Abstract
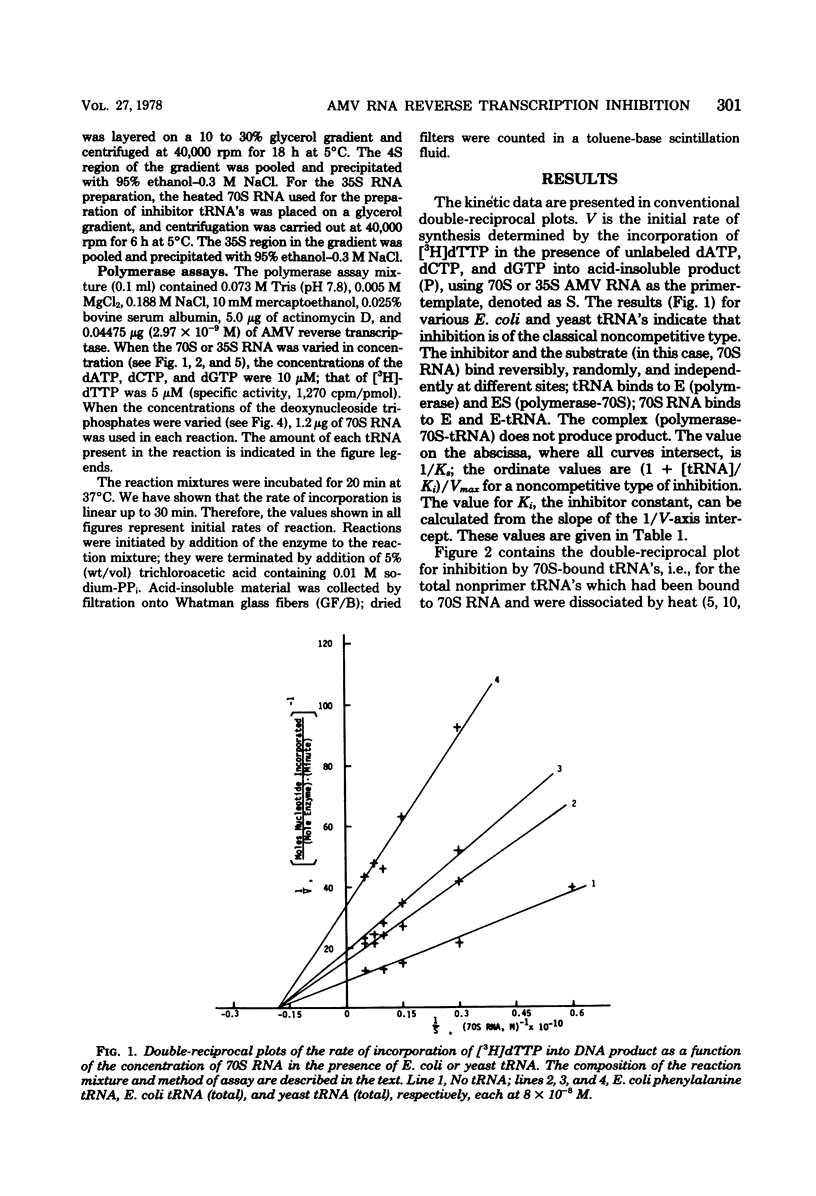
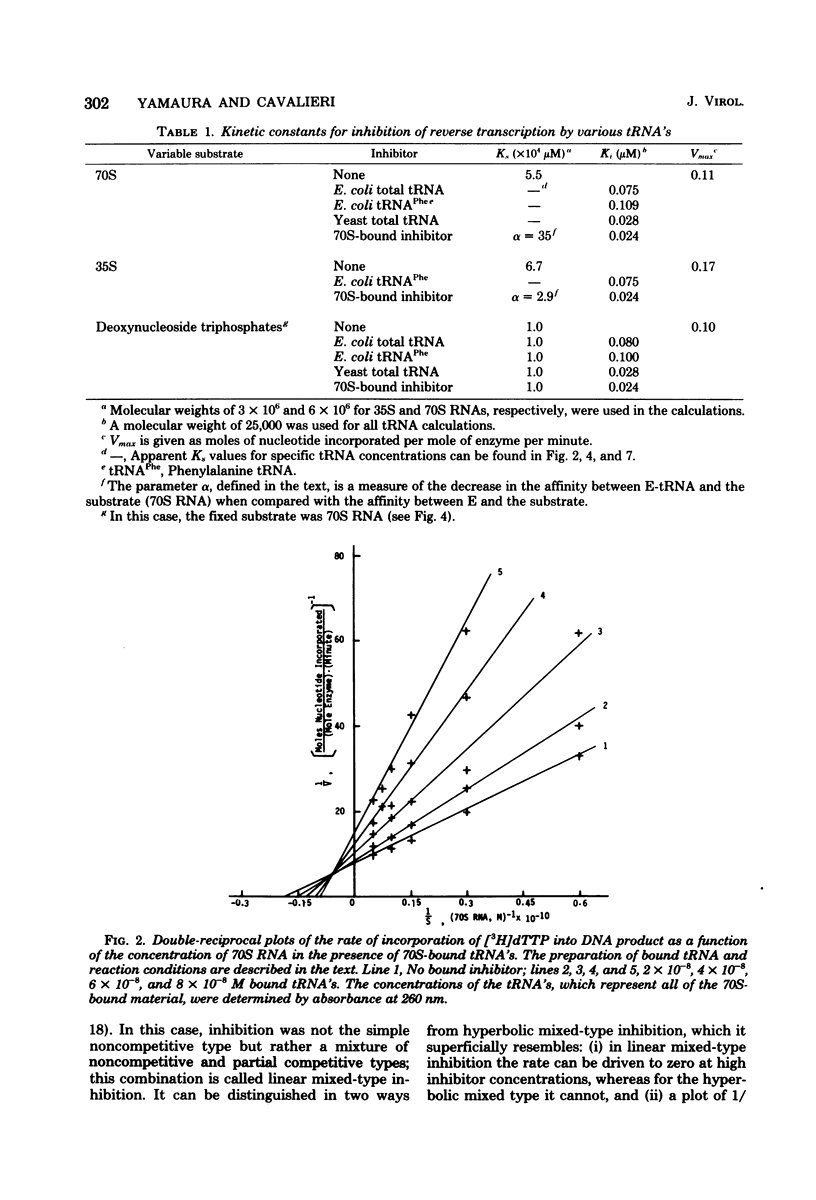
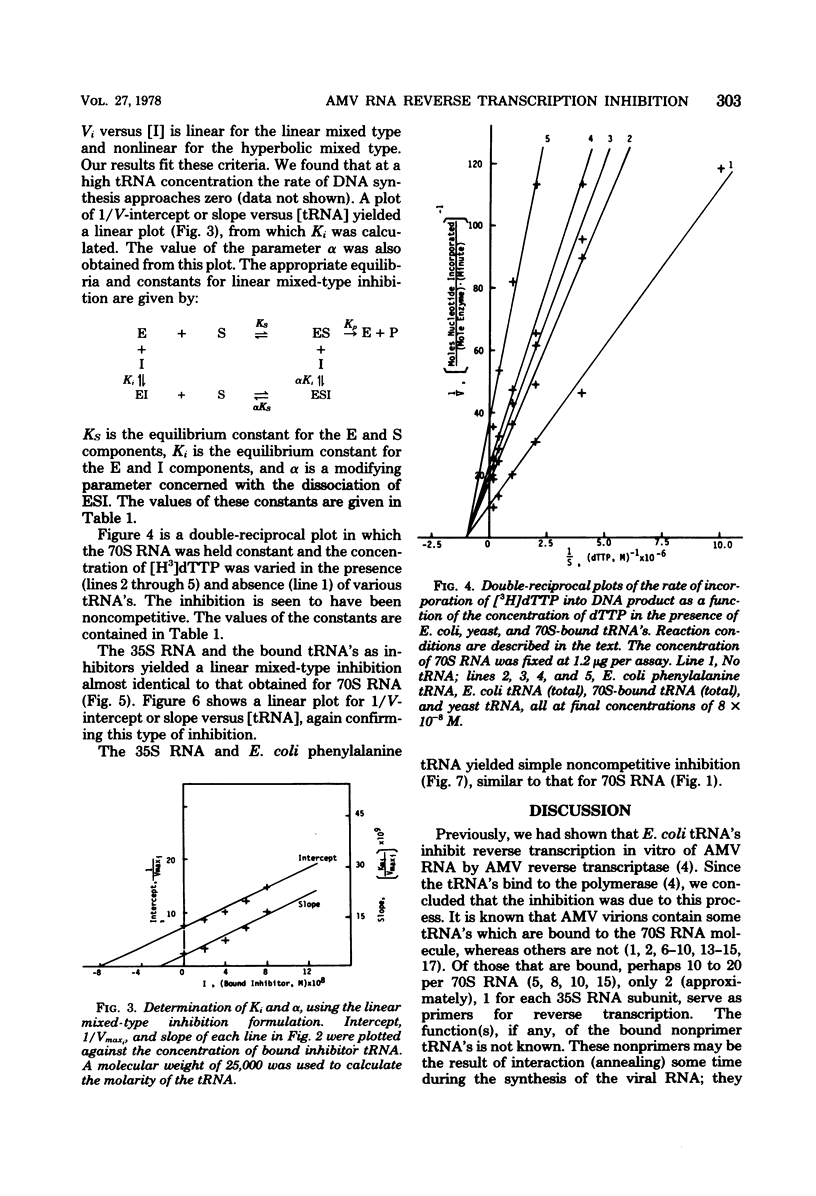
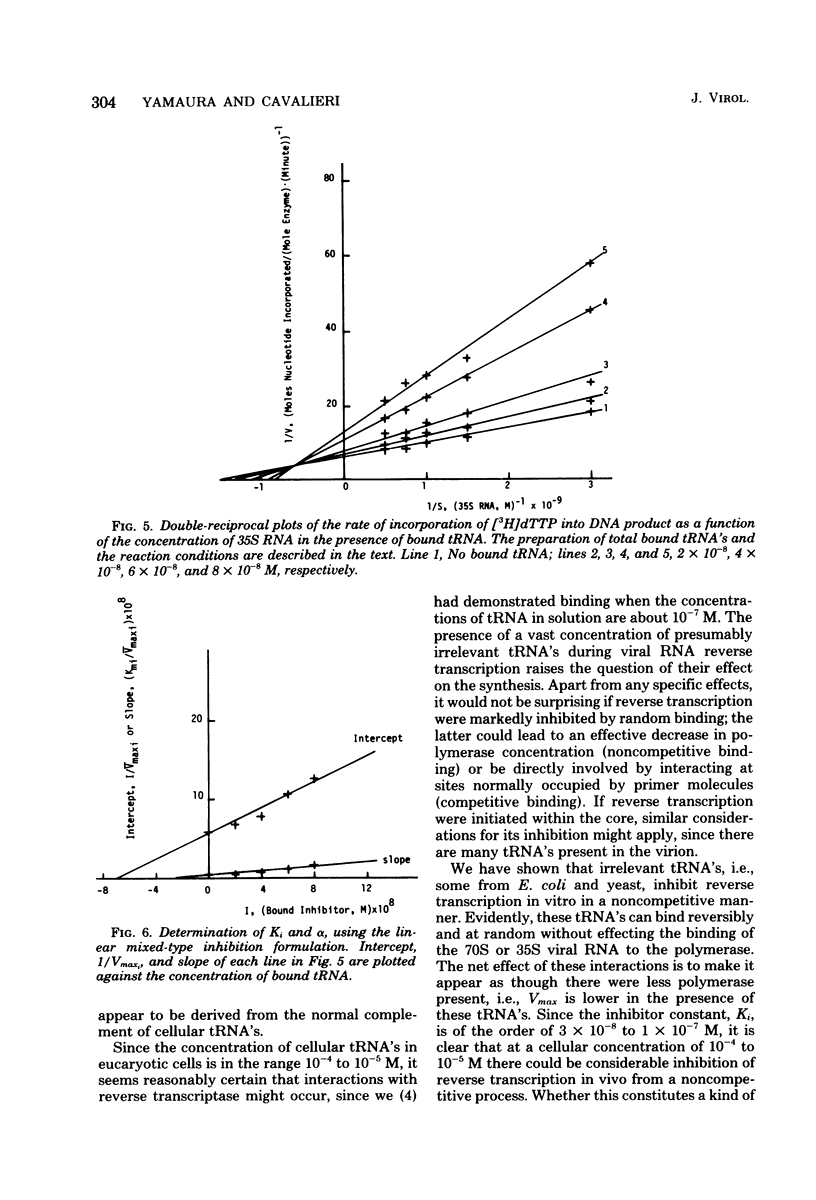
We studied the kinetics of the reverse transcription of 70S and 35S RNA of avian myeloblastosis virus in the presence and absence of various tRNA's. All tRNA's inhibited synthesis. tRNA's from Escherichia coli and yeast exhibited a noncompetitive type of inhibition, i.e., they bound reversibly and randomly and did not alter the affinity of the viral RNA for the polymerase. Nonprimer tRNA's obtained from 70S RNA molecules produced a complex pattern of inhibition. The results show that the nonprimer tRNA's which bound to the reverse transcriptase decreased the affinity of the viral RNA for the enzyme. The maximum rate of synthesis with 70S RNA as the template was less than that with 35S RNA, presumably because the former contains nonprimer tRNA's which can interact with the polymerase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bonar R. A., Sverak L., Bolognesi D. P., Langlois A. J., Beard D., Beard J. W. Ribonucleic acid components of BAI strain A (myeloblastosis) avian tumor virus. Cancer Res. 1967 Jun;27(6):1138–1157. [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri L. F., Yamaura I. E. coli tRNAs as inhibitors of viral reverse transcription in vitro. Nucleic Acids Res. 1975 Dec;2(12):2315–2328. doi: 10.1093/nar/2.12.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Zamecnik P. C. Amino-acid acceptor activity of the "70S-associated" 4S RNA from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1184–1185. doi: 10.1073/pnas.70.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing N., Grantham C. A., Kaminski F., Lindley I. J. Protection of mice against encephalomyocarditis virus infection by preparations of transfer RNA. J Gen Virol. 1977 Jan;34(1):73–85. doi: 10.1099/0022-1317-34-1-73. [DOI] [PubMed] [Google Scholar]
- Wang S., Kothari R. M., Taylor M., Hung P. Transfer RNA activities of Rous sarcoma and Rous associated viruses. Nat New Biol. 1973 Apr 4;242(118):133–135. doi: 10.1038/newbio242133a0. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C., Ho T., Yang W. K. Ability of tryptophan tRNA to hybridize with 35S RNA of avian myeloblastosis virus and to prime reverse transcription in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2155–2159. doi: 10.1073/pnas.72.6.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]


