Abstract
Activity-dependent trafficking of AMPA receptors to synapses regulates synaptic strength. Activation of the NMDA receptor induces several second messenger pathways that contribute to receptor trafficking-dependent plasticity, including the NO pathway, which elevates cGMP. In turn, cGMP activates the cGMP-dependent protein kinase type II (cGKII), which phosphorylates the AMPA receptor subunit GluA1 at serine 845, a critical step facilitating synaptic delivery in the mechanism of activity-dependent synaptic potentiation. Since cGKII is expressed in the striatum, amygdala, cerebral cortex, and hippocampus, it has been proposed that mice lacking cGKII may present phenotypic differences compared to their wild-type littermates in emotion-dependent tasks, learning and memory, and drug reward salience. Previous studies have shown that cGKII KO mice ingest higher amounts of ethanol as well as exhibit elevated anxiety levels compared to wild-type (WT) littermates. Here, we show that cGKII KO mice are significantly deficient in spatial learning while exhibiting facilitated motor coordination, demonstrating a clear dependence of memory-based tasks on cGKII. We also show diminished GluA1 phosphorylation in the postsynaptic density (PSD) of cGKII KO prefrontal cortex while in hippocampal PSD fractions, phosphorylation was not significantly altered. These data suggest that the role of cGKII may be more robust in particular brain regions, thereby impacting complex behaviors dependent on these regions differently.
1. Introduction
Environmental stimuli modify the functions of brain circuits that encode information surrounding the event. These modifications can be stabilized through the formation of memories. In rodents, the role of the hippocampus in memory has been studied extensively (Eichenbaum, 1999) but it is also thought that the prefrontal cortex, which connects to the hippocampus, plays a role in the maintenance of mental representations that guide goal-directed behaviors (Lynch, 2004). Activity within the prefrontal cortex is involved in the assignment of temporal position to spatial and nonspatial events alike (Lynch, 2004). The molecular mechanisms contributing to long-term potentiation (LTP), an activity-dependent form of synaptic plasticity that may underlie forms of nondeclarative memory, cognition and behavior, are only beginning to be understood (Mayford, Mansuy, Muller, and Kandel, 1997). Signaling pathways in the molecular component of memory have been identified, and among those of particular interest are the pathways that convert short-term memories to long-term memories, including the cyclic nucleotide mediated protein kinase pathways that phosphorylate ionotropic glutamate receptors and control their subsequent trafficking to the synapse.
α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPARs) play an important role in the regulation of synaptic strength, and are believed to be an essential component of the memory formation mechanism. AMPARs are tetramers that contain GluA1-4 subunits (Malinow and Malenka, 2002). Insertion of GluA2-lacking, Ca2+-permeable AMPARs into the postsynaptic membrane may be induced by the activation of NMDA receptors (Derkach, Oh, Guire, and Soderling, 2007). The influx of Ca2+ induces signaling cascades that result in the phosphorylation of the intracellular C-terminal domain of GluA1 (Roche, O'Brien, Mammen, Bernhardt, and Huganir, 1996; Serulle, Zhang, Ninan, Puzzo, McCarthy, Khatri, Arancio, and Ziff, 2007). Increases in excitatory neuronal transmission that lead to spatial memory, learning, and retention are mediated by phosphorylation of GluA1, with phosphorylations of S831 and S845 contributing to the mechanism (Lee, Takamiya, Han, Man, Kim, Rumbaugh, Yu, Ding, He, Petralia, Wenthold, Gallagher, and Huganir, 2003). Deletion of αCAMKII, the kinase that phosphorylates GluA1 at serine 831 (Fu, Zhang, Meng, and Zhang, 2004), impairs LTP in the hippocampus and disrupts spatial learning (Fu et al., 2004). It has also been shown that mice with knockin mutations substituting alanine for serines 831 and 845 of GluA1 exhibit deficient memory retention as well as disruptions in LTP and LTD (Lee et al., 2003).
Two closely-related cyclic nucleotide regulated protein kinases, cAMP-dependent PKA and cGMP-dependent protein kinase II (cGKII) phosphorylate serine 845, inducing delivery of GluA1 to the synapse (Serulle et al., 2007). PKA plays a key role in memory formation and the induction of LTP (Selcher, Weeber, Varga, Sweatt, and Swank, 2002). In transgenic mice expressing a dominant negative form of the regulatory subunit of PKA, deficits were observed in long-term contextual fear learning (Selcher et al., 2002). In addition to PKA’s known role in GluA1 phosphorylation, it is also a regulator of cAMP response binding-element (CREB) and triggers signaling cascades that drive transcription of proteins necessary for strengthening synapses (Michel, Kemenes, Muller, and Kemenes, 2008). cGKII is inactive when cGMP is not present (Serulle et al., 2007). Upon NMDA receptor activation and the subsequent binding of cGMP to cGKII, a conformational change of the kinase occurs, whereby the autoinhibitory domain is auto-phosphorylated, released from the catalytic domain, and the kinase function is activated (Serulle et al., 2007). This series of molecular events enables the GluA1 C-terminus to bind to the kinase and GluA1 is phosphorylated at S845, leading to an increase in receptor surface levels (Serulle et al., 2007), a step that is required for receptor entry into the synapse.
The closely related roles of cGKII and PKA in GluA1 phosphorylation and trafficking raise the question of the relative contributions of these kinases to memory and behavior formation. Of the two, cGKII has been less well studied.Serulle et al. (2007) found that cGKII activation increased surface levels of GluA1 and that this effect was blocked by KT5823, a selective inhibitor of cGMP-dependent protein kinase. Also, LTP was blocked when KT5823 was applied to hippocampal slices. Interestingly, previous studies byKleppisch et al. (1999) demonstrated that LTP was normal in the cGKII KO animals. Even though LTP was shown to be normal in cGKII KO animals, distinct phenotypes have been found in various behavioral assays.Werner et al. (2004) found that cGKII KO mice exhibited hyposensitivity and elevated preference for ethanol as well as heightened anxiety levels compared to wild-type littermates. Modifications of kinase activity as well as phosphorylation sites on GluA1 have been shown to disrupt LTP and learning (Lee et al., 2003). S845 of GluA1 presents a novel situation in which two kinases under the control of the NMDA receptor and cyclic nucleotides, PKA and cGKII, both phosphorylate this critical residue. Furthermore, acute inhibition of cGKII blocks LTP in hippocampal slices, while KO of the gene leaves LTP apparently unchanged (Kleppisch, Pfeifer, Klatt, Ruth, Montkowski, Fassler, and Hofmann, 1999; Serulle et al., 2007).
To begin to understand the role of cGKII in memory formation, we examined the behavior of cGKII KO mice in a battery of assays in order to identify phenotypic changes. The assays used to assess phenotypes were Prepulse Inhibition (PPI), Acoustic Startle (ASR), the Rotating Rod Test, Open Field Assay (OFA), and the Morris Water Maze (MWM). Although acute inhibition of cGKII has been shown to decrease levels of LTP significantly (Serulle et al., 2007) while knocking out the cGKII gene has revealed normal LTP (Kleppisch et al., 1999), we could not anticipate whether behavioral assays would indicate phenotypic differences in comparison to wild type. Here we have shown that cGKII KO mice exhibit deficits in spatial learning in the Morris Water Maze, heightened acoustic startle response, and improved motor coordination on the Rotating Rod Test as compared to wild-type (WT) littermates. Our immunoblot studies showed that GluA1 phosphorylation at serine 845 in the hippocampus of cGKII KO animals was unchanged, but was significantly reduced in the prefrontal cortex. Thus, cGKII KO animals could show synaptic impairments in a brain region-specific manner.
2. Material and Methods
2.1. Mice
The generation of cGKII knockout has been previously described (Pfeifer, Aszodi, Seidler, Ruth, Hofmann, and Fassler, 1996). cGKII KO mice were either kept on the Sv / 129 background or back-crossed to C57BL / 6N. Mice were bred in the Skirball Animal Facility at New York University School of Medicine. Mice were kept under standard housing conditions (20–24 °C, 30–70% relative humidity, 12-h dark : 12-h light cycle) and were housed at an average of ~four animals per cage. At the conclusion of the behavioral assays, the animals were weighed and the following weight averages were recorded: cGKII KO: 24.7 ± 1.6g and WT: 38.9 ± 0.6g, p<0.0001. All behavioral tests were performed on adult male mice from the ages of 27–41 weeks, with an average age of 33 weeks.
The animal experiments were conducted in accordance with the New York University School of Medicine’s Institutional Animal Care and Use Committee, New York University School of Medicine. Experiments were conducted in the Smilow Barrier Facility. Assays were conducted in the following order: OFA, the Rotating Rod Test, ASR, PPI, and MWM using four cohorts in MWM and five cohorts for all other assays.
2.2. Prepulse inhibition and acoustic startle
In prepulse inhibition and acoustic startle (Graham, Putnam, and Leavitt, 1975; Lang, Bradley, and Cuthbert, 1990), the animal is removed from its home cage and placed into a soundproof chamber, where the startle response tone (120 decibels for 20 ms) is given and startle response is scored by an automated system (San Diego Instruments). Mild prepulse tones (74, 82, and 90 dB and 20ms in duration) are then played shortly before response to the 120 dB tone is scored. Five trials of each prepulse and 120 dB tone pairing are performed in a randomized order with intervening segments and unpaired 120dB test pulses.
Here, mice were acclimated in cages alone for half an hour before being placed into the soundproof chamber containing a Plexiglas tube sitting atop a plastic frame. An accelerometer detected the motion within the tube as animals were undergoing testing. The acoustic startle tone was controlled by startle software and delivered by a speaker mounted inside the chamber. Animals were placed inside the tube and the startle pulse was given. The animal’s response was calibrated by the system. Five randomized trials were given from each pairing with interspersed silent periods and the animal’s response was scored as described. In wild-type mice, the acoustic startle response decreases as the prepulse tone is increased.
2.3. The Rotating Rod Test
In the Rotating Rod Test (Bogo, Hill, and Young, 1981), the mouse is placed on a rotating horizontal rod (Columbus) which is approximately three cm in diameter. The rod accelerates from four to 40 rpm during the five-minute trial. A single test lasts from the time the animal is placed on the rod until it drops off (approximately 12 cm onto the rest platform) or until five minutes have elapsed. Promptly afterwards, the animal is returned to the home cage and the rod and the rest platform are cleaned with 70% ethanol and water. Each animal is tested for four trials per day for two consecutive days. The animals rest in their home cages for 30 minutes to one hour between each trial.
In our study, mice were displaced from their home cages and placed into acclimation cages for 30 minutes prior to testing. Mice were then positioned on the rotating rod as described. Latency to fall from the rod was recorded for each animal for each trial.
2.4. Open Field
In Open Field (Hall, 1936), animals to be tested are removed from their room and brought to the behavioral testing area in their home cages. A test mouse is placed in the center of a clear, Plexiglas chamber (43X43X18 cm) enclosed in an acoustically protected chamber (Med-Associates). The animal remains in the chamber for 20 minutes to explore the novel environment. Exploration by the animal is monitored by infrared (IR) beam breaks. After 20 minutes, the animal is removed from the chamber and returned to its home cage. Multiple variables are measured (time and distance moved in zones, resting time, jumps, vertical and stereotypy movements). The Plexiglas chamber is then wiped clean with 70% ethanol and water before the next mouse is tested. The test is carried out in a square chamber positioned in the same room as other testing devices.
Mice were placed into the center of the chamber after having been acclimated alone in cages for thirty minutes. Animals’ movements were tracked as described. Constant lighting (60 lux) and background sound (55 dB) were presented during the testing.
2.5. Morris Water Maze
During Morris Water Maze (Morris, 1984), animals are removed from their home room and taken to the behavioral testing rooms where they remain for only a few hours before being returned to the home room. Most of the time spent in the behavior room involves the animals resting comfortably in standard mouse cages. The periods of behavioral testing and observation, which last a few minutes for any individual animal, involve the animal learning to use visible cues to navigate in a swimming test. Care is taken to minimize any stress the animals might experience in order to maximize learning in the maze. The water in the water tank is room temperature. The mice are dried to keep them warm after they are in the water. Mice removed from the maze are placed onto paper towels to dry them off, and then they are placed into a specific drying cage with extra paper towels placed there to help dry them and keep them warm.
Mice were trained in the Morris water task to locate a hidden escape platform in a circular pool (1.38 m Nalgene pool) of opaque water (made opaque with white tempera paint) using distal visual cues outside the pool. Each mouse was given one block of four trials a day for 10 consecutive days (the length of the entire task, including acquisition, reversal, and visible test training). Subjects were released into the pool from one of four starting positions, and the location of the platform remained constant throughout five days during acquisition. Time to find the escape platform (escape latency) was measured. The amount of time any individual mouse spent in the water was limited to 60 seconds. If the mice did not find the platform in that time, they were placed by hand onto the platform for a few seconds to help them learn where the platform was located. Following the training block on day five (on day six for cohort three), a probe test was given. In the probe test, the platform was removed and animals were allowed to search the pool for 60 seconds. Quadrant search time and platform crossings were assessed to characterize the mouse’s search behavior during the probe trial.
On day six, the platform was moved to the opposite quadrant (reversal) and the mice were trained to locate the new position of the platform using the same approach as used in acquisition. After reversal, which lasted for three consecutive days, mice were trained on the visible platform task (days nine and ten). In this test, animals were required to locate a platform whose position was marked by a post extending 10 cm out of the water topped by a large flag (colored with contrasting images, ~ 450 cm2). The location of the platform remained constant throughout training. Training consisted of four trials a day for two consecutive days, as described. Escape latencies were determined for each trial. The behavior of the animals was assessed using a commercial video monitoring apparatus (Noldus).
2.6. Postsynaptic Density Purification
Crude PSD was purified from hippocampal and PFC fractions according to the procedure described by Jordan et al. (2004). 10–20 μg of PSD were separated by 10% SDS-PAGE gel and transferred to a nitrocellulose membrane prior to being immunoblotted with the following antibodies: Anti-GluA1 (1:5000, Millipore), P-GluA1 (1:2000, Millipore), actin (1:2000, Sigma), and cGKII, (1:500, Covance).
2.7. Statistics
For behavior and biochemistry experiments, analysis of variance (ANOVA) and t tests were applied as appropriate. Significant main effects or interactions were followed by posthoc testing as appropriate. All statistical tests are two-tailed at significance level 0.05. Data on graphs represent mean + SEM. Statistical analysis was performed using Prism and Excel software.
3. Results
We analyzed the performance of cGKII KO mice on a battery of assays: the Morris Water Maze, Open Field, Prepulse Inhibition, and the Rotating Rod Test and compared them with WT. We also performed Western blot analysis on KO and WT animals to probe for levels of GluA1 and phosphorylated GluA1 at serine 845 [pGluA1(S845)] in the postsynaptic density (PSD) of hippocampal and prefrontal cortex (PFC) fractions.
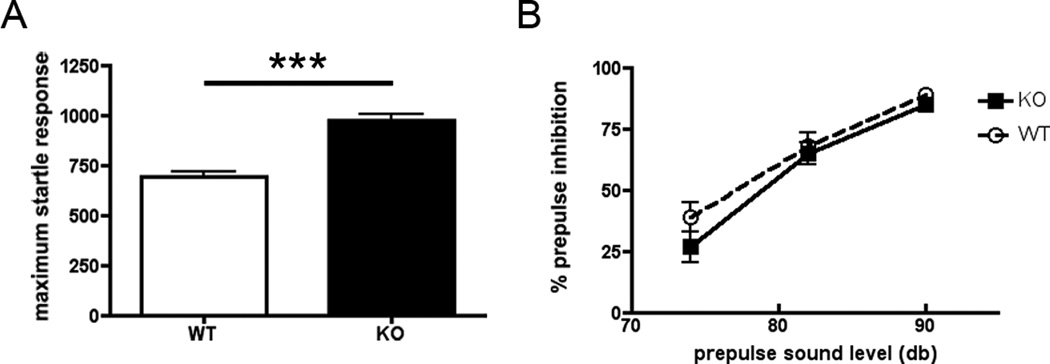
3.1 cGKII KO mice show enhanced startle response but no differences in Prepulse Inhibition
Acoustic startle response (ASR) is a measure of an animal’s sensorimotor response to a startling auditory stimulus. PPI tests sensorimotor gating, during which the intensity of an animal’s response to an initial startling auditory stimulus is diminished when the pulse is preceded by a weaker tone. cGKII KO animals exhibited a heightened response to the auditory stimuli relative to WT but no differences were observed between groups in prepulse inhibition (PPI). The results, presented in Figure 1, showed differences between groups in ASR but not in PPI. A two-tailed t test revealed a significant difference in ASR between WT and KO (p < 0.0001). This finding suggests that there was an elevated startle response in cGKII KO animals compared to WT. In PPI, a two-way ANOVA with repeated measures for decibel levels indicated that there was no significant main effect of genotype [F1,399 = 2.759, p = 0.0975]. The Decibel Level X Genotype interaction was also not significant [F2,399 = 0.5501, p = 0.5773].
Figure 1.
(A) Acoustic startle response to 120 db stimulus. Data are the average (±SEM) for wild type (WT), n=13 (white bar) and knockout cGKII (KO), n=14 (black bar). (B) Prepulse inhibition (PPI) to acoustic startle response. The averages (±SEM) for levels (%) of PPI of the startle response in WT (black dotted line) and KO (black solid line).
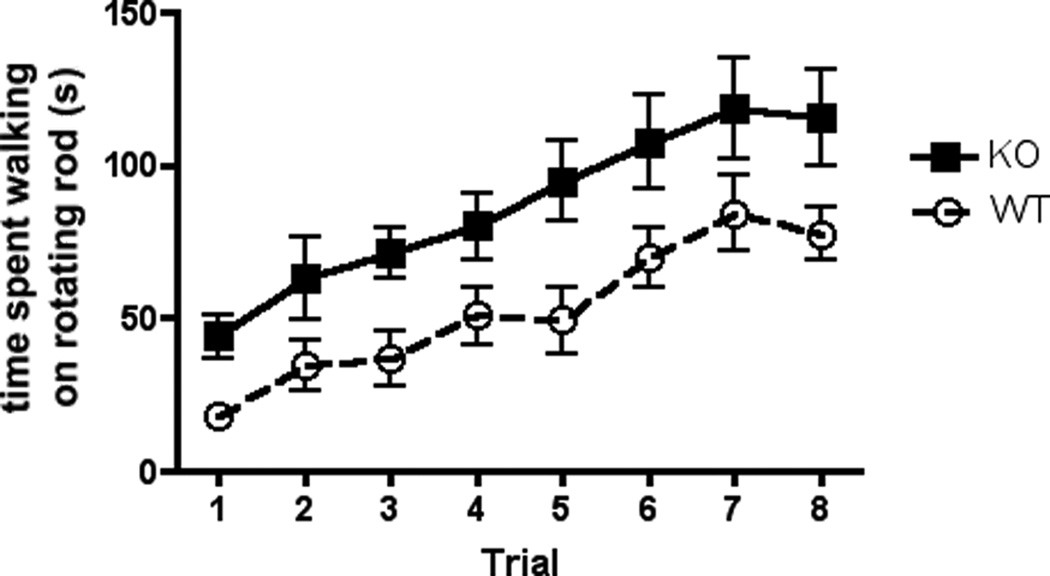
3.2 cGKII KO animals exhibit facilitated motor coordination as revealed by the Rotating Rod Test
The Rotating Rod Test is used to assess coordination and motor learning. In Figure 2, we show time spent walking by WT and KO mice on a rotating rod whereby the rotational speed is slowly increased throughout the length of a five-minute test trial. A two-way ANOVA with repeated measures for trials showed a significant main effect of trial [F7,200 = 9.351, p < .0001] and a significant main effect of genotype [F1,200 = 35.79, p < 0.0001], while the Trial X Genotype interaction was not significant [F7,200 = 0.1538, p = 0.9933]. These results indicate that the KO performed significantly better than WT.
Figure 2.
The Rotating Rod Test. The averages (±SEM) for time spent on the Rotating Rod Test across eight trials (four trials per day for two days) for WT, n=13, (dashed line) and KO, n=14 (solid line) animals.
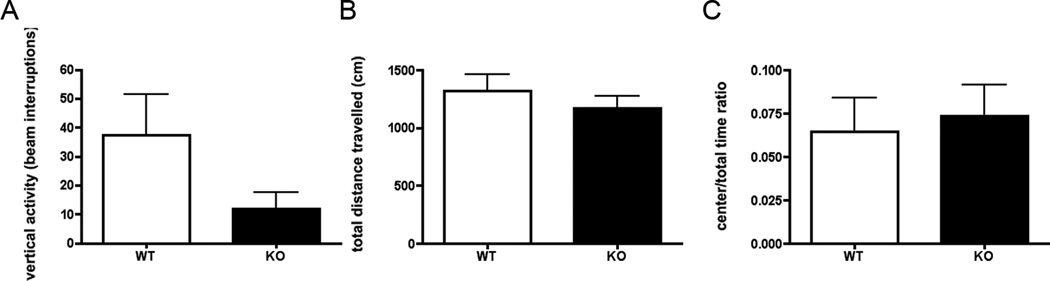
3.3 cGKII KO mice show normal anxiety displays in Open Field Assay
The open field assay (OFA) measures locomotor activity of a rodent when it is presented to a novel space. The test divides the testing arena into a ‘center’ zone which is surrounded by a residual zone. This assay is routinely used to assess exploratory and locomotor activity as well anxiety, as mice that display higher levels of innate anxiety will show lower occupancy times and distance moved in the more anxiogenic center zone. No significant differences were observed between cGKII KO and WT animals in vertical activity, total distance travelled, and center to total time ratio in the open field assay (Figures 3A, 3B, 3C). Two-tailed t tests performed in vertical activity and total distance travelled (locomotor activity) for WT and KO animals revealed no significant differences (p = 0.0945 and p = 0.4201, respectively, Figs. 3A and 3B). In center to total time ratio, a behavioral measure corresponding to anxiety levels, no significant differences were observed between groups (p = 0.7259, 3C). This suggests that WT and KO had similar levels of anxiety as well as similar patterns of locomotor activity.
Figure 3.
Open field exploration. (A) Vertical activity in open field assay. Data are the average (±SEM) for wild type (WT), n=13, (white bar) and knockout cGKII (KO), n=14 (black bar) animals. (B) Total distance travelled. Data are the average in centimenters (±SEM) for WT, n=13, (white bar) and KO, n=14 (black bar) animals. (C) Center to total time ratio. Data are the average (±SEM) for WT, n=13, (white bar) and KO, n=14 (black bar) animals.
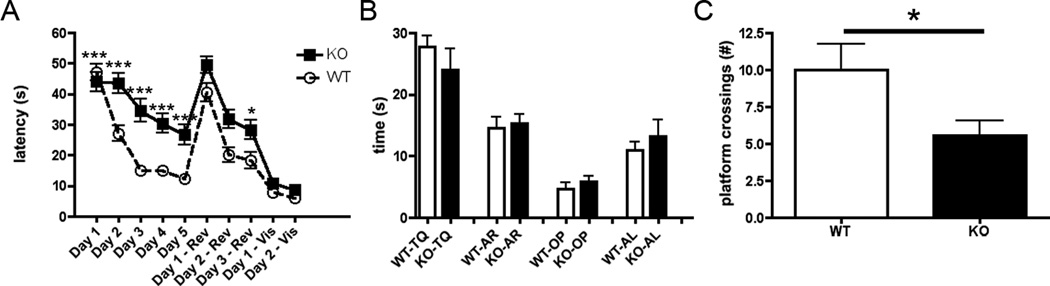
3.4 cGKII KO animals show significant deficits in spatial learning in the Morris Water Maze
The Morris Water Maze (MWM) is used to assess spatial learning and memory. The MWM assay is a hippocampus-dependent spatial learning task that measures the ability of a rodent to learn and remember the relationship between distal spatial cues and the location of a hidden escape platform (Morris, 1984). Our MWM experiments revealed significant differences between WT and cGKII KO mice in spatial learning, with KO mice exhibiting deficits. A two-way ANOVA with repeated measures shown in Figure 4A revealed significant main effects of trial [F9,860 = 54.40, p < 0 .0001] and of genotype [F1,860 = 79.07, p < 0.0001] as well as a significant Trial X Genotype interaction [F9,860 = 3.080, p <0.0001]. Using the Bonferroni post-hoc test, on Days 2–5 of acquisition, there were significant differences in spatial learning between WT and KO (p < 0.0001) as well as on Day 3 of reversal (p < .05). During the probe trial, no significant differences were observed between groups in search time spent in each quadrant (Fig. 4B) however there were significant differences in the number of platform crossings in the target quadrant (Fig. 4C) as revealed by a two-tailed t test (p = 0.0407). No significant differences were observed between WT and KO on the days where the platform was visible. On Visible Day 2 Trial 3, no significant differences were observed in swimming speed or distance travelled (data not shown). These results indicate that neither visual problems nor motor and swimming differences accounted for the observed deficits.
Figure 4.
Spatial learning in the Morris Water Maze. (A) Across 10 days, presented is the average (±SEM) latency (seconds) to locate the platform for wild type (WT), n=12, (dashed line) and cGKII knockout (KO), n=10 (solid line) animals. Days 1–5 of acquisition, the platform is hidden, Days 1–3 – Rev indicate latency to find the hidden platform during the reversal trials, and Days 1–2 – Vis indicate the latency to find the visible platform. (B) Presented is the average (±SEM) time spent in seconds during the probe trial in each quadrant for WT (white bar) and cGKII KO (black bar) animals. TQ = target quadrant, AR = adjacent right quadrant, OP = opposite quadrant, and AL = adjacent left quadrant. (C) Presented is the average (±SEM) number of platform crossings during the probe trial in WT (white bar) and cGKII KO animals (black bar).
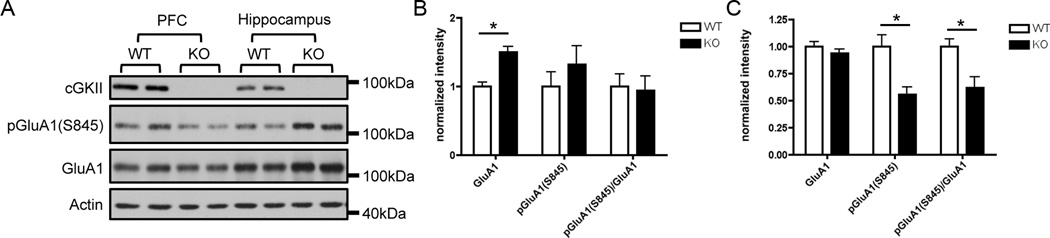
3.5 Decreased GluA1 phosphorylation in the PSD of cGKII KO prefrontal cortex
We conducted a Western blot analysis to compare levels of GluA1 and pGluA1(S845) in the PSD of PFC and hippocampus in WT and KO animals (Figure 5). In hippocampal PSD fractions, total GluA1 levels were significantly elevated in the KO (p < .001) while pGluA1(S845) levels were not significantly different (Figures 5A and 5B). There were also no observed differences between WT and KO in pGluA1(S845)/total GluA1 ratio in hippocampal PSD fractions (Figure 5B). In the PSD of PFC fractions, GluA1 levels were not significantly different in KO and WT (Figures 5A and 5C). However, significant decreases (p < .001) were found in pGluA1(S845) in the KO compared to WT as well as in pGluA1(S845) /total GluA1 ratio (p < .05) (Figures 5A and 5C).
Figure 5.
Immunoblot analysis of the PSD from hippocampus and PFC in WT and KO animals. (A) Representative immunoblots of the PSD of hippocampus and PFC in WT and KO animals (n=3 animals, duplicated). (B) Presented is the average (± SEM) of normalized intensity of hippocampal GluA1 S845 phosphorylation in WT (white bar) and KO (black bar). (C) Presented is the average (± SEM) of normalized intensity of PFC GluA1 S845 phosphorylation in WT (white bar) and KO (black bar).
4. Discussion
Phosphorylation of GluA1 S845 has been linked to AMPA receptor trafficking that is required for LTP, a mechanism of synapse potentiation thought to underlie the formation of learning and memory (Derkach et al., 2007). Acute inhibition of phosphorylation of S845 by cGKII blocked LTP, suggesting a strong requirement for this kinase in the hippocampus (Serulle et al., 2007). However in KO hippocampal slices, LTP was apparently normal (Kleppisch et al., 1999). This could reflect a compensatory mechanism, and the PKA-dependent pathway for phosphorylation of GluA1 S845 could potentially be capable of restoring function in the absence of cGKII. This made uncertain the nature and extent of phenotypic differences between the KO and the WT that would be observed. It was also unknown as to whether the role of cGKII varied from region to region, leaving unanswered the question as to how any such differences might impact GluA1-mediated synaptic plasticity and related behavior.
Here we report that genetic deletion of cGKII in mice caused pronounced and highly specific phenotypic behavioral changes. cGKII removal in one instance produced an apparent behavioral benefit, specifically improved motor coordination on the Rotating Rod Test, but striking deficits in others: decreased performance in spatial learning and heightened sensitivity in the acoustic startle response. In in situ hybridization assays of cGKII expression in c57BL/6 wild-type mice,Werner et al. (2004) found high mRNA signals of cGKII in the frontal and anterior cingulate cortices, raphe nuclei, amygdala, septal hippocampal nucleus, basal forebrain, somatosensory and motor cortices as well as the thalamus (ventrolateral group and anterior corsomedial group) (Werner, Raivich, Cowen, Strekalova, Sillaber, Buters, Spanagel, and Hofmann, 2004). Because the kinase is normally expressed in the somatosensory and motor cortices, a knockout could alter signaling pathways that modify synaptic plasticity in those areas. Connections governing motor response to sensory stimuli could be affected, thereby altering animal behavior on the Rotating Rod Test and acoustic startle response. An additional possibility is that the improved performance observed in the KO on the Rotating Rod Test was a result of the animals’ dwarfism, a trait previously described byPfeifer et al. (1996). KO animals weighed significantly less than WT (see section 2.1). However, this would not explain the deficits in MWM and combined with the overall normal locomotor and anxiety measures (Figures 3B, 3C), this suggests that alteration of behavior by cGKII KO is the consequence of synaptic dysfunction rather than differences in body weight.
GluA1 phosphorylation in the hippocampus of KO animals suggests that a compensatory mechanism may exist, which could potentially explain the previous finding of unchanged LTP between WT and KO mice (Kleppisch et al., 1999); however, any such mechanism may not occur in the PFC. Decreased pGluA1(S845) in the PFC of KO animals suggests that PFC-mediated physiological functions could be altered in KO animals. The activity of the PFC is involved in the decision-making component necessary for appropriate behavioral responses during spatial learning (Carvalho, Pereira, Pires, Ferraz, Romano-Silva, Oliveira-Silva, and Ribeiro, 2006; Dias and Aggleton, 2000; Martinet, Sheynikhovich, Benchenane, and Arleo, 2011; Ragozzino, Wilcox, Raso, and Kesner, 1999). Thus, cGKII removal-mediated reduction of GluA1 phosphorylation in the PFC could account for spatial learning deficits.
5. Conclusions
Our study shows that cGKII KO mice are deficient in spatial learning while exhibiting facilitated motor coordination, findings that demonstrate the involvement of cGKII in memory-based tasks. Decreased GluA1 phosphorylation in the PSD of the prefrontal cortex in cGKII KO animals indicates that cGKII may function in a brain region-specific manner, thereby contributing differently to complex behavioral phenotypes.
cGKII KO mice are significantly deficient in spatial learning in the Morris Water Maze
cGKII KO mice exhibit facilitated motor coordination in the Rotating Rod test
Decreased S845 GluA1 phosphorylation in the PSD of cGKII KO prefrontal cortex
Acknowledgements
This work was supported by National Institutes of Health Grant R01MH067229, (to EBZ). RT was supported by NIH MSTP Training Grant 5T32GM007308, SK by the Blas Frangione Foundation Fellowship, and CW by NIH Training Grant 5T32NS069514. We would like to thank Sarah Vunck, Melissa Blank, and Paul Lipton for their enthusiastic assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogo V, Hill TA, Young RW. Comparison of accelerod and rotarod sensitivity in detecting ethanol- and acrylamide-induced performance decrement in rats: review of experimental considerations of rotating rod systems. Neurotoxicology. 1981;2:765–787. [PubMed] [Google Scholar]
- Carvalho FM, Pereira SRC, Pires RGW, Ferraz VP, Romano-Silva MA, Oliveira- Silva LF, Ribeiro AM. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacology Biochemistry and Behavior. 2006;83:481–489. doi: 10.1016/j.pbb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature reviews. Neuroscience. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. The European journal of neuroscience. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural brain research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Fu XZ, Zhang QG, Meng FJ, Zhang GY. NMDA receptor-mediated immediate Ser831 phosphorylation of GluR1 through CaMKIIalpha in rat hippocampus during early global ischemia. Neuroscience research. 2004;48:85–91. doi: 10.1016/j.neures.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Graham FK, Putnam LE, Leavitt LA. Lead-stimulation effects of human cardiac orienting and blink reflexes. Journal of experimental psychology. Human perception and performance. 1975;104:175–182. [PubMed] [Google Scholar]
- Hall CS. Emotional behavior in the rat III The relationship between emotionality and ambulatory activity. Journal of comparative psychology. 1936;22:345–352. [Google Scholar]
- Kleppisch T, Pfeifer A, Klatt P, Ruth P, Montkowski A, Fassler R, Hofmann F. Long-term potentiation in the hippocampal CA1 region of mice lacking cGMP- dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. Journal of Neuroscience. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiological reviews. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annual review of neuroscience. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Martinet LE, Sheynikhovich D, Benchenane K, Arleo A. Spatial Learning and Action Planning in a Prefrontal Cortical Network Model. PLoS computational biology. 2011;7 doi: 10.1371/journal.pcbi.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Mansuy IM, Muller RU, Kandel ER. Memory and behavior: a second generation of genetically modified mice. Current biology : CB. 1997;7:R580–R589. doi: 10.1016/s0960-9822(06)00287-9. [DOI] [PubMed] [Google Scholar]
- Michel M, Kemenes I, Muller U, Kemenes G. Different phases of long-term memory require distinct temporal patterns of PKA activity after single-trial classical conditioning. Learning & memory. 2008;15:694–702. doi: 10.1101/lm.1088408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a Water-Maze Procedure for Studying Spatial-Learning in the Rat. Journal of neuroscience methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behavioral neuroscience. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M. Protein kinase signal transduction cascades in mammalian associative conditioning. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2002;8:122–131. doi: 10.1177/107385840200800208. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, Raivich G, Cowen M, Strekalova T, Sillaber I, Buters JT, Spanagel R, Hofmann F. Importance of NO/cGMP signalling via cGMP-dependent protein kinase II for controlling emotionality and neurobehavioural effects of alcohol. The European journal of neuroscience. 2004;20:3498–3506. doi: 10.1111/j.1460-9568.2004.03793.x. [DOI] [PubMed] [Google Scholar]