Abstract
Background
Glycemic index (GI) and glycemic load (GL) have been associated with coronary heart disease (CHD) risk in some but not all cohort studies. We therefore assessed the association of GI and GL with CHD risk in prospective cohorts.
Methods and Results
We searched MEDLINE, EMBASE, and CINAHL (through April 5, 2012) and identified all prospective cohorts assessing associations of GI and GL with incidence of CHD. Meta-analysis of observational studies in epidemiology (MOOSE) methodologies were used. Relative measures of risk, comparing the group with the highest exposure (mean GI of cohorts=84.4 GI units, range 79.9 to 91; mean GL of cohorts=224.8, range 166 to 270) to the reference group (mean GI=72.3 GI units, range 68.1 to 77; mean GL=135.4, range 83 to 176), were pooled using random-effects models, expressed as relative risk (RR) with heterogeneity assessed by χ2 and quantified by I2. Subgroups included sex and duration of follow-up. Ten studies (n=240 936) were eligible. Pooled analyses showed an increase in CHD risk for the highest GI quantile compared with the lowest, with RR=1.11 (95% confidence interval [CI] 0.99 to 1.24) and for GL, RR=1.27 (95% CI 1.09 to 1.49), both with evidence of heterogeneity (I2>42%, P<0.07). Subgroup analyses revealed only a significant modification by sex, with the female cohorts showing significance for GI RR=1.26 (95% CI 1.12 to 1.41) and for GL RR=1.55 (95% CI 1.18 to 2.03).
Conclusions
High GI and GL diets were significantly associated with CHD events in women but not in men. Further studies are required to determine the relationship between GI and GL with CHD in men.
Keywords: coronary heart disease, glycemic index and load, meta-analysis, nutrition, prospective cohort
Introduction
High-risk lifestyle and dietary patterns have been proposed to account for more than 80% of all coronary events in Western nations.1 The predominant concern in heart disease prevention has been saturated fatty acid (SFA) reduction, leading to widespread therapeutic adoption of low-total-fat, high-carbohydrate diets as the standard dietary approach for the reduction of coronary heart disease (CHD) risk.2,3 However, recent prospective cohort meta-analyses suggest an even greater increase in CHD risk when highly refined and readily absorbed carbohydrates replaced SFAs.4,5 As a result, SFAs per se no longer appeared to be associated with CHD, emphasizing the potentially deleterious effects of refined, rapidly absorbed carbohydrates.4,5 In addition, replacement of SFAs with unsaturated fatty acids and complex carbohydrates is associated with favorable changes in CHD risk factors.6–8 These findings have intensified the focus on carbohydrates, because diets rich in highly processed carbohydrates can lead to raised triglycerides (TGs),9 reductions in high-density lipoprotein cholesterol (HDL-C),10 and increasing CHD risk.11
Carbohydrates with differing physical form, particle size, chemical structure, and fiber content alter the rate of starch digestion and their physiological response. The glycemic index (GI) was developed to characterize the rate of digestion of a carbohydrate food compared with a reference carbohydrate food.12 Over the last 3 decades, clinical trials have demonstrated that reducing the GI or glycemic load (GL), the product of GI and the available carbohydrate content of a food,13 in the context of diets low in saturated fat, can improve CHD risk factors including body mass index (BMI), blood pressure, and serum cholesterol.14–24 Similar favorable effects have been seen with lower SFAs, higher poly-/monounsaturated fatty acids, and higher complex carbohydrate diets.6–8 These randomized controlled trials provide data that are harmonious with the emerging, albeit inconsistent, cohort literature on the unfavorable relationships between higher GI and GL dietary patterns and CHD risk. Cohort studies have also shown an association between low GI diets25,26 and reduced development of hyperglycemia and diabetes, further implicating the GI in the progression to CHD.25,26 This dietary pattern is also likely to have the additional advantage of reduced LDL cholesterol by lower intake of SFAs and dietary cholesterol.
Despite the proposed physiological mechanisms,27 published cohort studies in the last decade have produced mixed results for the associations of GI and GL with CHD.28–31 We therefore undertook a systematic review and meta-analysis of prospective cohort studies with healthy populations at baseline to determine whether associations exist between GI and GL with CHD.
Methods
Data Sources and Study Selection
We conducted separate searches for all prospective cohort studies that assessed potential associations between glycemic index or load and primary incidence of CHD (including myocardial infarction [MI] or death due to CHD) in adults. We followed the meta-analysis of observational studies in epidemiology (MOOSE) guidelines for this report.32 Electronic databases (MEDLINE 1946-March, Week 4, 2012; EMBASE 1980–2012, Week 13; CINAHL 1982–December 2011) were searched by 2 individuals independently; searches were supplemented by manual searches through the reference lists of original publications and review articles. The following search terms were used: ([Glycemic or Glycaemic Index] or [Glycemic or Glycaemic Load]) and (CHD or Cardiovascular Disease or CVD or MI) and (Prospective or Cohort). Titles and abstracts were initially reviewed to identify relevant reports by 2 independent reviewers (A.M., L.C., both investigators); reviewers conducted a subsequent full-text assessment of all studies in which there was uncertainty about the assessment of relevance. Disagreements regarding eligibility were resolved through discussion with 2 additional adjudicators (D.J.A.J/R.J.D.).
Data Extraction
Two reviewers (A.M., L.C.) independently reviewed and extracted relevant data employing a standardized pro forma sheet with the first author and year of publication used as study identifiers for convenience. Data extracted from each cohort included information about sample size, population characteristics (age and sex), country of origin, follow-up duration, method of collecting dietary information, outcome measures, exposure quantification, and analytical methods, including adjustment parameters used for confounding factors. The most complete multivariate adjusted risk estimates from eligible studies assessing GI and GL associations with CHD events with their corresponding confidence intervals (CIs) were extracted to provide the main end points. All authors of eligible reports were contacted to acquire any missing data for each exposure level including number of events, person-years, mean or median dose of GI and GL (all values were converted to bread scale [GI=100] if not already reported as such,31,33–36 with bread scale=glucose scale/0.7),37 as well as risk estimates with corresponding CIs.
Data Synthesis
Data were analyzed using Review Manager (RevMan) 5.1.4 (Cochrane Library software, Oxford, UK) and STATA version 11.0 (StataCorp, College Station, TX). The natural log-transformed relative risks of CHD events (including MI) with corresponding standard errors comparing the highest exposure level with the reference group from each cohort, irrespective of the number of quantile divisions in the original analysis, were pooled in separate analyses for GI and GL. The generic inverse variance method with random-effects models in RevMan were used to allow for heterogeneity assessment. Interstudy heterogeneity was tested by Cochrane's Q (χ2) and quantified by the I2 statistic. Regardless of P value, sensitivity analyses were performed to identify sources of heterogeneity.38 Potential publication bias was assessed visually by inspecting funnel plots of effect size against the standard error and formally tested using Begg's and Egger's tests in STATA.39,40 Our a priori stratified analyses included sex and duration of follow-up, that is, whether studies were more or less than 10 years, consistent with the 10-year Framingham Risk Score41 approach, and analyzed using meta-regression in STATA. Statistical significance was defined as P<0.05 for all comparisons, except for Cochrane's Q (χ2), where significance was set at <0.10.
Results
Search Results
Figure 1 shows the flow of the literature applying the systematic search and selection strategies. In all, 473 eligible studies were identified by the search. A total of 10 studies with 12 GI reports28–31,33,35,36,42,43 and 12 GL reports28–31,33–36,42,43 were selected for analyses. Two reports28,34 on the Nurses' Health Study provided data on the GL exposure; only the report with the larger subject numbers and longer follow-up was included in the GL analyses.34,44
Figure 1.
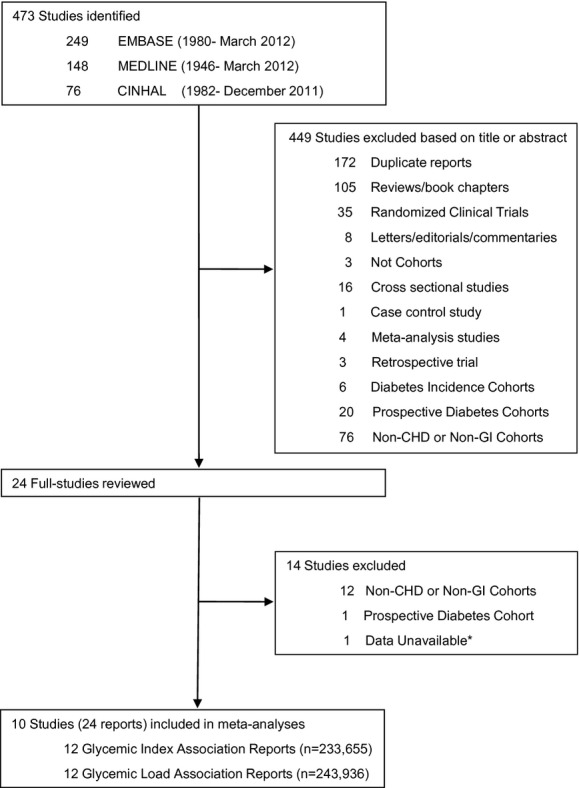
Literature search and review flow. CHD indicates coronary heart disease; GI, glycemic index.*The Hardy et al study, for which data were unavailable, only reported a rate of change in risk of CHD per 5 and 30 units of GL.
Cohort Characteristics
The study characteristics and their diet compositions are shown in Tables 1 and 2, respectively. Of the 10 studies identified, 12 GI and 12 GL reports with CHD risk estimation were included in our analyses, with a total of 233 65528–31,33,35,36,42,43 and 240 93628–31,33,34,35,36,42,43 subjects, respectively. During 6 to 25 years of follow-up, 6940 coronary events were recorded. All studies used Cox proportional hazard models for CHD risk estimation analyses, except for 1 study43 which used restricted cubic spline models. The most common confounders adjusted for included age, BMI, and cigarette smoking, with full multivariate analyses outlined in Table 1. All cohorts excluded those with documented CHD or major CHD risk factor at time of enrollment, with the exception of 1 that included a population 5% of whom had diabetes35 but adjusted for diabetes status. All analyses were stratified by sex. The majority of the studies (7 of 10) used either a semiquantitative or quantitative Food Frequency Questionnaire (SFFQ or FFQ),28,30,31,33,34,36,42 but 3 used 4- or 7-day diet records or diet history interviews for food consumption patterns.29,35,43 All studies used the International Tables of Glycemic Index for assessing the GI of different foods; 328,29,34 used the 1995 iteration,45 630,31,33,36,42,43 used the 2002 iteration,46 and 135 used the 2008 iteration. One study33 further supplemented the 2002 International GI tables with GI values for 159 local food items that were tested at an academic institution following the International GI table methodologies. The majority of the included reports provided dietary pattern data for GL quantiles. The dietary patterns across the different quantiles of GL were similar between studies, with a trend of increasing carbohydrate and decreasing protein and fat content at higher GL quantiles (Table 2).
Table 1.
Study Characteristics
| Reference | N | Age Range | Country | Years Range | Mean Duration of Follow-Up, years | Number of Questionnaires Administered* | Total Number of New Events | Quantile Division | Mean/Median GI (GL) Interquantile Ranges | Method for Reporting Events | Adjustments† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al28 | 75 521 (W) | 38 to 63 | United States | 1984–1994 | 10 | 3 (SFFQ) | 761 CHD | Quintiles | 68.1 to 82.7 (117 to 206) | Death certificates, medical/autopsy records | Hypertension; hypercholesterolemia; parental history of MI; menopause; use of multivitamins, vitamin E, and ASA; dietary intake of folate, vitamin E, trans fat, PUFA, and total protein |
| van Dam et al29 | 646 (M) | 64 to 84 | Netherlands | 1985–1995 | 10 | 1 (Interview) | 94 CHD | Tertiles | 77.0 to 85.0 (161 to 230) | Death and hospital discharge registries | ‡Prescribed diet (yesn/no); dietary intake (g/day) of PUFA and CH2O |
| Levitan et al30 | 36 246 (M) | 45 to 79 | Sweden | 1998–2003 | 6 | 1 (FFQ) | 1324 MI | Quartiles | 73.0 to 82.9 (176 to 255) | Death and hospital discharge registries | Hypertension; hypercholesterolemia; parental history of MI; living alone; use of ASA; dietary intake of PUFA, total protein, CH2O |
| Beulens et al31 | 15 714 (W) | 49 to 70 | Netherlands | 1993–2005 | 9 | 1 (FFQ) | 556 CHD | Quartiles | 68.6 to 81.4 (112 to 174) | Death and hospital discharge registries | Hypertension; SBP; menopause; education; use of vitamin E; dietary intake of PUFA, MUFA, total protein |
| Sieri et al33 | 13 637 (M) | 35 to 64 | Italy | 1993–2004 | 7.9 | 1 (SFFQ) | 305 CHD | Quartiles | 71.9 to 80.7 (168.6 to 270) | Death certificates, hospital discharge registries, and clinical record | Hypertension; education |
| Sieri et al33 | 30 495 (W) | 35 to 74 | Italy | 1993–2004 | 7.9 | 1 (SFFQ) | 158 CHD | Quartiles | 71.9 to 80.9 (175.4 to 265.4) | Death certificates, hospital discharge registries, and clinical record | Hypertension; education |
| Halton et al34 | 82 802 (W) | 30 to 55 | United States | 1980–2000 | 20 | 6 (SFFQ) | 1994 CHD | Deciles | GI N/A (83 to 256) | Death certificates, medical/autopsy records | Hypertension; hypercholesterolemia; parental history of MI; menopause; use of multivitamins, vitamin E, and ASA; dietary intake of trans fat, PUFA, MUFA, total protein |
| Mursu et al35 | 1981 (M) | 42 to 60 | Finland | 1984–2005 | 16.1 | 1 (FR) | 376 MI | Quartiles | 70.4 to 89.0 (146.1 to 256.7) | Hospital discharge registries | Diabetes; SBP; hypertension medications; hypercholesterolemia; TAG; family history of CVD; education; dietary intake of folate, vitamin C, and PUFA |
| Burger et al36 | 8855 (M) | 21 to 64 | Netherlands | 1993–2008 | 11.9 | 1 (FFQ) | 581 CHD | Quartiles | 72.9 to 85.0 (143.4 to 208.0) | Municipal administration registries, statistics Netherlands | Hypertension; education; dietary MUFA, PUFA, and energy-adjusted vitamin C, CH2O, and protein intake; plasma total cholesterol and HDL-C |
| Burger et al36 | 10 753 (W) | 21 to 64 | Netherlands | 1993–2008 | 11.9 | 1 (FFQ) | 300 CHD | Quartiles | 73.3 to 84.9 (144.7 to 208.3) | Municipal administration registries, statistics Netherlands | Hypertension; education; dietary MUFA, PUFA, and energy-adjusted vitamin C, CH2O, and protein intake; plasma total cholesterol, and HDL-C |
| Levitan et al42 | 36 234 (W) | 48 to 83 | Sweden | 1998–2006 | 9 | 1 (FFQ) | 1138 MI | Quartiles | 73.3 to 79.9 (128 to 188) | Death and hospital discharge registries | Hypertension; hypercholesterolemia; parental history of MI; menopause; education; marital status; use of multivitamin, vitamin E, and ASA; dietary intake of trans fat, PUFA, MUFA, total protein |
| Grau et al43 | 1684 (M) | 30 to 70 | Denmark | 1974–1999 | 6 to 25 | 1 (Interview/FR) | NR | Quintiles | 75.0 to 91.0 (102 to 220) | Hospital discharge registries | Education; energy-adjusted intake of fat, total protein, and CH2O |
| Grau et al43 | 1889 (W) | 30 to 70 | Denmark | 1974–1999 | 6 to 25 | 1 (Interview/FR) | 114 CHD | Quintiles | 72.0 to 89.0 (84 to 166) | Hospital discharge registries | Education; energy-adjusted intake of fat, total protein, and CH2O |
M indicates men; W, women; SFFQ, Semiquantitative Food Frequency Questionnaire (validated); FFQ, Food Frequency Questionnaire (validated); Interview, Diet History Interview; FR, food record; GI, glycemic index; GL, glycemic load; BMI, body mass index; CHD, coronary heart disease; MI, myocardial infarction; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; TAG, triacylglyceride; SBP, systolic blood pressure; ASA, aspirin; PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; CH2O, carbohydrate; NR, not reported; N/A, not available.
All studies used Cox proportional hazard models in their CHD risk estimation association analyses with GI and GL, except for Grau et al.43
All studies had a healthy starting population at the beginning of follow-up except for Mursu et al,35 who had a 5% diabetic population at the start.
Number of Questionnaires Administered denotes number of administered questionnaires at the beginning of and throughout the study.
All studies adjusted for age, BMI, physical activity, alcohol intake, total energy, saturated fat intake.
Dietary fiber intake was adjusted for in all but 1 study.29
Table 2.
Dietary Composition Patterns for Each GL Quantile by Total Energy (E), Percent Energy From Carbohydrates, Protein, and Fat and Types of Fat (SFA, PUFA, MUFA)*
| Reference | First GL Quantile (CH2O:Prt:Fat) | Second GL Quantile (CH2O:Prt:Fat) | Third GL Quantile (CH2O:Prt:Fat) | Fourth GL Quantile (CH2O:Prt:Fat) | Fifth GL Quantile (CH2O:Prt:Fat) |
|---|---|---|---|---|---|
| Liu et al28 (W) | E: 1702 kcal/day | E: 1783 kcal/day | E: 1797 kcal/day | E: 1767 kcal/day | E: 1676 kcal/day |
| (34:19:NR) | (38:17:NR) | (41:16:NR) | (45:15:NR) | (54:15:NR) | |
| SFA: 13 | SFA: 12 | SFA: 11 | SFA: 11 | SFA: 10 | |
| PUFA: 7 | PUFA: 6 | PUFA: 6 | PUFA: 6 | PUFA: 5 | |
| MUFA: 13 | MUFA: 12 | MUFA: 12 | MUFA: 11 | MUFA: 10 | |
| Fiber/1000 kcal: 8.2 g | Fiber/1000 kcal: 9.0 g | Fiber/1000 kcal: 9.5 g | Fiber/1000 kcal: 9.6 g | Fiber/1000 kcal: 10.7 g | |
| van Dam et al29 (M) | E: 2272 kcal/day | E: 2321 kcal/day | E: 2177 kcal/day | — | — |
| (40:14:NR) | (42:14:NR) | (45:14:NR) | |||
| SFA: 17 | SFA: 17 | SFA: 18 | |||
| PUFA: 6 | PUFA: 6 | PUFA: 6 | |||
| MUFA: NR | MUFA: NR | MUFA: NR | |||
| Fiber/1000 kcal: 11.0 g | Fiber/1000 kcal: 10.9 g | Fiber/1000 kcal: 10.2 g | |||
| Levitan et al30 (M) | E: 2703 kcal/day | E: 2728 kcal/day | E: 2710 kcal/day | E: 2705 kcal/day | — |
| (35:14:NR) | (39:13:NR) | (42:13:NR) | (46:12:NR) | ||
| SFA: 13 | SFA: 12 | SFA: 11 | SFA: 9 | ||
| PUFA: 3 | PUFA: 3 | PUFA: 3 | PUFA: 3 | ||
| MUFA: 9 | MUFA: 9 | MUFA: 8 | MUFA: 7 | ||
| Fiber/1000 kcal: 5.0 g | Fiber/1000 kcal: 6.0 g | Fiber/1000 kcal: 6.53 g | Fiber/1000 kcal: 7.0 g | ||
| Beulens et al31 (W) | E: 1797 kcal/day | E: 1828 kcal/day | E: 1819 kcal/day | E: 1789 kcal/day | — |
| (36:16:37) | (41:16:35) | (45:15:34) | (51:15:31) | ||
| SFA: 16 | SFA: 15 | SFA: 14 | SFA: 13 | ||
| PUFA: 7 | PUFA: 7 | PUFA: 6 | PUFA: 6 | ||
| MUFA: 14 | MUFA: 13 | MUFA: 12 | MUFA: 11 | ||
| Fiber/1000 kcal: 11.1 g | Fiber/1000 kcal: 12.0g | Fiber/1000 kcal: 12.6 g | Fiber/1000 kcal: 13.4 g | ||
| Sieri et al33 (M) | E: 2562 kcal/day | E: 2387 kcal/day | E: 2409 kcal/day | E: 2677 kcal/day | — |
| (45:18:40) | (50:17:36) | (54:16:33) | (59:15:29) | ||
| SFA: 14 | SFA: 12 | SFA: 11 | SFA: 10 | ||
| PUFA: 5 | PUFA: 4 | PUFA: 4 | PUFA: 4 | ||
| MUFA: 19 | MUFA: 17 | MUFA: 16 | MUFA: 14 | ||
| Fiber/1000 kcal: 9.0 g | Fiber/1000 kcal: 10.1 g | Fiber/1000 kcal: 10.9 g | Fiber/1000 kcal: 13.1 g | ||
| Sieri et al33 (W) | E: 2194 kcal/day | E: 1998 kcal/day | E: 2023 kcal/day | E: 2300 kcal/day | — |
| (42:18:42) | (48:17:38) | (52:16:34) | (58:15:30) | ||
| SFA: 15 | SFA: 13 | SFA: 12 | SFA: 10 | ||
| PUFA: 5 | PUFA: 4 | PUFA: 4 | PUFA: 4 | ||
| MUFA: 20 | MUFA: 18 | MUFA: 16 | MUFA: 14 | ||
| Fiber/1000 kcal: 9.6 g | Fiber/1000 kcal: 10.5 g | Fiber/1000 kcal: 11.1 g | Fiber/1000 kcal: 12.0 g | ||
| Halton et al34 (W) | NR | NR | NR | NR | NR |
| Mursu et al35 (M) | E:2490 kcal/day | E: 2310 kcal/day | E: 2306 kcal/day | E: 2494 kcal/day | — |
| (36:16:42) | (42:16:40) | (45:15:37) | (49:15:35) | ||
| SFA: 20 | SFA: 18 | SFA: 17 | SFA: 16 | ||
| PUFA: 5 | PUFA: 5 | PUFA: 5 | PUFA: 4 | ||
| MUFA: 13 | MUFA: 12 | MUFA: 11 | MUFA: 10 | ||
| Fiber/1000 kcal: 8.8 g | Fiber/1000 kcal: 10.4 g | Fiber/1000 kcal: 11.3 g | Fiber/1000 kcal: 11.2 g | ||
| Burger et al36 (W) | NA | NA | NA | NA | — |
| Burger et al36 (M) | NA | NA | NA | NA | — |
| Levitan et al42 (W) | E: 1765 kcal/day | E: 1727 kcal/day | E: 1728 kcal/day | E: 1745 kcal/day | — |
| (41:18:NR) | (47:17:NR) | (50:16:NR) | (55:14:NR) | ||
| SFA: 17 | SFA: 15 | SFA: 13 | SFA: 11 | ||
| PUFA: 4 | PUFA: 4 | PUFA: 4 | PUFA: 4 | ||
| MUFA: NR | MUFA: NR | MUFA: NR | MUFA: NR | ||
| Fiber/1000 kcal: 10.8 g | Fiber/1000 kcal: 12.5 g | Fiber/1000 kcal: 13.4 g | Fiber/1000 kcal: 14.0 g | ||
| Grau et al43 (M) | E: 2536 kcal/day | NR | E: 2608 kcal/day | NR | E: 2584 kcal/day |
| (29:14:43) | (36:14:43) | (45:13:39) | |||
| SFA: NR | SFA: NR | SFA: NR | |||
| PUFA: NR | PUFA: NR | PUFA: NR | |||
| MUFA: NR | MUFA: NR | MUFA: NR | |||
| Fiber/1000 kcal: 5.9 g | Fiber/1000 kcal: 7.3 g | Fiber/1000 kcal: 8.1 g | |||
| Grau et al43 (W) | E: 1818 kcal/day | NR | E: 1867 kcal/day | NR | E: 1842 kcal/day |
| (31:16:46) | (38:15:43) | (45:14:39) | |||
| SFA: NR | SFA: NR | SFA: NR | |||
| PUFA: NR | PUFA: NR | PUFA: NR | |||
| MUFA: NR | MUFA: NR | MUFA: NR | |||
| Fiber/1000 kcal: 6.6 g | Fiber/1000 kcal: 8.0 g | Fiber/1000 kcal: 8.7 g | |||
GL indicates glycemic load; SFA, saturated fatty acids; PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; CH2O, carbohydrate; Prt, protein; NR, not reported; NA,36 author was contacted for data, but data were not available; E, total energy (kcal/day); Fiber, g/1000 kcal; W, women; M, men.
Percent energy for each component in each quantile was calculated from reported intake in grams multiplied by energy per gram (4 kcal/g for protein and CH2O, and 9 kcal/g for fat) and expressed as a percentage of the total energy in the respective quantile.
Diet composition depicted in percent energy at every exposure level according to glycemic load quantiles.
Glycemic Index and Coronary Heart Disease
Figure 2 (overall analysis) shows the overall pooled relative risk estimation of GI with CHD events. The CHD incidence rate was increased at the highest level of GI exposure (mean GI of 84.4 GI units, range 79.9 to 91) relative to the lowest (mean GI of 72.3 GI units, range 68.1 to 77), RR=1.11, 95% CI 0.99 to 1.24, and approached significance (P=0.09) but with significant evidence of heterogeneity (I2=45%, P=0.05). Sensitivity analyses identified the Grau et al43 report on men as the largest contributor to heterogeneity. The removal of this study changed the risk estimate for the association of GI with CHD (RR=1.14 [95% CI 1.02 to 1.26], P=0.02) and also improved the precision of the estimate and eliminated much of the heterogeneity (I2=30%, P=0.16). A priori stratification revealed no significant modification of association for the duration of follow-up analysis with cohorts of ≥10 years28,29,35,36,43 (RR=1.08 [95% CI 0.89 to 1.31]) versus those of <10 years30,31,33,42 (RR=1.13 [95% CI 0.97 to 1.31]) as subsets (β=0.95 [95% CI 0.71 to 1.26]), both with evidence of heterogeneity (I2>44% for both, figure not shown). Sex, however, was a significant modifier of the association of GI with CHD (β=0.77 [95% CI 0.65 to 0.93]; P=0.004, Figure 2, sex-specific subgroups). The pooled female cohorts28,31,33,36,42,43 showed a larger, statistically significant association (ΔGI between mean of highest exposure and mean of reference=11.9±1.5 SE, RR=1.26 [95% CI 1.12 to 1.41]), whereas the male cohorts29,30,33,35,36,43 showed no association (ΔGI between mean of highest exposure and mean of reference=12.2±1.7 SE, RR=0.96 [95% CI 0.84 to 1.11]), with no significant evidence of heterogeneity in either subset.
Figure 2.
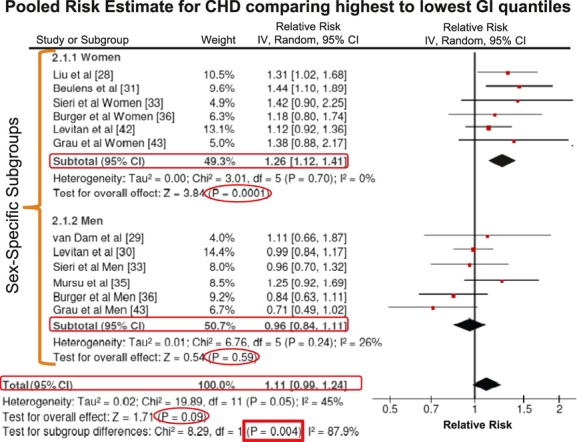
Pooled risk estimate of all prospective cohorts investigating the association of highest GI exposure with CHD events (including death and myocardial infarctions) relative to the reference exposure (ΔGI between mean of highest exposure and mean of reference=12.1±1.1 SE). The figure is stratified by sex-specific subgroups with subtotal boxes in 2.1.1 and 2.1.2 summarizing the pooled analysis for women28,31,33,36,42,43 and for men,29,30,33,35,43 respectively. The total analysis box represents the overall pooled analysis for both men and women. P values in circles are based on generic inverse variance (IV) methods in random-effects models and represent the significance for association of high-GI diets with CHD. The P value in a rectangle depicts the significance of differences between the subgroups. Interstudy heterogeneity was tested by Cochrane's Q (χ2) at a significance level of P<0.10 and quantified by I2.38 CHD indicates coronary heart disease; GI, glycemic index.
Glycemic Load and Coronary Heart Disease
Figure 3 (overall analysis) shows the overall pooled relative risk estimation of GL with CHD events. The pooled risk estimation showed a significant increase in CHD risk (RR=1.27 [95% CI 1.09 to 1.49], P=0.002), with significant heterogeneity (I2=43%, P=0.06), for the highest level of GL exposure (mean GL of 224.8 GL units, range 166 to 270) relative to the lowest (mean GL of 135.4 GL units, range 83 to 176). Sensitivity analyses identified the Grau et al43 and Sieri et al33 reports on women as the largest contributors to heterogeneity when removed individually. The removal of Grau et al43 somewhat reduced the estimate of the association of GL with CHD (RR=1.21 [95% CI 1.05 to 1.38], P=0.007; I2=23%, P=0.23), as did the removal of Sieri et al33 (RR=1.22 [95% CI 1.06 to 1.40], P=0.005; I2=27%, P=0.19). Similar to the GI analyses, no modification in the association was revealed by the duration of follow-up analysis, ≥10 years29,34–36,43 (RR=1.26 [95% CI 1.01 to 1.59]) versus <10 years30,31,33,42 (RR=1.29 [95% CI 1.02 to 1.63]) with β=0.99 (95% CI 0.68 to 1.44), with evidence of heterogeneity (I2>47%, figure not shown) for both subgroups. Congruent with the GI analysis, sex was a significant modifier of the association of GL with CHD (β=0.73 [95% CI 0.56 to 0.96]; P=0.02, Figure 3, sex-specific subgroups). The female cohorts31,33,34,36,42,43 showed a larger, statistically significant association (ΔGL between mean of highest exposure and mean of reference=88.4±17.6 SE, RR=1.55 [95% CI 1.18 to 2.03]), whereas in the male cohorts,29,30,33,35,36,43 the association was not significant (ΔGL between mean of highest exposure and mean of reference=90.4±9.2 SE, RR=1.08 [95% CI 0.93 to 1.26]). There was significant evidence of heterogeneity in the female subset but not in the male subset.
Figure 3.
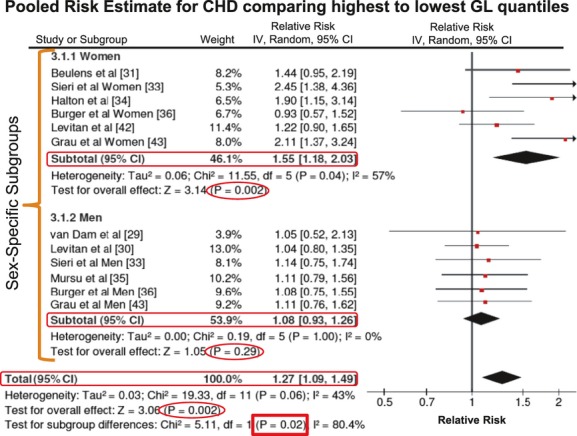
Pooled risk estimate of all prospective cohorts investigating the association of highest GL exposure with CHD events (including death and myocardial infarctions) relative to the reference exposure (ΔGL between mean of highest exposure and mean of reference=89.4±9.5 SE). The figure is stratified by sex-specific subgroups with subtotal boxes in 3.1.1 and 3.1.2 summarizing the pooled analysis for women31,33,34,36,42,43 and for men,29,30,33,35,43 respectively. The total analysis box represents the overall pooled analysis for both men and women. P values in circles are based on generic inverse variance (IV) methods in random-effects models and represent the significance for association of high-GL diets with CHD. The P value in a rectangle depicts the significance of differences between the subgroups. Interstudy heterogeneity was tested by Cochrane's Q (χ2) at a significance level of P<0.10 and quantified by I2.38 CHD indicates coronary heart disease; GI, glycemic index.
Publication Bias
Funnel plots for each of the overall analyses were inspected for presence of publication bias (Figures 4 and 5). Neither Begg's nor Egger's tests revealed significant evidence of publication bias in the overall analyses of GI and GL (P>0.115 for all). However, in the visual inspection of the GI funnel plot, the Grau et al43 report on men appears to be an outlier and in the GL funnel plot, and the Grau et al43 report on women appears to be an outlier (outside the pseudo 95% confidence limits).
Figure 4.
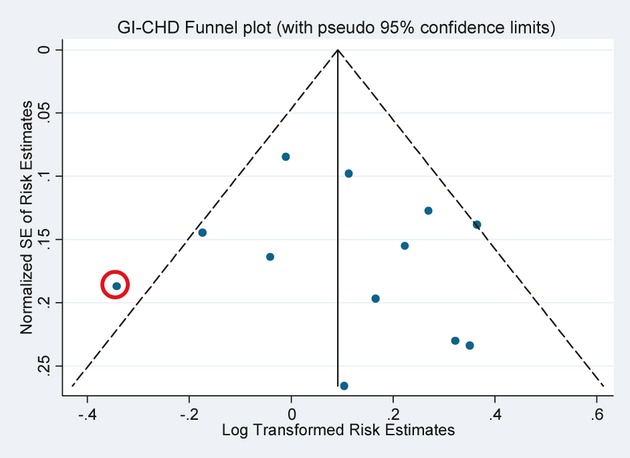
Test for publication bias in the overall pooled analysis of CHD risk estimates associated with the highest GI quantiles; Grau et al43 report on men was identified outside the 95% pseudo–confidence limits. Neither Begg's test (P>0.837) nor Egger's test (P=0.621) revealed evidence of publication bias.39,40 CHD indicates coronary heart disease; GI, glycemic index.
Figure 5.
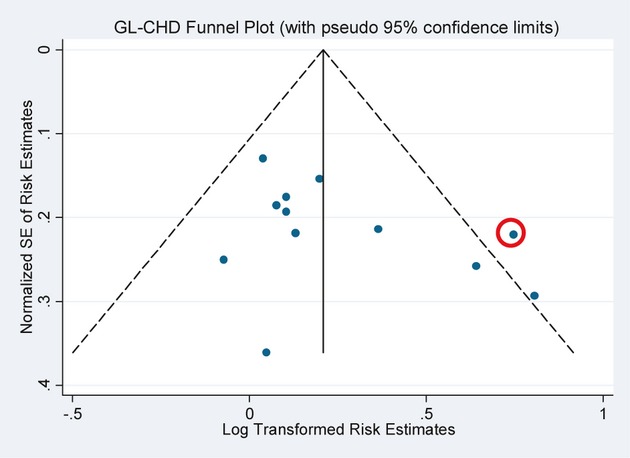
Test for publication bias in the overall pooled analysis of CHD risk estimates associated with highest GL quantiles; Grau et al43 report on women was identified outside the 95% pseudo–confidence limits. Begg's (P>0.115) and Egger's (P=0.134) tests approached significance for evidence of publication bias.39,40 CHD indicates coronary heart disease; GL, glycemic load.
Discussion
We believe that this analysis represents the most comprehensive meta-analysis of the data presented as quantiles of GI and/or GL. However, we were unable to include 2 studies in this analysis because of the expression of results as either the substitution of low GI carbohydrates for SFAs, rather than dietary GI analysis, or the incremental association of dietary GI with CHD rather than quantile presentation (ie, CHD risk per 5 GI unit increments). These studies indicated that in Danish47 and Black American44 men, positive benefits for cardiovascular events were seen related to consumption of lower GI foods. We demonstrated an overall increased relative risk of CHD of 11% in the comparison of the highest versus lowest quantile of GI and a 27% increased relative risk of CHD for the highest versus the lowest quantile of GL. The effect was seen only in women with 26% increased relative risk of CHD for GI and 55% for GL.
The sex difference in the CHD response to the glycemic index was unexpected and may be the result of the larger total number of subjects in the female cohorts (n=177 887, CHD events=4260) than in the male cohorts (n=63 049, CHD events=2680) and our inability to include 2 studies, 1 from Denmark47 and 1 from the United States,44 both of which demonstrated adverse effects of high GI foods or diets on CHD outcomes in men. Furthermore, the 1 study43 of men in the present analysis that showed a near-significant deleterious effect of low-GI diets was also responsible for the heterogeneity in the analysis. This study differed from the other studies of men in several respects. The proportion (54%) of smokers was almost twice that of the other studies, and BMI was somewhat lower (25.3 versus 26.1 kg/m2); men with lower BMIs have been shown to be less susceptible to the effects of GI on CHD risk.48 There may also be another potential explanation for the difference between men and women, possibly a result of differences in diet reporting. For example, if women report more precisely, there would be less measurement error and hence better power. In addition, there may be more homogeneity (insufficient heterogeneity) in the diets of men and therefore a lack of the necessary power to detect potentially important relationships between dietary GI/GL and CHD risk in men.
Despite these reservations there are reasons why women may be potentially more vulnerable to high glycemic index diets. Part of the protection that women have from CHD may be related to their high HDL-C levels.49,50 Higher glycemic index diets tend to reduce circulating HDL-C concentrations and thus disproportionately increase CHD risk in women, especially when postmenopausal.51 At the same time, high-GI diets may raise TG levels,52 which may also carry more risk for CHD in women than in men.53,54
Other factors that in general may contribute to the increased CHD risk with high-GI diets are blood pressure and CRP, both of which may be raised by high-GI diets.52,55 Conversely, acarbose, the α-glucosidase inhibitor that converts dietary carbohydrate to a low glycemic index form, has been shown to prevent hypertension and CHD events in the STOP NIDDM trial.56
The link between low GI and GL diets and lower CHD risk is also substantiated by randomized clinical trials (RCTs) assessing the effects of dietary strategies low in GI and GL with low saturated fat content. A systematic review of such RCTs in overweight and obese subjects found that replacing refined carbohydrates with low-GI complex carbohydrates conferred more beneficial effects on CHD lipid risk factors when compared with complex starchy carbohydrates with higher GI.57 There has also been emerging evidence on the positive effects of low-GI diets on CHD risk factors such as oxidative damage58 and inflammation22 in overweight/obese and type 2 diabetic individuals, respectively. In addition, meta-analyses of low-GI RCTs have shown beneficial effects on body weight and lipid profiles in obese and overweight subjects21 as well as on glycemic control in type 2 diabetic subjects.59
The current studies do not suggest a latency effect of GI or GL on CHD risk. Because no studies have a time frame shorter than 6 years, it is not possible to determine whether the effect is early, possibly from alterations in clotting factors,60 or later due to reduction in the rate of atheroma formation secondary to oxidative damage.61
An earlier meta-analysis that assessed the effect of dietary GI on several health outcomes including diabetes, CHD, and cancer also concluded that low-GI diets were protective for diabetes, CHD, and colon and breast cancers.62 However, only 2 studies28,29 were available to assess CHD outcome at the time of that analysis.
In general, the glycemic index and glycemic load data were in agreement, although the magnitude of the CHD risk was greater based on the difference between the extreme quantiles of glycemic load. In the overall GL extreme quantile analysis there was significant heterogeneity that became nonsignificant when either the Grau et al43 or the Sieri et al33 reports on women were removed. The Grau et al43 report was visually an outlier on the publication bias funnel plot, which could potentially be a result of the difference in the analytical approach, using restricted cubic splines in Grau et al,43 compared with the methods of analyses used in other reports. We could not find an explanation for the heterogeneity in the overall GL analysis due to the Sieri et al33 report on women.
The weaknesses of the present study include the limited number of studies, the inability to include potentially relevant studies44,47 because of lack of necessary data, and the heterogeneity in the overall analyses, especially in the likely underpowered analyses of men. Another inherent limitation of observational analyses is the potential problem for residual confounding as well as the possibility of overadjusting, which remains an area of debate in epidemiology.63 Perhaps, the most common limitation in meta-analyses of dietary studies is the combination of dietary data collected using multiple instruments. Although no change was found in our overall conclusions with post hoc sensitivity analyses removing the 429,35,43 reports with food diaries and interviews, because of the time frame of published reports, the GI and GL data were still compiled from various data sources. As such, our findings should be considered with caution, and further studies should be undertaken to allow sufficient power for subgroup analyses of dietary data sources.
Although the reports included men and women from Holland,29,31,36 Finland,35 Denmark,43 Sweden,30,42 Italy,33 and the United States,28,34 the majority of the population was white, limiting the racial diversity of this meta-analysis. However, it is noteworthy that our systematic review captured a report on African American men that, although not included because of lack of necessary data (ie, quantile analyses), showed that a 5-unit increase in GI conferred a 16% increase in CHD risk.44 Further studies are required with a wider range of ethnic groups and more racial diversity both in women and especially in men because cohorts of men were likely underpowered.
The strength of our study included the use of random-effects models to allow assessment of heterogeneity to guide the sensitivity analyses. We further believe that our systematic review was strengthened by our efforts to acquire as much data as possible by contacting all study authors to include all relevant studies in our analyses.
Conclusion
We conclude that a reduction in the glycemic index and glycemic load may favorably affect CHD outcomes in women. Further studies are required to determine the effect of the glycemic index and load on CHD risk in men.
Acknowledgments
We thank all the authors who corresponded with us to acquire the most complete data set for our analyses.
Sources of Funding
Dr Jenkins and Mr Mirrahimi were provided unrestricted research and travel grants by the Barilla company. Mr Mirrahimi was supported in this work by a Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship. Dr de Souza was also supported by a postdoctoral fellowship from the CIHR. Dr Jenkins was funded by the Government of Canada through the Canada Research Chair Endowment. None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
Dr Sievenpiper has received several unrestricted travel grants to present research at meetings from The Coca-Cola Company and is a coinvestigator on an unrestricted research grant from The Coca-Cola Company. Dr Sievenpiper has also received travel funding and honoraria from Archer Daniels Midland and the International Life Sciences Institute (ILSI) North America and research support, consultant fees, and travel funding from Pulse Canada. Drs de Souza, Beyene, and Kendall are coinvestigators on an unrestricted grant from The Coca-Cola Company. Dr Kendall has served on the scientific advisory board of or received research grants, travel funding, consultant fees, or honoraria from Pulse Canada, Barilla, Solae, Unilever, Hain Celestial, Loblaws Inc., Oldways Preservation Trust, the Almond Board of California, the International Nut Council, Paramount Farms, the California Strawberry Commission, the Canola and Flax Councils of Canada, and Saskatchewan Pulse Growers. Dr Jenkins holds unrestricted research grants from The Coca-Cola Company and the Calorie Control Council and has served on the scientific advisory board for or received research support, consultant fees, honoraria, or travel grants from Barilla, Solae, Unilever, Unilever Canada, Haine Celestial, Loblaws Supermarket, Sanitarium Company, Herbalife International, Nutritional Fundamentals for Health, Pacific Health Laboratories Inc., Metagenics/MetaProteomics, Bayer Consumer Care, Dean Foods, Oldways Preservation Trust, the Almond Board of California, The Canola and Flax Councils of Canada, International Tree Nut Council Nutrition Research and Education Foundation, Peanut Institute, The American Peanut Council, The California Strawberry Commission, Orafti, Science Advisory Council Agriculture and Agrifoods, Canadian Agriculture Policy Institute (CAPI), Agriculture Bioproducts Innovation Program (ABIP) through Pulse Research Network Canada (PURENet), Soy Advisory Board, Alpro Foundation, Kellogg's Company, Quaker Oats, Procter and Gamble Technical Center, Coca-Cola, Pepsi, NuVal Griffin Hospital, Abbott Laboratories, the Canola and Flax Councils of Canada, Pulse Canada, and the Saskatchewan Pulse Growers. Dr Jenkins has stock options with Pacific Health Laboratories Inc. Dr Jenkins also holds additional grant support from the CIHR, Canadian Foundation for Innovation (CFI), Ontario Research Fund (ORF), Advanced Foods and material Network (AFMNet), International Tree Nut Council Nutrition Research and Education Foundation (INC NREF), and the Peanut Institute. Dr Jenkins' wife is a director of Glycemic Index Laboratories, Toronto, Ontario, Canada. Dr Chiavaroli is a casual Clinical Research Coordinator at GI Laboratories, Toronto, Ontario, Canada. Drs Mirrahimi, Beyene, Hanley, and Augustin have no declared conflicts of interest related to this article.
References
- 1.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16-22. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Dietary guidelines for healthy American adults. A statement for physicians and health professionals by the Nutrition Committee, American Heart Association. Circulation. 1986;74:1465A-1468A. [PubMed] [Google Scholar]
- 3.Krauss RM, Deckelbaum RJ, Ernst N, Fisher E, Howard BV, Knopp RH, Kotchen T, Lichtenstein AH, McGill HC, Pearson TA, Prewitt TE, Stone NJ, Horn LV, Weinberg R. Dietary guidelines for healthy American adults. A statement for health professionals from the Nutrition Committee, American Heart Association. Circulation. 1996;94:1795-1800. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146-1155. [DOI] [PubMed] [Google Scholar]
- 7.Van Horn L, McCoin M, Kris-Etherton PM, Burke F, Carson JA, Champagne CM, Karmally W, Sikand G. The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc. 2008;108:287-331. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the omniheart randomized trial. JAMA. 2005;294:2455-2464. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, Willett WC. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560-566. [DOI] [PubMed] [Google Scholar]
- 10.Mancini M, Mattock M, Rabaya E, Chait A, Lewis B. Studies of the mechanisms of carbohydrate-induced lipaemia in normal man. Atherosclerosis. 1973;17:445-454. [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen J, Schaaf P, Jones C, Zhou MY, Chen YD, Reaven GM. Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women. Am J Clin Nutr. 1997;65:1027-1033. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362-366. [DOI] [PubMed] [Google Scholar]
- 13.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728-2732. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson S, Hancock DP, Petocz P, Ceriello A, Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappab activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008;87:1188-1193. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S, Banach MS, Ares S, Mitchell S, Emam A, Augustin LS, Parker TL, Leiter LA. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742-2753. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins DJ, Srichaikul K, Kendall CW, Sievenpiper JL, Abdulnour S, Mirrahimi A, Meneses C, Nishi S, He X, Lee S, So YT, Esfahani A, Mitchell S, Parker TL, Vidgen E, Josse RG, Leiter LA. The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes. Diabetologia. 2011;54:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luscombe ND, Noakes M, Clifton PM. Diets high and low in glycemic index versus high monounsaturated fat diets: effects on glucose and lipid metabolism in NIDDM. Eur J Clin Nutr. 1999;53:473-478. [DOI] [PubMed] [Google Scholar]
- 18.McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466-1475. [DOI] [PubMed] [Google Scholar]
- 19.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482-2490. [DOI] [PubMed] [Google Scholar]
- 20.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27:1866-1872. [DOI] [PubMed] [Google Scholar]
- 21.Thomas D, Elliott E, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolever TM, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, Leiter LA, Maheux P, Rabasa-Lhoret R, Rodger NW, Ryan EA. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87:114-125. [DOI] [PubMed] [Google Scholar]
- 23.Wolever TM, Mehling C. High-carbohydrate-low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br J Nutr. 2002;87:477-487. [DOI] [PubMed] [Google Scholar]
- 24.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, Stender S, Holst C, Saris WH, Astrup A, Diet O, Genes P. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472-477. [DOI] [PubMed] [Google Scholar]
- 26.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545-550. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414-2423. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455-1461. [DOI] [PubMed] [Google Scholar]
- 29.van Dam RM, Visscher AW, Feskens EJ, Verhoef P, Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr. 2000;54:726-731. [DOI] [PubMed] [Google Scholar]
- 30.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr. 2007;85:1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14-21. [DOI] [PubMed] [Google Scholar]
- 32.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [DOI] [PubMed] [Google Scholar]
- 33.Sieri S, Krogh V, Berrino F, Evangelista A, Agnoli C, Brighenti F, Pellegrini N, Palli D, Masala G, Sacerdote C, Veglia F, Tumino R, Frasca G, Grioni S, Pala V, Mattiello A, Chiodini P, Panico S. Dietary glycemic load and index and risk of coronary heart disease in a large Italian cohort: the EPICOR study. Arch Intern Med. 2010;170:640-647. [DOI] [PubMed] [Google Scholar]
- 34.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991-2002. [DOI] [PubMed] [Google Scholar]
- 35.Mursu J, Virtanen JK, Rissanen TH, Tuomainen TP, Nykanen I, Laukkanen JA, Kortelainen R, Voutilainen S. Glycemic index, glycemic load, and the risk of acute myocardial infarction in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Nutr Metab Cardiovasc Dis. 2011;21:144-149. [DOI] [PubMed] [Google Scholar]
- 36.Burger KN, Beulens JW, Boer JM, Spijkerman AM, van der AD. Dietary glycemic load and glycemic index and risk of coronary heart disease and stroke in Dutch men and women: the EPIC-MORGEN study. PLoS ONE. 2011;6:e25955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005;18:145-171. [DOI] [PubMed] [Google Scholar]
- 38.Higgins J, Green S. The Cochrane Collaboration; 2008. Cochrane handbook for systematic reviews of interventions version 5.0.0 [updated February 2008] [Google Scholar]
- 39.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293-298. [DOI] [PubMed] [Google Scholar]
- 42.Levitan EB, Mittleman MA, Wolk A. Dietary glycaemic index, dietary glycaemic load and incidence of myocardial infarction in women. Br J Nutr. 2010;103:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grau K, Tetens I, Bjornsbo KS, Heitman BL. Overall glycaemic index and glycaemic load of habitual diet and risk of heart disease. Public Health Nutr. 2011;14:109-118. [DOI] [PubMed] [Google Scholar]
- 44.Hardy DS, Hoelscher DM, Aragaki C, Stevens J, Steffen LM, Pankow JS, Boerwinkle E. Association of glycemic index and glycemic load with risk of incident coronary heart disease among whites and African Americans with and without type 2 diabetes: the Atherosclerosis Risk in Communities study. Ann Epidemiol. 2010;20:610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster-Powell K, Miller JB. International tables of glycemic index. Am J Clin Nutr. 1995;62:871S-890S. [DOI] [PubMed] [Google Scholar]
- 46.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5-56. [DOI] [PubMed] [Google Scholar]
- 47.Jakobsen MU, Dethlefsen C, Joensen AM, Stegger J, Tjonneland A, Schmidt EB, Overvad K. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91:1764-1768. [DOI] [PubMed] [Google Scholar]
- 48.Ding EL, Malik VS. Convergence of obesity and high glycemic diet on compounding diabetes and cardiovascular risks in modernizing China: an emerging public health dilemma. Global Health. 2008;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knopp RH, Paramsothy P, Retzlaff BM, Fish B, Walden C, Dowdy A, Tsunehara C, Aikawa K, Cheung MC. Gender differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Atheroscler Rep. 2005;7:472-479. [DOI] [PubMed] [Google Scholar]
- 50.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8-15. [DOI] [PubMed] [Google Scholar]
- 51.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641-646. [DOI] [PubMed] [Google Scholar]
- 52.Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, Manson JE, Liu S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reardon MF, Nestel PJ, Craig IH, Harper RW. Lipoprotein predictors of the severity of coronary artery disease in men and women. Circulation. 1985;71:881-888. [DOI] [PubMed] [Google Scholar]
- 54.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B-12B. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492-498. [DOI] [PubMed] [Google Scholar]
- 56.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso MSTOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494. [DOI] [PubMed] [Google Scholar]
- 57.Gastrich M, Lasser N, Wien M, Bachmann G. Dietary complex carbohydrates and low glycemic index/load decrease levels of specific metabolic syndrome/cardiovascular disease risk factors. Top Clin Nutr. 2008;23:76-96. [Google Scholar]
- 58.Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring). 2009;17:1664-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009CD006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10-18. [DOI] [PubMed] [Google Scholar]
- 61.Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr. 2006;84:70-76quiz 266-267 [DOI] [PubMed] [Google Scholar]
- 62.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk – a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627-637. [DOI] [PubMed] [Google Scholar]
- 63.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]


