
Nrf2-dependent radioresistance was not P53 dependent in A549 and H460 lung cancer cells. (A, B) A549 and H460 cells constitutively expressing control luciferase shRNA or Nrf2 shRNA were exposed to ionizing radiation, and the expression of p53 and p21 were analyzed at 6 h and 24 h post irradiation. GAPDH was used as loading control. (C, D) Densitometric quantization of p53 and p21 relative protein expression in radiation exposed A549 (10 Gy and 20 Gy) and H460 (5 Gy and 10 Gy) cells as compared to 0 Gy exposed control A549 and H460 cells (arbitrary units [A.U.]). Protein band intensities were quantified using Image-J software (National Institutes of Health).
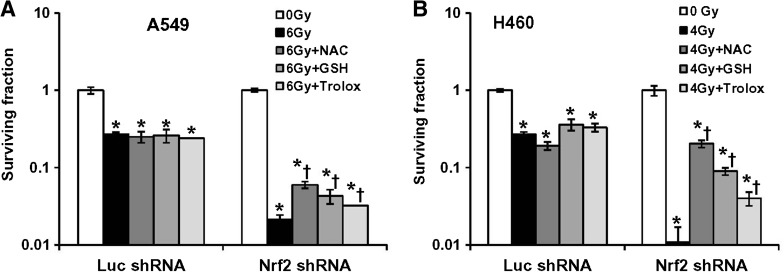
Antioxidant supplementation protects Nrf2-depleted lung cancer cells against ionizing radiation-induced cytotoxicity
We further examined whether lowering endogenous ROS levels in cells expressing Nrf2 shRNA by antioxidant treatment could abrogate the increased sensitivity to ionizing radiation (Figs. 6A and 6B). We used three antioxidants in this study: GSH ester, NAC, and Trolox. Glutathione (L-gamma-glutamyl-L-cysteinylglycine) is the predominant antioxidant in the aqueous cytoplasm of cells. Glutathione can scavenge peroxynitrite and hydroxyl radicals and convert hydrogen peroxide to water. NAC is an aminothiol and synthetic precursor of intracellular cysteine and GSH. NAC also acts a powerful direct antioxidant. Trolox is a water-soluble derivative of vitamin E. It is an antioxidant, like vitamin E, and is used in biological or biochemical applications to reduce oxidative stress or damage. A549 and H460 control cells (A549–LucshRNA and H460–LucshRNA) and Nrf2 shRNA expressing (A549–Nrf2shRNA and H460–Nrf2shRNA) were pretreated with GSH (5 mM), NAC (10 mM for H460 cells and 20 mM for A549 cells) and Trolox (1 mM), followed by γ-irradiation. The cell clonogenic survival assay showed that restoring the cellular redox status in Nrf2shRNA cells by antioxidant supplementation prior to radiation exposure attenuated endogenous ROS levels, rescued cell death as demonstrated by increase in the number of colonies in the clonogenic assay (Figs. 6A and 6B). Of the three antioxidant agents tested, NAC pretreatment produced the maximum radioprotective effect in Nrf2-depleted H460–Nrf2shRNA cells. In A549–Nrf2shRNA cells, NAC and GSH supplementation conferred a similar level of protection against radiation-induced lethality. As anticipated, the treatment of control luciferase shRNA expressing A549 and H460 cells with antioxidants prior to radiation exposure had no effect on their survival. These results clearly indicate that downregulation of Nrf2 causes radiosensitization in a ROS-dependent manner.
FIG. 6.
Pretreatment with antioxidants before radiation treatment restores the redox balance and rescues colony formation defect in the Nrf2-depleted lung cancer cells. (A, B) A549 and H460 stable cells were pretreated with three different antioxidants (NAC, GSH, and Trolox). Cells were incubated with growth medium containing NAC (10–20 mM), GSH (5 mM), and Trolox (1 mM) for 1 h prior to radiation treatment. Antioxidant containing medium was replaced 6 h post irradiation. At the end of Day 14, surviving fractions were calculated. *p ≤ 0.01 relative to the 0 Gy exposed Luc-shRNA and Nrf2shRNA cells; *†p < 0.05 relative to the radiation exposed cells (6 Gy for A549 and 4 Gy for H460) cells.
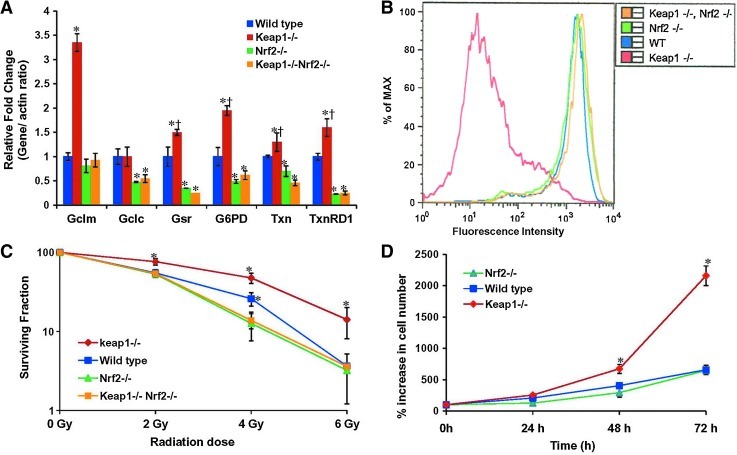
Keap1-/- MEF cells with constitutive activation of Nrf2 are radioresistant
To further establish the role of Nrf2 in radioprotection, we used genetic knockout models such as Nrf2-/- MEFs to determine the effect of loss of Nrf2 expression, Keap1-/- MEFs, which have increased Nrf2 expression and Keap1-/- ;Nrf2-/- double knockout MEFs (28, 41). Expression of Nrf2-dependent antioxidant genes were significantly upregulated in Keap1-/- cells as measured by real time RT-PCR (Fig. 7A). Next, we measured the intracellular ROS levels in these four MEF cell lines using c-H2DCFDA staining and flow cytometry. As anticipated, Keap1-/- cells demonstrated dramatically lower (∼100 fold) H2DCFDA fluorescence, suggesting dramatically lower intracellular ROS levels. The endogenouos ROS levels in wild-type, Nrf2-/-, and Keap1-/-;Nrf2-/- double knockout MEFs were similar (Fig. 7B). To assess the affect of γ-irradiation on survival of MEF cells, we exposed Keap1-/-, wild-type, Nrf2-/-, and keap1-/-;Nrf2-/- double knockout cells to different doses of ionizing radiation and determined clonogenic cell survival. At a dose of 2 Gy, the surviving fraction of the Keap1-/- cells was approximately 75% as compared with 50% for wild-type, Nrf2-/-, and Keap1-/-;Nrf2-/- double knockout cells. Similarly, at a dose of 4 Gy, the survival fraction of the Keap1-/- cells was reduced to approximately 50% compared to 25% for the wild-type and less than 15% for Nrf2-/- and Keap1-/-;Nrf2-/- double knockout cells (Fig. 7C). Keap1-/- cells showed significant (p < 0.001) radioresistance at all doses compared to wild-type, Nrf2-/-, and double knockout Keap1-/-;Nrf2-/- cells.
FIG. 7.
Keap1-/- mouse embryonic fibroblast cells with constitutive activation of Nrf2 express high levels of antioxidant genes correspond to a lower endogenous ROS level and are radioresistant. (A) Real time RT-PCR-based analysis of Gclc, Gclm, Gsr, G6PD, Txn, and Txnrd1, in Keap1-/-, wild-type, Nrf2-/-, and Keap1-/-Nrf2-/- double knockout MEF cells. Gene expression values in wild-type cells were used as baseline to calculate the fold change. β-Actin was used as normalization control. *p ≤ 0.01 relative to the WT and Keap1-/- cells; *†p < 0.01, relative to the WT, Nrf2-/-, and Keap1-/-Nrf2-/- cells. (B) Reduced endogenous ROS levels in Keap1-/- MEF cells as compared to wild-type, Nrf2-/-, and Keap1-/-Nrf2-/- MEF cells. (C) Clonogenic survival of Keap1-/-, wild-type, Nrf2-/-, and Keap1-/-Nrf2-/- double knockout MEF cells exposed to different doses (2 Gy, 4 Gy, and 6 Gy) of ionizing radiation. Surviving fractions were calculated as the plating efficiency of treated cells divided by the plating efficiency of untreated cells. *p ≤ 0.01 relative to the radiation exposed WT, Nrf2-/-, and Keap1-/-Nrf2-/- cells; *†p < 0.05 relative to the radiation exposed Nrf2-/- and Keap1-/-Nrf2-/- cells. (D) Comparison of in vitro cellular proliferation rate of WT, Nrf2-/-, and Keap1-/- MEF cells. *p ≤ 0.01 relative to the WT and Nrf2-/- MEF cells.
To further demonstrate that gain of Nrf2 function in Keap1-/- MEF cells confers growth advantage, we compared the in vitro cellular proliferation rate of Keap1-/-, wild-type, and Nrf2-/- MEFs. We plated 1000 cells/well in 96-well plates and measured the cellular proliferation over a period of 72 h. As shown in Figure 7D, Keap1-/- cells with high Nrf2 grew faster than wild-type and Nrf2-/- cells. Similar observations were made in lung cancer cells, where constitutive activation of Nrf2 promotes in vitro and in vivo cellular proliferation (29). In summary, the results of the current study demonstrate that gain of function confers radioresistance and facilitates cell growth.
Discussion
Nrf2, a redox sensitive bZIP transcription factor, activates cytoprotective pathways against oxidative injury, inflammation, apoptosis, and carcinogenesis through transcriptional induction of a broad spectrum of genes involved in electrophile/drug detoxification and antioxidant protection (8, 23, 36). Keap1 mediates proteasomal degradation of Nrf2 and thus negatively regulates Nrf2 activity. We and others have shown that Nrf2 is constitutively activated in NSCLC cells due to somatic mutations in Keap1 gene (21, 30, 32). Loss of Keap1 activity leading to gain of Nrf2 function in lung cancer cells upregulates the expression of cytoprotective antioxidants and anti-apoptotic proteins (21, 30). Recently, somatic activating mutations in Nrf2 gene leading to constitutive activation of Nrf2 were reported in squamous carcinoma of lung (27). This study demonstrates that constitutive activation of Nrf2-dependent signaling in lung cancer cells promotes radioresistance. The most important finding is that shRNA-mediated reduction of Nrf2 expression induces ROS generation and increases protein oxidation resulting in enhanced sensitivity to radiation-induced cell death.
Increased ROS is common in cancer cells and is believed to be attributable at least in part to high metabolism and hyperactive glycolytic metabolism driven by oncogenic proliferative signals (38). The intrinsic ROS associated with oncogenic transformation renders the cancer cells highly dependent on antioxidant systems to maintain redox balance. ROS must be neutralized by antioxidants to avoid potential damage to cellular DNA, protein, and lipids. Glutathione and thioredoxin systems constitute the major thiol-based antioxidant and repair systems, and Nrf2 regulates the expression of majority of these genes. Enzymes involved in GSH synthesis (GCLc and GCLm), members of the glutathione reductase and peroxidase families (GSR, GPx2, and GPx3), and genes that constitute the thioredoxin system (TXN, TXNRD1, and Prx1) have been shown to be the transcriptional targets of Nrf2 (8, 9, 23, 35, 36). The members of these redox systems interact with various transducer and effector molecules, leading to antioxidant-specific responses. The regeneration of reduced TXN and GSH by TXNRD1 and GSR, respectively, utilizes NADPH as a reducing equivalent generated by glucose-6-phosphate dehydrogenase and malic enzyme, which have been shown to be expressed in an Nrf2-dependent manner (8, 23, 36). Increased formation of NADPH may prove beneficial for the cell survival because it is involved in the reductive biosynthesis, maintenance of redox state, and it also acts as a potent antioxidant (10). The coordinated expression of all of these genes involved in electrophile detoxification and antioxidant status suggest an important role for Nrf2 in regulating cellular defenses against cytotoxic and genotoxic agents by increasing the reductive capacity of the cell (36). Inhibition of Nrf2 expression by shRNA approach attenuated the expression of antioxidants, several electrophile, and xenobiotic drug detoxification proteins (29). Blocking of Nrf2-dependent gene expression by shRNA dramatically enhanced the steady-state ROS levels in lung cancer cells, making them vulnerable to death induced by cytotoxic agents. Treatment with a nonspecific free radical scavenger NAC significantly reduced the endogenous ROS levels (29).
Ionizing radiation triggers the formation of free radicals, which interact among themselves and critical biological targets with the formation of a plethora of newer free radicals (43). Some of these free radicals damage genomic DNA (13, 42, 43). ROS damages cellular DNA through oxidative stress-induced destruction of pyrimidine and purine bases, single- and double- strand DNA breaks, and oxidation of protein thiols and lipids (5). Nonenzymatic antioxidants (glutathione and thioredoxin) and several antioxidant enzymes such as glutathione-S-transferases, aldehyde dehydrogenases, glutathione peroxidases, thioredoxin, and peroxiredoxins constitute the electrophile detoxification system that scavenges the radiation-induced electrophiles, thereby causing cellular resistance. Radioprotective effects by modification of antioxidant enzyme expression or by addition of free radical scavengers have been reported (2, 14, 39, 42, 43). Protective effects of Bcl2 (17) and HSP25 (1) against irradiation have been demonstrated to be mediated by GSH. Conversely, thiol depletion can result in a higher incidence of radiation-induced apoptosis (17).
In this study, we found that alteration of redox status by Nrf2 inhibition enhanced the sensitivity of lung cancer cells to ionizing radiation through depletion of antioxidants and electrophile detoxification enzymes. Pretreatment with antioxidants, glutathione ester, N-acetyl-L-cysteine, or a vitamin E analogue, Trolox, followed by radiation exposure significantly increased the survival of Nrf2-depleted cells but had no effect on colony-forming ability of control Nrf2-proficient wild-type cells. It is observed that antioxidants rescue the radiation-induced damage in cancer cells expressing Nrf2 shRNA, which indicates that the global decrement in the expression of electrophile detoxification systems has led to increased sensitivity to ROS in cancer cells. To demonstrate that Nrf2-mediated radioresistance is applicable to other cell types, we used MEF cells with gain of Nrf2 function (Keap1-/-) as well loss of Nrf2 function (Nrf2-/-). As expected, Keap1-/- MEF cells with high antioxidant capacity and low endogenous ROS levels were found to be resistant to ionizing radiation-induced cell death. Lower expression of antioxidant genes coupled with higher endogenous ROS levels in Nrf2-/- MEF cells as well as double knockout Keap1-/-;Nrf2-/- cells wild-type cells resulted in enhanced sensitivity to γ-irradiation, leading to decreased survival. Overall, the results from cancer cells and nontumorigenic MEF cells unequivocally establish that high levels of Nrf2 confer resistance to radiotherapy.
In conclusion, we have identified Nrf2 as a novel radioresistance factor. Ionizing radiation is one of the most commonly employed modalities of cancer treatment; cancer cells increasingly become resistant to consecutive administration of radiation. Our findings suggest that targeting Nrf2 by combining RNAi and/or small-molecule inhibitors approach will enhance the susceptibility to ionizing radiation-induced cell death by altering cellular redox status and will likely have significance in treatment of radioresistant tumors. Due to the additive efficacy of Nrf2 gene knockdown and ionizing radiation on cell death in NSCLC, this combined treatment modality has the potential to improve the anticancer efficacy and thus reduce radiation dose to attenuate collateral damage to normal tissues. Furthermore, the fact that Nrf2 promotes resistance to both chemotherapy and irradiation suggests that Nrf2 activity might have predictive value for response to chemotherapy and/or irradiation.
Abbreviations Used
- ARE
antioxidant response element
- G6PD
glucose-6-phosphate dehydrogenase
- GCLc
γ-glutamyl cysteine synthetase catalytic subunit
- GCLm
γ-glutamyl cysteine synthetase modifier subunit
- GSH
glutathione
- GSR
glutathione reductase
- GST
glutathione-S-transferase
- IR
ionizing radiation
- Keap1
Kelch-like ECH-associated protein 1
- MEF
mouse embryonic fibroblast
- MFI
mean fluorescence intensity
- MRP
multidrug resistance protein
- NAC
N-acetyl-L-cysteine
- NQO1
NADP(H) quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid-2 related factor 2
- NSCLC
non-small-cell lung cancer
- PRDX
peroxiredoxin
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- TXN
thioredoxin-1
- TXNRD1
thioredoxin reductase 1
Acknowledgments
This work was supported by NIH Grants P50 CA058184, NIEHS center grant P30 ES 038819, RO1 CA140492 (SB), R01 CA104253 (FB), and Flight Attendant Research Institute (SB and AS). We thank Ping Zhang and Deepti Malhotra for help with immunoblot and real time PCR assays.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baek SH. Min JN. Park EM. Han MY. Lee YS. Lee YJ. Park YM. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell Physiol. 2000;183:100–107. doi: 10.1002/(SICI)1097-4652(200004)183:1<100::AID-JCP12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Bravard A. Ageron–Blanc A. Alvarez S. Drane P. Le Rhun Y. Paris F. Luccioni C. May E. Correlation between antioxidant status, tumorigenicity and radiosensitivity in sister rat cell lines. Carcinogenesis. 2002;23:705–711. doi: 10.1093/carcin/23.5.705. [DOI] [PubMed] [Google Scholar]
- 3.Brognard J. Clark AS. Ni Y. Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 4.Diehn M. Cho RW. Lobo NA. Kalisky T. Dorie MJ. Kulp AN. Qian D. Lam JS. Ailles LE. Wong M. Joshua B. Kaplan MJ. Wapnir I. Dirbas FM. Somlo G. Garberoglio C. Paz B. Shen J. Lau SK. Quake SR. Brown JM. Weissman IL. Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gromer S. Urig S. Becker K. The thioredoxin system—From science to clinic. Med Res Rev. 2004;24:40–89. doi: 10.1002/med.10051. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi A. Suzuki H. Itoh K. Yamamoto M. Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 7.Hensley K. Floyd RA. Reactive oxygen species and protein oxidation in aging: A look back, a look ahead. Arch Biochem Biophys. 2002;397:377–383. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 8.Kensler TW. Wakabayashi N. Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ. Ahn JY. Liang P. Ip C. Zhang Y. Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch M. De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi A. Kang MI. Okawa H. Ohtsuji M. Zenke Y. Chiba T. Igarashi K. Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotlo KU. Yehiely F. Efimova E. Harasty H. Hesabi B. Shchors K. Einat P. Rozen A. Berent E. Deiss LP. Nrf2 is an inhibitor of the Fas pathway as identified by Achilles' Heel Method, a new function-based approach to gene identification in human cells. Oncogene. 2003;22:797–806. doi: 10.1038/sj.onc.1206077. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KS. Vaishnav YN. Weiss JF. Radioprotection by antioxidant enzymes and enzyme mimetics. Pharmacol Ther. 1988;39:301–309. doi: 10.1016/0163-7258(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 14.Lee HC. Kim DW. Jung KY. Park IC. Park MJ. Kim MS. Woo SH. Rhee CH. Yoo H. Lee SH. Hong SI. Increased expression of antioxidant enzymes in radioresistant variant from U251 human glioblastoma cell line. Int J Mol Med. 2004;13:883–887. [PubMed] [Google Scholar]
- 15.Lee JM. Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci USA. 1993;90:5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TD. Yang H. Whang J. Lu SC. Cloning and characterization of the human glutathione synthetase 5'-flanking region. Biochem J. 2005;390:521–528. doi: 10.1042/BJ20050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirkovic N. Voehringer DW. Story MD. McConkey DJ. McDonnell TJ. Meyn RE. Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene. 1997;15:1461–1470. doi: 10.1038/sj.onc.1201310. [DOI] [PubMed] [Google Scholar]
- 18.Morito N. Yoh K. Itoh K. Hirayama A. Koyama A. Yamamoto M. Takahashi S. Nrf2 regulates the sensitivity of death receptor signals by affecting intracellular glutathione levels. Oncogene. 2003;22:9275–9281. doi: 10.1038/sj.onc.1207024. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T. Sherratt PJ. Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 20.Nioi P. Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan B. Tong KI. Ohta T. Nakamura Y. Scharlock M. Ohtsuji M. Kang MI. Kobayashi A. Yokoyama S. Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Pleshakova OV. Kutsyi MP. Sukharev SA. Sadovnikov VB. Gaziev AI. Study of protein carbonyls in subcellular fractions isolated from liver and spleen of old and gamma-irradiated rats. Mech Ageing Dev. 1998;103:45–55. doi: 10.1016/s0047-6374(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 23.Rangasamy T. Cho CY. Thimmulappa RK. Zhen L. Srisuma SS. Kensler TW. Yamamoto M. Petrache I. Tuder RM. Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangasamy T. Guo J. Mitzner WA. Roman J. Singh A. Fryer AD. Yamamoto M. Kensler TW. Tuder RM. Georas SN. Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy NM. Kleeberger SR. Cho HY. Yamamoto M. Kensler TW. Biswal S. Reddy SP. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata T. Kokubu A. Gotoh M. Ojima H. Ohta T. Yamamoto M. Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 27.Shibata T. Ohta T. Tong KI. Kokubu A. Odogawa R. Tsuta K. Asamura H. Yamamoto M. Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin S. Wakabayashi N. Misra V. Biswal S. Lee GH. Agoston ES. Yamamoto M. Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: Iinfluence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A. Boldin-Adamsky S. Thimmulappa RK. Rath SK. Ashush H. Coulter J. Blackford A. Goodman SN. Bunz F. Watson WH. Gabrielson E. Feinstein E. Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A. Misra V. Thimmulappa RK. Lee H. Ames S. Hoque MO. Herman JG. Baylin SB. Sidransky D. Gabrielson E. Brock MV. Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A. Rangasamy T. Thimmulappa RK. Lee H. Osburn WO. Brigelius-Flohe R. Kensler TW. Yamamoto M. Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjoblom T. Jones S. Wood LD. Parsons DW. Lin J. Barber TD. Mandelker D. Leary RJ. Ptak J. Silliman N. Szabo S. Buckhaults P. Farrell C. Meeh P. Markowitz SD. Willis J. Dawson D. Willson JK. Gazdar AF. Hartigan J. Wu L. Liu C. Parmigiani G. Park BH. Bachman KE. Papadopoulos N. Vogelstein B. Kinzler KW. Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann NY Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 34.Sun SY. Yue P. Wu GS. El-Deiry WS. Shroot B. Hong WK. Lotan R. Implication of p53 in growth arrest and apoptosis induced by the synthetic retinoid CD437 in human lung cancer cells. Cancer Res. 1999;59:2829–2833. [PubMed] [Google Scholar]
- 35.Tanito M. Agbaga MP. Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Thimmulappa RK. Mai KH. Srisuma S. Kensler TW. Yamamoto M. Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 37.Thimmulappa RK. Scollick C. Traore K. Yates M. Trush MA. Liby KT. Sporn MB. Yamamoto M. Kensler TW. Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trachootham D. Zhou Y. Zhang H. Demizu Y. Chen Z. Pelicano H. Chiao PJ. Achanta G. Arlinghaus RB. Liu J. Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Tuttle S. Stamato T. Perez ML. Biaglow J. Glucose-6-phosphate dehydrogenase and the oxidative pentose phosphate cycle protect cells against apoptosis induced by low doses of ionizing radiation. Radiat Res. 2000;153:781–787. doi: 10.1667/0033-7587(2000)153[0781:gpdato]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Vollrath V. Wielandt AM. Iruretagoyena M. Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakabayashi N. Itoh K. Wakabayashi J. Motohashi H. Noda S. Takahashi S. Imakado S. Kotsuji T. Otsuka F. Roop DR. Harada T. Engel JD. Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 42.Weiss JF. Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 43.Weiss JF. Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 44.Yang CT. Li JM. Weng HH. Li YC. Chen HC. Chen MF. Adenovirus-mediated transfer of siRNA against survivin enhances the radiosensitivity of human non-small cell lung cancer cells. Cancer Gene Ther. 2010;17:120–130. doi: 10.1038/cgt.2009.55. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DD. Lo SC. Cross JV. Templeton DJ. Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]