Abstract
As a minimally invasive technique, endoscopic resection may benefit patients diagnosed with early stage gastrointestinal neuroendocrine tumors (NETs). However, no studies have yet been published in which endoscopic submucosal dissection (ESD) has been applied for gastric NETs. For the first time a research group in China applied ESD to remove gastric NETs, and indicated that ESD should be considered for treatment of eligible gastric NETs because the technique shows a high histologically complete resection rate, provides accurate histopathological evaluation, has a low complication rate, and can be performed within a reasonable timeframe.
1. Introduction
Gastric NETs have been previously thought to be extremely rare lesions. However, over the last 50 years, the incidence of gastric neuroendocrine tumors (NETs) has been increasing in most countries because of better awareness and an increased widespread use of upper gastrointestinal endoscopy [1]. In the preendoscopic era, they comprised only 0.3% of all gastric tumors and 1.9% of all gastrointestinal NETs. More recent studies have shown that as many as 10–30% of all NETs may occur in the stomach [1]. Nowadays, more and more gastric NETs are usually diagnosed at an early stage (tumor size < 11–20 mm and limited to the mucosa/submucosa) [2] and thus can be managed with local excision including endoscopic treatment because of a low frequency of lymph node and distant metastasis. As a minimally invasive technique, endoscopic resection may benefit patients diagnosed with gastric NETs. It offers the promise of localized treatment of these tumors, with relatively few complications and low mortality.
Various endoscopic resection procedures such as endoscopic polypectomy, strip biopsy, aspiration resection, and band-snare resection have been described as potential treatment procedures for gastric NETs [3–5]. However, complete resection of NETs is difficult with conventional polypectomy and endoscopic mucosal resection (EMR) because most gastrointestinal NETs are not confined to the mucosa but, rather, invade the submucosa [6], which results in frequent involvement of the resection margin. Polypectomy may not provide adequate resection margins, and additional surgical intervention may be needed.
Endoscopic submucosal dissection (ESD) is a method of endoscopic resection and has the advantage of a high probability of en bloc and histologically complete resection even in submucosal tumors because the technique involves dissection of the submucosal tissue beneath the lesion [7]. To date, the fact that ESD can facilitate histologically complete resection of NETs has been verified on the use of ESD for treatment of rectal NETs [8–10]. However, limited systematic studies in which ESD has been applied for gastric NETs have been published. The purpose of this paper was to provide a better understanding of the endoscopic features of these tumors and to retrospectively evaluate the clinical impact of ESD for gastric NETs.
2. Patients and Methods
2.1. Patients
With the approval of the institutional review board, from January 2008 to January 2012, 25 patients with confirmed histological diagnosis of gastric neuroendocrine neoplasms were treated with ESD. None had regional lymph node enlargement and distant metastases to the liver or lung on computerized tomography (CT) scanning or endoscopic ultrasonography (EUS) before ESD. Tumor characteristics, complete resection rate, complications, local recurrences, and distant metastases were evaluated in all patients. Informed patient's consent was obtained prior to the procedures.
2.2. ESD Procedures
Preoperative EUS (high-frequency miniprobe, UM-2R, 12 MHz; UM-3R, 20 MHz, Olympus) was performed to evaluate the depth of tumor invasion and the involvement of regional lymph nodes. The existence of lymph node and distant metastasis was surveyed by contrast-enhanced CT, abdomen ultrasound, and chest X-ray.
To dissect the tumor, endoscopic submucosal dissection was attempted with a single-channel gastroscope (GIF-H260, Olympus) and an insulated-tip electrosurgical knife (KD-611L, Olympus) or hook knife (KD-620LR, Olympus). A transparent cap (D-201-11304, Olympus) was attached to the tip of the gastroscope to provide direct views of the submucosal layer. Other equipment included injection needle (NM-4L-1, Olympus), grasping forceps (FG-8U-1, Olympus), snare (SD-230U-20, Olympus), hot biopsy forceps (FD-410LR, Olympus), clips (HX-610-90, HX-600-135, Olympus), high-frequency generator (ICC-200, ERBE), and argon plasma coagulation unit (APC300, ERBE).
Patients were treated under general anesthesia. After making several marking dots with argon plasma coagulation around the lesion, a mixture solution (including 100 mL of normal saline, 1 mL of indigo carmine, and 1 mL of epinephrine) was injected into the submucosa. The mucosa was incised outside the marking dots. Direct dissection of the submucosal layer beneath the tumor was then performed under direct vision to achieve complete en bloc resection of the specimen. The tumor was dissected along the capsule, and saline solution was injected repeatedly during the dissection when necessary. The resultant artificial ulcer was managed routinely with argon plasma coagulation to prevent delayed bleeding, and hemoclips were used to close the deeply dissected areas as needed (Figure 1).
Figure 1.
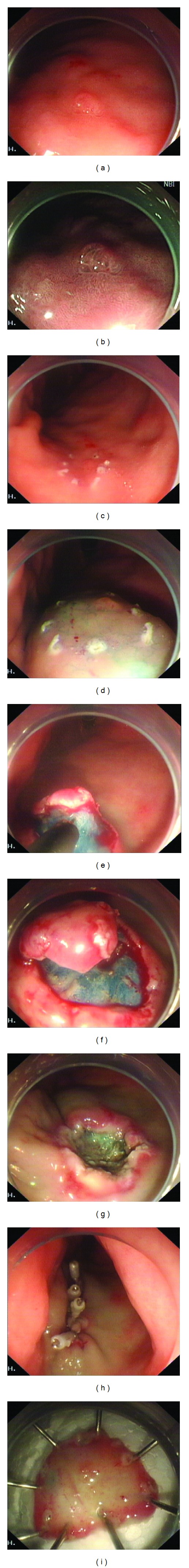
The procedure of endoscopic submucosal dissection for a gastric neuroendocrine tumor. (a, b) A sessile polyp with a reddened surface of the gastric body. (c) Making. (d) Injection. (e, f) Dissection. (g) Resultant artificial ulcer. (h) Closure of the defect with metallic clips. (i) Completely resected specimen.
2.3. Clinicopathological Categorization and Pathological Evaluation
There is a clinicopathological categorization of the gastric NETs which distinguishes four types of neuroendocrine neoplasms of the stomach [2]: type I is those arising in chronic atrophic gastritis with hypergastrinemia; type II occurs in patients with hypergastrinemia due to the Zollinger-Ellison syndrome in association with multiple endocrine neoplasia type I; type III is gastric NET not associated with any specific pathogenetic background; poorly differentiated neuroendocrine carcinomas are nowadays classified as type IV neuroendocrine neoplasms of the stomach.
The WHO 2010 classification of tumours of the digestive system was used for histopathologic evaluation [11]. Mitotic count per 10 high-power field (HPF) or Ki-67 Index per 400–2000 cells was used for grading and staging. On the basis of proliferative activity, gastric neuroendocrine neoplasms are graded as G1, G2, or G3. Low to intermediate grade tumors (G1-G2) are defined as NETs (previously referred to as carcinoids) whereas high-grade carcinomas (G3) are termed neuroendocrine carcinomas (NECs).
En bloc resection refers to a resection in one piece. A resection with a tumor-free margin in which both the lateral and basal margins were free of tumor cells was considered as a complete resection. A resection in which the tumor extended into the lateral or basal margin, or the margins were indeterminate because of artificial burn effects, was considered as an incomplete resection.
2.4. Followup
Patients underwent followup endoscopy and/or EUS at 1, 3, 6, and 12 months after ESD and annually thereafter to view the healing of the wound and to check any tumor residual or recurrence. Close followup by abdomen ultrasound, contrast-enhanced CT, and chest radiography were carried out to evaluate distant metastasis every 6 months. A final checkup was performed via telephone questionnaires in August 2012. At that time, followup data for 100.0% of the patients were available for evaluation.
3. Results
Patient characteristics, lesion features, and clinical outcomes are summarized in Table 1. The study cohort consisted of 8 men and 17 women, aged from 35 to 82 years. 4 cases had 2 tumors, and 2 cases had 3 tumors. Of the 33 lesions, 1 of them located in the cardia, 5 in the gastric fundus, 26 in the gastric body, and 1 in the gastric antrum. All lesions were found incidentally during routine upper gastrointestinal endoscopy for other indications such as anemia, reflux symptoms, or nonspecific abdominal symptoms. None had symptoms of carcinoid syndrome. With respect to macroscopic appearance, 12 patients had submucosal tumors with a central depression or erosion on top, 10 patients had sessile polyps with a reddened surface, 2 patients had erosion-type tumors, and 1 patient had a tumor with superficial ulcer.
Table 1.
Clinicopathological characteristics of 25 patients with gastric neuroendocrine tumors treated by endoscopic submucosal dissection.
| Case no. | Age, y | Gender | Location | Macroscopic appearance | Clinicopathological type | Size, mm | En bloc resection | Depth of invasion | WHO classification | Lateral margin | Vertical margin | Vessel invasion | Complication | Additional surgery | Followup, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | Gastric body | Submucosal tumor | I | 4, 4, 3 | Yes | Mucosa | NET-G1 | (−) | (−) | Absent | None | None | 55 |
| 2 | 41 | M | Gastric antrum | Submucosal tumor | III | 10 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 52 |
| 3 | 71 | M | Gastric body | Submucosal tumor | III | 18 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | Reject | 45 |
| 4 | 53 | M | Gastric body | Submucosal tumor | I | 5 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 44 |
| 5 | 55 | F | Gastric body | Submucosal tumor | III | 10 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 41 |
| 6 | 82 | F | Gastric fundus | Submucosal tumor | III | 30 | Yes | Submucosa | NET-G2 | (−) | (−) | Absent | None | Reject | 40 |
| 7 | 35 | F | Gastric fundus | Polyp | I | 6 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 38 |
| 8 | 40 | F | Gastric body | Polyp | I | 6 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 35 |
| 9 | 47 | F | Gastric body | Submucosal tumor | I | 4 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 35 |
| 10 | 56 | F | Gastric body | Polyp | I | 3, 2 | Yes | Mucosa | NET-G1 | (−) | (−) | Absent | None | None | 31 |
| 11 | 51 | F | Gastric body | Submucosal tumor | III | 8 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 29 |
| 12 | 55 | M | Gastric body | Polyp | I | 8, 3, 3 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 28 |
| 13 | 55 | M | Gastric body | Polyp | I | 4 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 28 |
| 14 | 57 | M | Gastric body | Submucosal tumor | III | 15 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | Delayed bleeding | Reject | 26 |
| 15 | 35 | F | Gastric body | Submucosal tumor | III | 25 | Yes | Submucosa | NET-G2 | (−) | (−) | Absent | None | Yes | 25 |
| 16 | 45 | F | Gastric body | Polyp | I | 6, 3 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 22 |
| 17 | 48 | F | Gastric body | Polyp | I | 5 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 21 |
| 18 | 55 | M | Gastric body | Polyp | I | 5 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 20 |
| 19 | 65 | F | Cardia | Submucosal tumor | III | 20 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | Reject | 20 |
| 20 | 35 | F | Gastric fundus | Erosion | I | 5, 3 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | None | 20 |
| 21 | 47 | M | Gastric fundus | Polyp | I | 4 | Yes | Mucosa | NET-G1 | (−) | (−) | Absent | None | None | 20 |
| 22 | 57 | F | Gastric body | Polyp | I | 8 | Yes | Mucosa | NET-G1 | (−) | (−) | Absent | None | None | 18 |
| 23 | 52 | F | Gastric body | Superficial ulcer | III | 15 | Yes | Submucosa | NET-G2 | (−) | (−) | Absent | None | Reject | 12 |
| 24 | 40 | F | Gastric body | Submucosal tumor | III | 20, 5 | Yes | Submucosa | NET-G1 | (−) | (−) | Absent | None | Reject | 11 |
| 25 | 70 | F | Gastric body | Erosion | I | 5 | Yes | Mucosa | NET-G1 | (−) | (−) | Absent | None | None | 7 |
NET: neuroendocrine tumor. Reject: the patient rejected the additional surgery.
With respect to clinicopathological categorization, 22 lesions in 15 patients were type I gastric NETs arising in chronic atrophic gastritis with hypergastrinemia, while other 11 lesions in 10 patients were type III because of absence of atrophic gastritis in these cases. None showed metastatic disease to lymph nodes or distal organs on preoperative examinations. Before ESD procedures, histological diagnosis of gastric NETs had been confirmed via biopsies in 4 cases.
All the tumors were removed in an en bloc fashion (33/33, 100%). The average maximum diameter of the lesions was 8.2 mm (range 2–30 mm), and the procedure time was 22.5 minutes (range 10–45 minutes). Results of pathological studies determined that 30 lesions were NET-G1 and 3 lesions were NET-G2. Complete resection was achieved in all the tumors (33/33, 100%). All of them were confined to the submucosa in histopathologic assessment, and no lymphovascular invasion was observed in any of the tumors.
Delayed bleeding occurred in one case 3 days after ESD. Successful hemostasis was achieved by coagulating forceps and spraying with thrombin during emergency endoscopy. The procedure-related perforation was not seen in any tumor.
Because type III gastric NETs with diameter larger than 10 mm may have high risks of metastasis, additional surgical intervention should be considered in 7 cases. However, only 1 of them underwent additional surgery, and we could not reveal residual lesions or metastatic lymph nodes in the surgical specimens. Other 6 cases refused additional surgery, citing their age, physical condition, or other personal reasons. Therefore, they were under careful followup.
During a mean of 28.9 months (range 7–55 months) followup periods, local recurrence occurred in two patients after initial ESD (case no. 1 and no. 12). Both of them then underwent repeat ESD successfully. Metastasis to lymph nodes or distal organs was not observed in any patient. No patients died during the study period.
4. Discussion
Because most gastrointestinal NETs are not confined to the mucosa but, rather, invade the submucosa [6], we, thus, chose ESD for the treatment of gastric NETs in the current study. Most of the lesions extended into the submucosa (75.8%, 25/33), and complete resection was achieved for all the tumors in this study. High histologically complete resection rate of ESD may give several advantages for the treatment of gastric NETs [8–10]. First, histologically complete resection can provide a substantial amount of submucosal tissue and accurate determination of lymphovascular invasion, and histological grading is possible and can inform decisions regarding subsequent therapy. Second, incomplete resection of tumors results in the need for additional surgery, and complete resection allows us to reduce the incidence of unnecessary surgery. Third, repeat endoscopic resection of remnant tumor after an initial incomplete endoscopic resection may be difficult because of fibrosis that prevents lifting of the lesion by submucosal injection. Therefore, we recommend histologically complete resection of gastric NETs even when lesions are small, and the present study indicates that ESD may maximize the likelihood of such an outcome because of complete resection.
Gastric neuroendocrine neoplasms are divided into four groups by the clinicopathological classification: type I: NETs associated with type A chronic gastritis; type II: carcinoids with endocrine neoplasia; type III: sporadic carcinoids without hypergastrinemia; type IV: poorly differentiated neuroendocrine carcinomas [2]. Type I is the most frequent and comprises approximately 65% of all gastric NETs, while Type III is less frequent (21%) [1]. Types I and II gastric NETs frequently show less lymph node involvement compared with type III [2, 12]. Gastric NETs with submucosal invasion and muscularis propria invasion also show high incidences of metastasis [13]. Therefore, the indication for rescue surgery after endoscopic resection is usually based upon the size, type, depth of invasion, grade, and stage of the gastric NET disease [3]. In general, additional surgical intervention is recommended in the case of type I or type II gastric NETs with positive margins, size > 20 mm, G2-G3 histological grading, invasion into the muscularis propria, or vessel infiltration of tumor cells. Additional surgery was also recommended in the case of type III gastric NETs with size > 10 mm irrespective of other risk factors. Surgery was the only treatment of choice in case of a localized type IV gastric NET. According to this, additional surgical intervention should be considered in 7 cases in our study; however, 6 of them refused additional surgery, citing their age, physical condition, or other personal reasons. During a two-year term followup, local recurrence or distal metastasis did not occur in these 6 patients.
Bleeding and perforation are the two main complications of ESD. In this study, only one case had delayed bleeding 3 days after ESD. Successful hemostasis was achieved by coagulating forceps and spraying with thrombin during emergency endoscopy. No patient had immediate or delayed perforation. The relatively low ESD complication rate most likely reflects the small size of lesions. Furthermore, we always keep our minds upon the prevention and handling of bleeding during procedure. Once bleeding occurs during ESD, hemostasis may take a long time, and the endoscopic view may be affected; blind hemostasis may eventually lead to perforation. In our study, immediate minor bleeding was treated successfully by grasping the bleeding vessels with hot biopsy forceps and coagulating them during ESD. Direct coagulation with the hook knife could be done for small vessels in the submucosa, and metallic clips were often deployed for more brisk bleeding.
In conclusion, the present study indicates that because of complete resection, ESD may reasonably serve as radical treatment for gastric NETs when lesions are within the existing criteria. It also provides enough histological information for tumor grading and staging even when lesions are beyond the selection criteria, which informs decisions regarding subsequent surgery. In addition, endoscopic treatment might be also considered in particular in patients with a high risk of perioperative complications due to old age or advanced comorbidity, for example, or if there are other contraindications to major surgery, even though the lesions are little beyond the existing criteria.
Conflict of Interests
The authors declare that they have no conflict of interests to report with respect to this paper.
Acknowledgments
This study was supported by Grants from the Medical Leading Project of Shanghai Municipal Science and Technology Committee (no. 10411969600) and the Major Project of Shanghai Municipal Science and Technology Committee (nos. 11411950502 and 11DZ2280400).
References
- 1.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: Small tumors, small problems? Endoscopy. 2010;42(8):664–671. doi: 10.1055/s-0030-1255564. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003;35(3):203–206. doi: 10.1055/s-2003-37256. [DOI] [PubMed] [Google Scholar]
- 4.Hopper AD, Bourke MJ, Hourigan LF, Tran K, Moss A, Swan MP. En-bloc resection of multiple type 1 gastric carcinoid tumors by endoscopic multi-band mucosectomy. Journal of Gastroenterology and Hepatology. 2009;24(9):1516–1521. doi: 10.1111/j.1440-1746.2009.05909.x. [DOI] [PubMed] [Google Scholar]
- 5.Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012;95(3):207–2213. doi: 10.1159/000329043. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikane H, Tsukamoto Y, Niwa Y, et al. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointestinal Endoscopy. 1993;39(3):375–383. doi: 10.1016/s0016-5107(93)70109-1. [DOI] [PubMed] [Google Scholar]
- 7.Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video) Gastrointestinal Endoscopy. 2012;75:1153–11158. doi: 10.1016/j.gie.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Zhou PH, Yao LQ, Qin XY, et al. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surgical Endoscopy and Other Interventional Techniques. 2010;24(10):2607–2612. doi: 10.1007/s00464-010-1016-z. [DOI] [PubMed] [Google Scholar]
- 9.Lee DS, Jeon SW, Park SY, et al. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy. 2010;42(8):647–651. doi: 10.1055/s-0030-1255591. [DOI] [PubMed] [Google Scholar]
- 10.Park HW, Byeon JS, Park YS, et al. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointestinal Endoscopy. 2010;72(1):143–149. doi: 10.1016/j.gie.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC; 2010. [Google Scholar]
- 12.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104(4):994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 13.Soga J. Gastric carcinoids: a statistical evaluation of 1094 cases collected from the literature. Surgery Today. 1997;27(10):892–901. doi: 10.1007/BF02388135. [DOI] [PubMed] [Google Scholar]


