Abstract
Photocrosslinkable biomaterials are promising for tissue engineering applications due to their capacity to be injected and form hydrogels in situ in a minimally invasive manner. Our group recently reported on the development of photocrosslinked alginate hydrogels with controlled biodegradation rates, mechanical properties, and cell adhesive properties. In this study, we present an affinity-based growth factor delivery system by incorporating heparin into photocrosslinkable alginate hydrogels (HP-ALG), which allows for controlled, prolonged release of therapeutic proteins. Heparin modification had minimal effect on the biodegradation profiles, swelling ratios, and elastic moduli of the hydrogels in media. The release profiles of growth factors from this affinity-based platform were sustained for 3 weeks with no initial burst release, and the released growth factors retained their biological activity. Implantation of bone morphogenetic protein-2 (BMP-2)-loaded photocrosslinked alginate hydrogels induced moderate bone formation around the implant periphery. Importantly, BMP-2-loaded photocrosslinked HP-ALG hydrogels induced significantly more osteogenesis than BMP-2-loaded photocrosslinked unmodified alginate hydrogels, with 1.9-fold greater peripheral bone formation and 1.3-fold greater calcium content in the BMP-2-loaded photocrosslinked HP-ALG hydrogels compared to the BMP-2-loaded photocrosslinked unmodified alginate hydrogels after 8 weeks implantation. This sustained and controllable growth factor delivery system, with independently controllable physical and cell adhesive properties, may provide a powerful modality for a variety of therapeutic applications.
Keywords: Alginate hydrogel, Controlled release, Heparin, Affinity binding, BMP-2, Bone formation
1. Introduction
Hydrogels are widely applied in many biomedical applications, such as drug delivery [1–3], cell transplantation [4–6], and tissue engineering [7–10]. Hydrogels are frequently used in an injectable format, which allows them to be administered to a site in a minimally invasive manner. The crosslinking method used to form the hydrogels is important as it should not damage or denature any encapsulated bioactive factors or be toxic to incorporated or surrounding host cells; a wide variety of crosslinking approaches have been explored [11]. Photocrosslinking has emerged as a popular method for crosslinking hydrogels, offering the advantages of the capacity to crosslink the material in situ upon application of UV light and to produce precise structures in two and three dimension using photopatterning [12]. Recently, biodegradable and photocrosslinked hydrogels have been developed [13–15]; hydrogels such as the photocrosslinked alginate and hyaluronic acid can have tunable biodegradation rates and tunable mechanical properties [13,14]. Our group has developed photocrosslinked alginate hydrogels in which the degradation rates and mechanical properties can be controlled by varying the degree of methacrylation of the alginate backbone [13], and the cell adhesive properties of the material can be independently modulated by covalently coupling cell adhesion ligands, such as those containing the Arg-Gly-Asp (RGD) amino acid sequence, to the polymer [16]. However, in spite of the promising capacity to regulate these physical and biochemical biomaterial properties, these hydrogels typically share a similar problem with many other hydrogel systems regarding delivery of small bioactive factors [17,18]: the release of growth factors from the hydrogels is completed within a few days due to rapid diffusion out of the water-swollen network [19–21] and is thus not sustained over a long period of time. For many tissue regeneration applications, the sustained presentation of growth factors may enhance the growth of new tissue, as the cells in the area may require extended exposure to a specific soluble factor in their microenvironment to elicit certain cellular behaviors or morphogenetic events [22]. The native extracellular matrix in which cells reside in the body stores bioactive growth factors and protects them from degradation [23]. The use of hydrogels, which are able to retain growth factors and then locally deliver them to a specific site over a prolonged time period, may mimic this native environment and be beneficial for tissue regeneration. The long-term release of growth factors would allow transplanted cells and cells in tissues adjacent to the hydrogel injection site to be exposed to bioactive growth factors for an extended time.
Several reports have tried to address this issue by introducing growth factor binding ligands to polymer delivery systems [24–26]. Heparin, a highly sulfated glycosaminoglycan, has been used extensively as it is able to bind to many growth factors through affinity interactions [27]. Heparin has been conjugated to natural hydrogels (i.e. fibrin [18], collagen [28], and alginate [29]) and synthetic hydrogels (i.e. poly(ethylene glycol) [30–32] and Pluronic F127 [33]) to elicit the sustained release of heparin-binding growth factors.
Alginate, a naturally derived biocompatible polysaccharide composed of repeating units of α-L guluronic acid and β-D mannuronic acid, has been used in a variety of tissue engineering applications, including for bone [34–36], cartilage [35,37], skin [38,39] and nerve regeneration [40,41]. As a result of its biocompatibility, hydrophilic nature, and ability to form a hydrogel under mild conditions, alginate has great potential as a material for regenerative medicine applications. Several different approaches have been taken to modify alginate systems with heparin. Chitosan-alginate polyelectrolyte scaffolds functionalized with heparin were found to delay the release of fibroblast growth factor-2 (FGF-2), although the majority of the growth factor was released after only 2 days [10]. Heparin has been mixed into alginate prior to making ionically-crosslinked microspheres, and its addition was found to delay the release of a neurotrophin, although again the majority was released within the first couple of days [42]. Alginate and heparin have also been covalently crosslinked by ethylenediamine to form a hydrogel, and the burst release of FGF-2 from these hydrogels was found to be less than that from covalently crosslinked alginate hydrogels without heparin; however these hydro-gels would likely not exhibit biodegradability over time due to the stable amide bond between ethylenediamine and alginate or heparin [29]. Alginate modified with sulfate groups using carbodiimide chemistry exhibits growth factor affinity binding capabilities similar to heparin due to the electrostatic interactions of growth factors with the sulfate groups [43], and when mixed into pre-formed freeze-dried calcium-crosslinked alginate scaffolds was shown to delay the release of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β) substantially [44].
In this study, we present an affinity-based growth factor delivery system using photocrosslinked heparin-alginate (HP-ALG) hydrogels to allow for the controlled, prolonged release of therapeutic proteins. Solutions of methacrylated alginate and methacrylated heparin are mixed together, and during the photopolymerization of the alginate hydrogels, the heparin is covalently bound to the alginate via free radical polymerization, and thus is covalently incorporated throughout the hydrogels. The alginate crosslinks and heparin linkages contain ester groups that are hydrolytically labile, thus permitting the degradation of the hydrogels by hydrolysis. Furthermore, growth factors incorporated into these hydrogels can bind to the heparin via affinity binding, and these bioactive factors can then be released through disassociation from the heparin and subsequent diffusion out of the hydrogels and through hydrolytic degradation of the heparin linkages and alginate crosslinks. Here, we determine if heparin modification has an effect on the biodegradation profiles, swelling ratios, and elastic moduli of the hydrogels and report on the release profiles and bioactivity of growth factors from these materials. We also examine the capacity of BMP-2-loaded HP-ALG hydrogels to enhance bone formation at an ectopic site in mice compared to BMP-2-laden alginate hydrogels without covalently coupled heparin. To our knowledge, this is the first report on an injectable alginate hydrogel system that offers the advantages of tunable physical properties [13], cell adhesive properties [16], and bioactive factor release properties; thus it may prove useful for a variety of therapeutic applications.
2. Materials and methods
2.1. Synthesis of methacrylated heparin
The methacrylated heparin was prepared by reacting heparin (Mw 17,000, Sigma, St. Louis, MO) with 2-aminoethyl methacrylate (AEMA, Sigma). To synthesize the methacrylated heparin with theoretical methacrylation of two carboxylic acid groups, heparin (1 g) was dissolved in a buffer solution (1% w/v, pH 6.5) of 50 mM 2-morpholinoethanesulfonic acid (MES, Sigma) containing 0.5 M NaCl. N-hydroxysuccinimide (NHS, 13.8 mg; Sigma) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC, 45.1 mg; Sigma) (molar ratio of NHS:EDC=1:2) were added to the solution to activate the carboxylic acid groups of the heparin. After 5 min, AEMA (21.7 mg) (molar ratio of NHS:EDC:AEMA=1:2:1) was added to the product and the reaction was maintained at room temperature for 24 h. The mixture was precipitated with the addition of excess acetone, dried under reduced pressure, and rehydrated to a 1% w/v solution in ultrapure deionized water (diH2O) for further purification. The methacrylated heparin was purified by dialysis (MWCO 3500; Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) against diH2O for 3 days, filtered (0.22 μm filter), and lyophilized. To verify the methacrylation of heparin, unmodified and methacrylated heparin were dissolved in deuterium oxide (D2O, Sigma) and placed in separate NMR tubes. The 1H-NMR spectra of the samples were recorded on a Varian Unity-300 (300 MHz) NMR spectrometer (Varian Inc., Palo Alto, CA, USA) using 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid (Sigma) as an internal standard.
2.2. Photocrosslinking for physical characterization of hydrogels
Low molecular weight sodium alginate (37,000 g/mol) was prepared by irradiating Protanal LF 20/40 (196,000 g/mol, FMC Biopolymer, Philadelphia, PA, USA) at a gamma dose of 5 Mrad. Methacrylated alginate (ALG) [13] and RGD-modified methacrylated alginate (RGD-ALG) at a theoretical methacrylation of 45% (25% actual) were prepared as previously reported [16]. To fabricate photocrosslinked HP-ALG hydrogels, methacrylated alginate (0.182 g) and methacrylated heparin (0.018 g) were dissolved in 10 ml of diH2O or Dulbecco’s Modified Eagle Medium (DMEM, Sigma) with 0.05% w/v photoinitiator (Irgacure-2959, Sigma). These solutions were placed between two glass plates separated by 0.75 mm spacers and photocrosslinked with 365 nm UV light (Model ENF-260C, Spectroline, Westbury, NY) at ~1 mW/cm2 for 10 min to form the hydrogels. To fabricate ALG hydrogels without heparin as a comparative group, methacrylated alginate (0.2 g) was dissolved in 10 ml of diH2O or DMEM with 0.05% w/v photoinitiator. These solutions were photocrosslinked as described above. Photocrosslinked hydrogel disks were created using a 6 mm diameter biopsy punch and placed in diH2O or DMEM for mechanical testing, swelling, and degradation studies. To verify the completeness of methacrylated heparin and methacrylated alginate photocrosslinking, methacrylated alginate (0.0182 g) and methacrylated heparin (0.0018 g) were dissolved in D2O (Sigma) with 0.05% w/v photoinitiator, placed in an NMR tube, and photocrosslinked as described above. The 1H-NMR spectra of the HP-ALG before and after crosslinking were then determined as described above.
2.3. Mechanical testing
The elastic moduli of the photocrosslinked HP-ALG or ALG hydrogels formed with diH2O or DMEM were determined by performing constant strain rate compression tests using a Rheometrics Solid Analyzer (RSAII, Rheometrics Inc., Piscataway, NJ, USA) equipped with a 10 N load cell. The photocrosslinked HP-ALG or ALG hydrogel disks were prepared as described above and maintained in DMEM or diH2O at 37 °C. After 24 h incubation in DMEM or diH2O, swollen hydrogel disks were punched once again to form 6 mm diameter disks, their thickness was measured using calipers, and uniaxial, unconfined compression tests were performed on the hydrogel disks at room temperature using a constant strain rate of 5%/s. Elastic moduli of photocrosslinked HP-ALG hydrogels were determined from the slope of stress vs. strain plots, limited to the linear first 5% strain of the plots (N=3 for each condition).
2.4. Swelling and in vitro degradation of HP-ALG hydrogels
The photocrosslinked HP-ALG or ALG hydrogels were lyophilized and initial dry weights (Wi) were measured. Dried hydrogel samples originally formed with diH2O or DMEM were immersed in 50 ml of diH2O or DMEM, respectively, and incubated at 37 °C to reach equilibrium swelling state. The diH2O or DMEM was replaced every week. Over the course of 8 weeks, samples were removed, rinsed with diH2O, and the swollen (Ws) hydrogel sample weights were measured. The swelling ratio (Q) was calculated by Q=Ws/Wi (N=3 for each condition per time point). After weighing the swollen hydrogels, they were lyophilized and weighed (Wd). The percent mass loss was calculated by (Wi−Wd)/Wi ×100 (N=3 for each condition per time point).
2.5. Release kinetics of growth factors
The release kinetics of four different growth factors [FGF-2 (generously provided by the NCI BRB Preclinical Repository), VEGF (generously provided by the NCI BRB Preclinical Repository), TGF-β1 (PeproTech Inc., Rocky Hill, NJ), and BMP-2 (GenScript, Piscataway, NJ)] from HP-ALG, ALG, HP-RGD-ALG, and RGD-ALG hydrogels were determined. ALG (27.3 mg) and methacrylated heparin (2.7 mg) or RGD-ALG (27.3 mg) and methacrylated heparin (2.7 mg) were dissolved in diH2O (1.5 ml) with 0.05% w/v photoinitiator. One of four different growth factors (0.75 μg) was added to the alginate solutions. After gently mixing for 5 min, aliquots (300 μl) of solution were placed in 96-well tissue culture plates and photocrosslinked with 365 nm UV light at ~1 mW/cm2 for 10 min to form the hydrogels. To prepare the ALG or RGD-ALG hydrogels, ALG (30 mg) or RGD-ALG (30 mg) was dissolved in diH2O (1.5 ml) with 0.05% w/v photoinitiator. Each of four different growth factors (0.75 μg) and unmodified heparin (18.75 μg) were added to the methacrylated alginate solution. After gently mixing for 5 min, aliquots (300 μl) of solution were photocrosslinked as described above. Each photocrosslinked hydrogel was then placed in a 15-ml conical tube containing 10 ml phosphate buffered saline (PBS, pH 7.4) and incubated at 37 °C. At predetermined time points over the course of 3 weeks, the supernatant was withdrawn and fresh buffer was replenished. The amount of growth factor in the supernatants was determined with sandwich enzyme-linked immunosorption assay (ELISA) kits (FGF-2, VEGF, and TGF-β1: Duoset, R&D Systems; BMP-2: Human BMP-2 Construction kit, Antigenix America Inc., New York, NY, USA). ELISA plates were coated with capture antibodies according to the manufacturer’s instructions, and were blocked with bovine serum albumin (1 % w/v) and sucrose (5 % w/v) for 1 h. After the appropriately diluted samples were added to the ELISA plates, bound growth factors were detected using biotinylated anti-human antibodies. Then, streptavidin-conjugated horseradish peroxidase was added to the plates. The substrate (tetramethylbenzidine) was subsequently added and incubated for 20 min. The enzyme reaction was stopped by addition of an acidic solution (2 N H2SO4). The absorbance of the samples was read at 450 nm on a plate reader (SAFIRE, Tecan, Austria). The amount of growth factor present in each sample was determined using calibration curves derived using known concentrations of the growth factors (N=3 for each condition per time point).
2.6. Bioactivity assessment of released growth factors in vitro
The bioactivity of VEGF released from HP-ALG hydrogels in vitro was examined by determining its ability to stimulate the proliferation of human umbilical vein endothelial cells (HUVECs, passage number 3; a generous gift from Andrew Putnam, Ph.D.) cultured in endothelial cell basal medium-2 (EBM-2, Cambrex Bio Science Inc., Walkersville, MD, USA) with 2% fetal bovine serum (FBS, Cambrex Bio Science Inc.) in a humidified incubator at 37 °C with 5% CO2. 1 μg of VEGF was loaded into the HP-ALG hydrogels, prepared as in Section 2.5. Cells (1×105 per well) were plated in each well of six-well tissue culture plates in 3 ml EBM-2+2% FBS, and 1 h hour later the VEGF-loaded hydrogels were placed on culture inserts (Transwell®, Corning Incorporated) in each well. The medium was changed every 3 days. At 1, 2, and 3 weeks, cell numbers were measured using a hemacytometer. ALG hydrogels containing 1 μg of VEGF and 25 μg of unmodified heparin in 3 ml EBM-2 with 2% FBS served as a comparative group. An additional comparative group without hydrogels was composed of VEGF (300 ng) with unmodified heparin (7.5 μg) supplied to HUVEC cultures in 3 ml EBM-2 with 2% FBS; these cultures were therefore exposed to 2.1 μg soluble VEGF over the course of 3 weeks. HUVECs cultured without hydrogels in endothelial cell growth medium (EGM-2, Cambrex Bio Science Inc.) or EBM-2 without VEGF served as positive and negative controls, respectively (N=3 for each condition per time point).
The bioactivity of BMP-2 released from HP-ALG hydrogels in vitro was assessed by determining its ability to stimulate the alkaline phosphatase (ALP) activity of MC3T3-E1 Subclone 4 cells (ATCC CRL 2593, Manassas, VA, USA), a mouse preosteoblast cell line, cultured in DMEM containing 10% FBS. 1 μg of BMP-2 was loaded into each photocrosslinked HP-ALG hydrogel, which was formed as in Section 2.5. Cells (3×104) were plated in each well of six-well tissue culture plates with 3 ml DMEM+10% FBS, and 1 h later the BMP-2-loaded hydrogels were placed on culture inserts in each well. The medium was changed every 3 days. BMP-2 (300 ng) mixed with 25 μg unmodified heparin added to MC3T3 cell cultures in 3 ml DMEM with 10% FBS served as a positive control. MC3T3 cells cultured in DMEM with 10% FBS and HP-ALG hydrogels without BMP-2, and DMEM with 10% FBS without either HP-ALG or BMP-2 served as a comparative and a negative control group, respectively (N=3 for each condition per time point). At predetermined time points over 2 weeks, the hydrogels were removed, and the ALP activity of the cells was measured using SensoLyte® pNPP ALP Assay kit (AnaSpec Inc., Fremont, CA) according to manufacturer’s instructions. At each time point, cells were lysed by adding 1 ml lysis buffer solution, and the lysates were cleared by centrifugation for 10 min at 16,200×g using an ultracentrifuge. 50 μl of supernatant was incubated with 50 μl of ALP substrate containing p-nitrophenylphosphate (pNPP) at room temperature for 30 min. The reaction was stopped by adding 50 μl of stop solution to the substrate reaction solution. The absorbance of the samples was read at 405 nm on a plate reader. Each ALP activity measurement was normalized to total sample protein content, which was quantified using a BCA protein assay kit as per the manufacturer’s instructions (Pierce Chemical, Rockford, IL, USA) (N=3 for each condition per time point).
2.7. In vivo bone formation
The capacity of the system to promote in vivo bone formation by host cells was tested. The following conditions were examined: BMP-2 (1 μg/hydrogel disk) loaded in HP-ALG hydrogels, BMP-2 (1 μg/hydrogel disk) mixed with unmodified heparin (25 μg/hydrogel disk) loaded in ALG hydrogels, and HP-ALG hydrogels without BMP-2. SCID mice (ICRSC, 4–5 weeks old; Taconic, city, USA) were anesthetized with xylazine (20 mg/kg) and ketamine (100 mg/kg), 2 small skin incisions were made on the right and left sides of the backs of the mice to create subcutaneous pouches, and photocrosslinked hydrogel disks (diameter=6 mm) were immediately implanted into the pouches (2 implants per mouse, N=10 implants per condition). Subsequently, the skin was closed with 6–0 silk sutures (Ethicon, Lenneke Marelaan, Belgium). Eight weeks after implantation, the mice were sacrificed, and the implants were retrieved. Three implants per condition were used for histomorphometric analysis and another three were used for quantification of total calcium content. The histological specimens were fixed in formalin, embedded in paraffin, sectioned at a thickness of 4 μm, and examined with hematoxylin and eosin (H&E) staining and Goldner’s trichrome staining. The bone formation (%) was calculated using three different Goldner’s trichrome stained images according to the following equation: Bone formation (%)=presence of bone on the perimeter of implant (mm)/perimeter of implant (mm)×100. The amount of calcium deposited in and directly surrounding the implants was measured using a calcium assay kit (Calcium Reagent Set, Pointe Scientific Inc., Canton, MI, USA) according to the manufacturer’s instructions. All animal procedures were carried out in accordance with a protocol approved by the Institutional Animal Care and Usage Committee at Case Western Reserve University.
2.8. Statistical analysis
All quantitative data are expressed as the mean±standard deviation. Statistical analysis was performed with one-way analysis of variance (ANOVA) with Tukey honestly significant difference post hoc test using Origin software (Origin Lab Co., Northampton, MA). A value of p<0.05 was considered statistically significant.
3. Results
3.1. Characterization of synthesized methacrylated heparin and photocrosslinked hydrogels
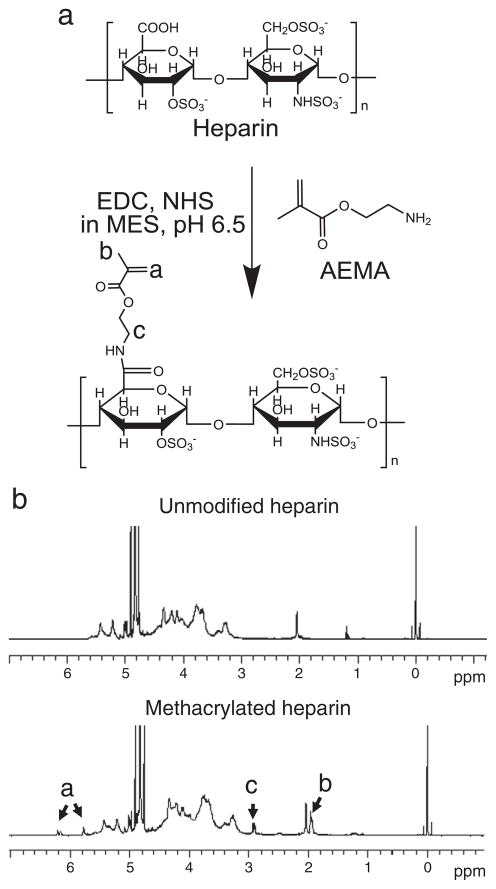
To incorporate heparin into photocrosslinked alginate hydrogels during the photopolymerization process, methacrylates were covalently coupled to the heparin main chains by reacting the carboxylic acid reactive groups of the heparin with the amines of the AEMA to form stable amide linkages using aqueous carbodiimide chemistry (Fig. 1a). The 1H-NMR spectra of methacrylated heparin (Fig. 1b) exhibit new peaks of vinyl methylene, methylene, and methyl protons that were newly formed by the reaction with AEMA, which are located at δ6.2 and 5.7, 2.9, and 1.95, respectively. The spectra demonstrate that the heparin was successfully methacrylated. The efficiency of heparin methacrylation was 70% as calculated from 1H-NMR spectra based on the ratio of the integrals for the internal standard protons to the methylene protons of methacrylate.
Fig. 1.
(a) Schematic illustration of the heparin methacrylation reaction and (b) 1H-NMR spectra of the unmodified and methacrylated heparin in D2O.
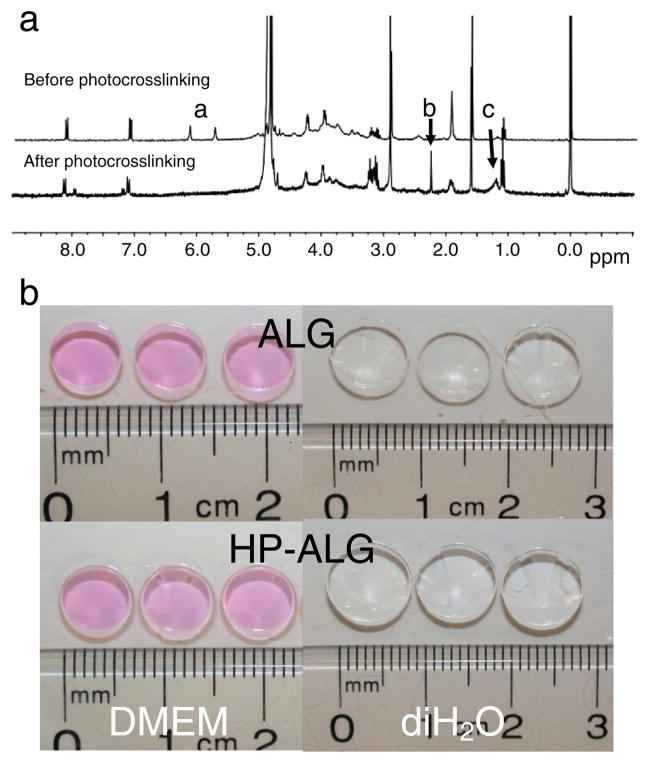
The completeness of photocrosslinking was verified with 1H-NMR. After photocrosslinking of methacrylated alginate and methacrylated heparin, the disappearance of the peaks of vinyl methylene (Fig. 2a, peaks labeled a) and shift of methyl peak to δ1.2 (Fig. 2a, peak labeled c) in the 1H-NMR spectrum indicates the complete reaction of the methacrylate groups. The peak of newly formed methylene proton (Fig. 2a, peak labeled b) following photocrosslinking appears at δ2.25.
Fig. 2.
(a) 1H-NMR spectra of HP-ALG before and after photocrosslinking in D2O. (b) Morphology of photocrosslinked ALG and HP-ALG hydrogel disks after 24 h equilibration in DMEM and diH2O.
Photocrosslinked alginate hydrogel disks (diameter=6 mm) were then formed using ALG or HP-ALG. The gross morphologies of the DMEM or diH2O-equilibrated ALG and HP-ALG hydrogel disks are exhibited in Fig. 2b, their mean diameters are 7.0±0.1 mm and 7.1±0.1 mm in DMEM, and 8.3±0.7 mm and 9.5±0.3 mm in diH2O, respectively. There are no significant differences in gross morphologies or size change between the hydrogel groups after 24 h equilibration in either solution.
3.2. Elastic moduli, swelling kinetics, and degradation of the photocrosslinked hydrogels
To compare mechanical properties of photocrosslinked ALG and HP-ALG hydrogels, constant strain-rate compression tests were performed after 24 h equilibration in DMEM or diH2O. There was no significant difference in compressive elastic modulus between the two hydrogel groups, however the hydrogels were significantly stiffer when equilibrated in diH2O compared to DMEM (Fig. 3a). The swelling ratio of the hydrogels was measured over time in DMEM (Fig. 3b) and diH2O (Fig. 3c) as it reflects changes in their physical and chemical structures. In DMEM both hydrogel conditions exhibited rapid swelling after 24 h, and then slightly increased over the course of 8 weeks. In diH2O both hydrogel conditions displayed rapid swelling after 24 h which was significantly greater than that observed in DMEM. The ALG hydrogels continued to swell to reach a maximum value at 2 weeks, and then the swelling ratio decreased to 0 after 5 weeks. In contrast, the HP-ALG hydrogels continued to swell and at the final time point of 8 weeks exhibited their highest recorded value. Compared to the ALG hydrogels, photocrosslinked HP-ALG hydrogels exhibited slower and decreased swelling kinetics in diH2O over 8 weeks. The mass loss of photocrosslinked hydrogels over time was then determined as a measure of degradation. The mass loss of the photocrosslinked ALG and HP-ALG hydrogels was similar in DMEM (Fig. 3d), indicating minimal impact of methacrylated heparin addition. Both hydrogel conditions lost approximately 20% of their mass by 2 weeks and demonstrated little additional mass loss up to 8 weeks. However, in diH2O, both hydrogel groups exhibited much more rapid degradation, with the photocrosslinked HP-ALG hydrogels losing almost 72% of their mass by 8 weeks and the ALG hydrogels completely degrading by 5 weeks (Fig. 3e). These differences in hydrogel swelling and degradation may be attributable to increased osmotic pressure in diH2O.
Fig. 3.
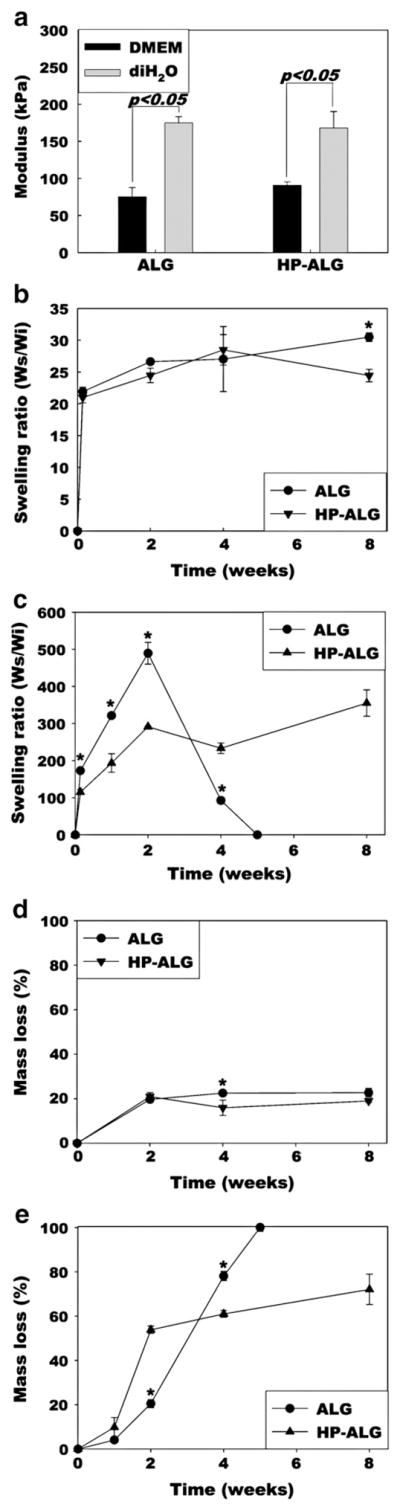
(a) Elastic moduli in compression, (b) swelling ratio in DMEM, (c) swelling ratio in diH2O, (d) in vitro degradation in DMEM, and (e) in vitro degradation in diH2O of photocrosslinked ALG and HP-ALG hydrogels. The values represent mean±standard deviation. *p<0.05 compared to HP-ALG at a specific time point.
3.3. Growth factor release kinetics
To examine whether incorporating heparin into the hydrogels during the UV crosslinking process could delay the release of growth factors through affinity binding interactions, release profiles of 4 different growth factors from photocrosslinked HP-ALG hydrogels in PBS were determined using ELISA assays and compared to those from photocrosslinked ALG hydrogels. The release of growth factors from the ALG hydrogels was more rapid than that from the HP-ALG hydrogels, with 82% to 100% being released within the first 5 to 7 days. In contrast, the release of all 4 growth factors from the photocrosslinked HP-ALG hydrogels was sustained over 3 weeks (Fig. 4).
Fig. 4.
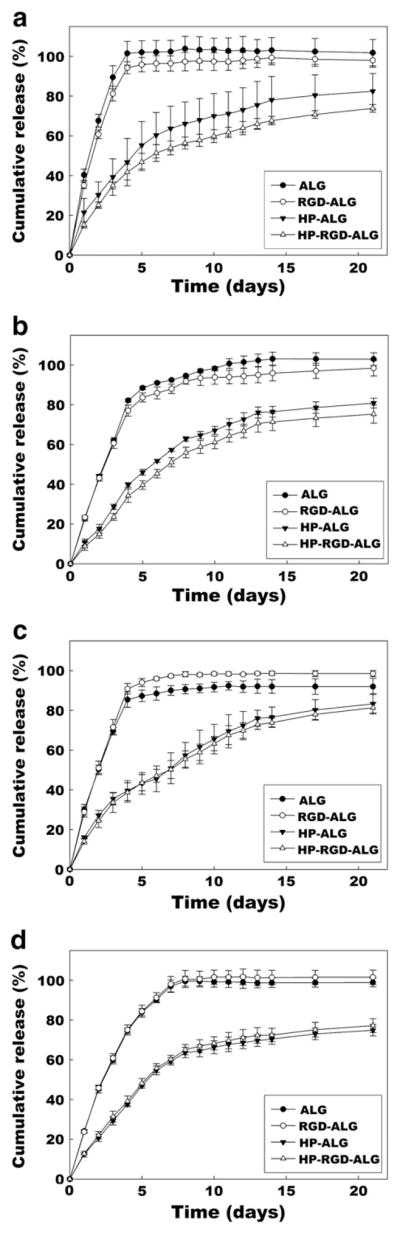
Release profiles of (a) TGF-β1, (b) FGF-2, (c) VEGF, and (d) BMP-2 from ALG, RGD-ALG, HP-ALG, and HP-RGD-ALG hydrogels. The values represent mean±standard deviation.
To ensure that the modification of the alginate with a cell adhesive peptide, GRGDSP, would not significantly influence the release rate of the growth factors, the release profiles of growth factors from photocrosslinked HP-RGD-ALG hydrogels in PBS were determined using ELISA. The release of growth factors from the RGD-ALG hydrogels was more rapid than that from HP-RGD-ALG hydrogel delivery systems. Almost all of the growth factors were released from the RGD-ALG hydrogels within the first 4 to 7 days. In contrast, the releases of growth factors from the HP-RGD-ALG hydrogels were slower and sustained over 3 weeks. However, there was little difference in release profiles when comparing the RGD-containing hydrogels to those without.
3.4. Bioactivity of growth factors released from the photocrosslinked hydrogels
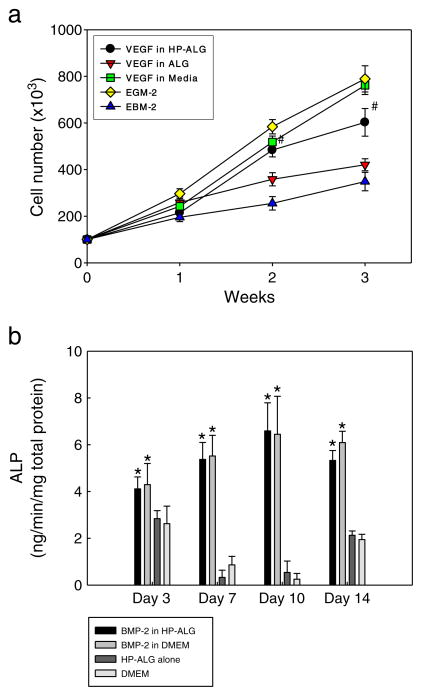
To determine whether growth factors released from the hydrogels remain bioactive, the biological activity of released VEGF was evaluated by measuring its ability to stimulate the proliferation of HUVECs (Fig. 5a). The HUVECs exhibited the lowest cell growth in the basal medium without VEGF. VEGF-loaded in ALG hydrogels promoted significantly increased HUVEC growth compared to the basal medium at 1 week. Moreover, VEGF released from photocrosslinked HP-ALG hydrogels significantly improved HUVEC growth compared to VEGF released from ALG hydrogels or EBM-2 by itself at both 2 and 3 weeks. Impressively, the HUVECs exhibited similar levels of proliferation in response to VEGF released from the HP-ALG hydrogels and to VEGF exogenously supplied in the EBM-2 at 1 and 2 weeks. The HUVECs exhibited greatest proliferation in EGM-2 or EBM-2 supplemented with VEGF at 3 weeks; the cell growth for these conditions at this time point was not significantly different. These results indicate that the VEGF released from photocrosslinked HP-ALG hydrogels was bioactive for up to 3 weeks.
Fig. 5.
The bioactivities of (a) VEGF and (b) BMP-2 released from the delivery systems, as assessed by measuring HUVEC proliferation and ALP activity of MC3T3 preosteoblasts, respectively, cultured in the presence of the delivery systems. The values represent mean±standard deviation. #p<0.05 compared to EGM-2 or VEGF in ALG hydrogel group. *p<0.05 compared to HP-ALG hydrogel alone in DMEM or DMEM group.
The biological activity of the BMP-2 released from the HP-ALG hydrogels was also evaluated by measuring its ability to stimulate the ALP activity of MC3T3 preosteoblasts (Fig. 5b). The ALP activity of MC3T3 cells did not increase over the entire culture period for photocrosslinked HP-ALG hydrogels without BMP-2, and the ALP activity mirrored that measured for cells cultured in DMEM alone. The addition of BMP-2 in a free form to the culture medium significantly enhanced the ALP activity over the entire culture period. The ALP activity resulting from BMP-2 released from photocrosslinked HP-ALG hydrogels was not significantly different from that resulting from exogenous BMP-2 supplementation in the media and was significantly greater than the control conditions without BMP-2 at all time points. This demonstrates that the BMP-2 released from photocrosslinked HP-ALG hydrogels was bioactive for at least 2 weeks.
3.5. Ectopic bone formation
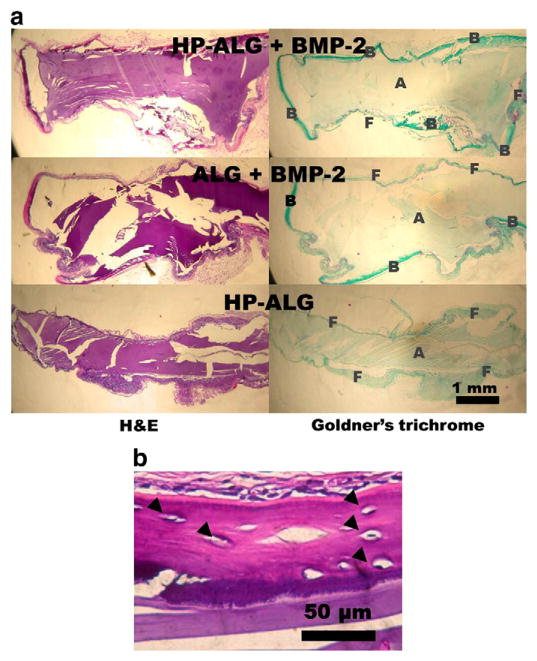
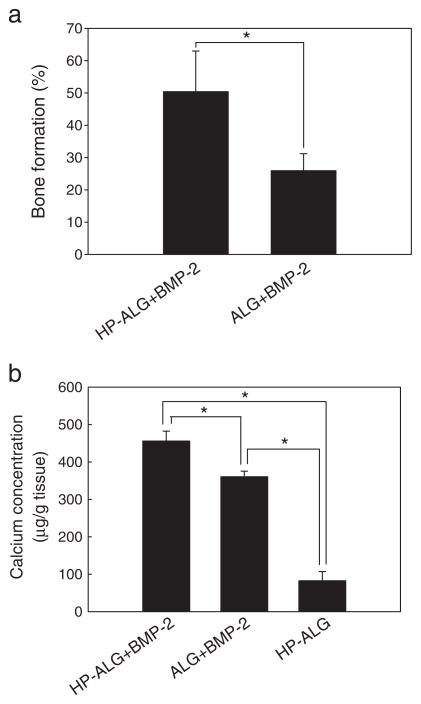
The capacity of sustained delivery of BMP-2 from photocrosslinked HP-ALG hydrogels to enhance ectopic bone formation compared to the delivery from photocrosslinked ALG hydrogels was examined. Histological examination shows no evidence of bone formation surrounding the photocrosslinked HP-ALG hydrogels without BMP-2 after 8 weeks (Fig. 6a). Implantation of BMP-2-loaded ALG hydrogel induced moderate bone formation around the hydrogel periphery (26%). Importantly, BMP-2-loaded photocrosslinked HP-ALG hydrogels induced peripheral bone formation to a much greater extent (1.9-fold higher) than did BMP-2-loaded photocrosslinked ALG hydrogels, with more than 50% of the constructs lined with new bone tissue.
Fig. 6.
(a) Photomicrographs of H&E and Goldner’s trichrome-stained histological sections of photocrosslinked alginate implants at 8 weeks. All photographs were taken at the same magnification. A: alginate hydrogel; B: bone; F: fibrous tissue (b) A photomicrograph at higher magnification of an H&E stained BMP-2-loaded HP-ALG implant at 8 weeks showing newly formed bone tissue surrounding the construct. Osteocytes are shown within lacunae (closed triangles).
Peripheral bone formation and calcium content in the implants were 1.9-fold and 1.3-fold higher, respectively, in the BMP-2-loaded photocrosslinked HP-ALG hydrogels compared to the BMP-2-loaded photocrosslinked ALG hydrogels after 8 weeks implantation (Fig. 7). The control photocrosslinked HP-ALG hydrogels without BMP-2 had significantly less calcium content than the 2 experimental groups releasing BMP-2.
Fig. 7.
Quantification of (a) bone formation surrounding and (b) calcium concentration in the constructs after 8 weeks implantation. The values represent mean±standard deviation. *p<0.05, bone formation (%)=presence of bone on the perimeter of implant (mm)/perimeter of implant (mm)×100.
4. Discussion
There is great need for the development of a drug delivery system for the sustained and controlled delivery of growth factors to increase the therapeutic efficacy of these growth factors [22]. Release of growth factors over a long period may not be expected from hydrogels, as they are water-swollen polymer networks frequently consisting of more than 90% water, and the growth factor release rate is generally controlled by diffusion through aqueous channels within hydrogels [45]. Recently, affinity-based drug delivery systems using heparin-functionalized hydrogels have been investigated [18,30,46], because heparin’s interactions with growth factors have permitted their controlled release [17,46]. In this study, we present an affinity-based growth factor delivery system using photocrosslinked HP-ALG hydrogels, which allow for controlled, prolonged release of therapeutic proteins such as FGF-2, VEGF, TGF-β1, and BMP-2.
The release of growth factors can be sustained by the introduction of heparin due to affinity binding between heparin and growth factors, via electrostatic interactions between heparin’s negatively charged sulfate groups and the proteins’ positively charged amino acid groups. Furthermore, all of the growth factors released from these hydrogels are bioactive and able to influence the behavior of cells. Additionally, it was demonstrated that the covalent modification of the HP-ALG with a cell adhesive peptide containing the RGD sequence had minimal influence on the release rate of these growth factors. Thus, these hydrogels could be modified to allow for cell adhesion without influencing the function and benefit of the covalently bound heparin.
BMP-2 typically has a short half-life in vivo, but heparin-bound BMP-2 may have increased stability and a prolonged half-life [47,48]. The delivery of BMP-2 from these photocrosslinked HP-ALG hydrogels resulted in greater ectopic bone formation in mice. This is likely due to the maintenance of BMP-2 bioactivity and the long-term release of BMP-2 from the photocrosslinked HP-ALG hydrogels. Ectopic bone formation seen with this system likely results from the osteogenic differentiation of circulating osteoblast progenitor or stem cells when exposed to the BMP-2 near the implant. The long-term release of BMP-2 would stimulate this osteogenic differentiation for an extended time period around the implant, thus further promoting bone regeneration in the area.
Photocrosslinked HP-ALG hydrogels have several advantages as growth factor delivery matrices for clinical use. Photocrosslinked HP-ALG hydrogels are biodegradable, and their degradation rate may be designed to match the rate of new tissue formation. Since transdermal UV illumination has been shown to be feasible for in situ hydrogel photocrosslinking [49], polymer solutions could be easily placed at the defect site in a minimally invasive manner by injection and then crosslinked transdermally or potentially with a UV fiber optic cable, which could obviate the need for open surgical treatment and reduce patients’ pain. The covalent modification of ALG with heparin does not substantially influence its physical properties such as swelling, degradation, and mechanical properties when the hydrogels are in media. Furthermore, we hypothesize that the release rate of growth factors from photocrosslinked HP-ALG hydrogels could be controlled by varying the concentration of heparin and/or alginate in the photocrosslinked HP-ALG hydrogels, the degree of heparin and/or alginate methacrylation, and the molecular weight of the alginate. Additionally, this system could be applied to deliver multiple growth factors that have affinities for heparin, such as VEGF, FGF-2, PDGF, EGF, TGF-β, and BMP-2, which when delivered together in a sustained manner could synergistically enhance tissue regeneration.
5. Conclusion
In this study, we have demonstrated the first naturally-derived biomaterial with independently controllable physical properties [13], cell adhesive properties [16], and bioactive growth factor delivery capacity. The photocrosslinked HP-ALG hydrogels released the heparin-binding growth factors in a sustained manner, and the growth factors were shown to retain their biological activity. Importantly, BMP-2 delivery using photocrosslinked HP-ALG hydrogels enhanced bone formation, compared to BMP-2 delivered from photocrosslinked ALG hydrogels. The photocrosslinked HP-ALG hydrogel system developed in this study offers great potential for use in bioactive factor delivery for tissue engineering and regenerative medicine.
Acknowledgments
The authors gratefully acknowledge funding support for this work from Biomedical Research and Technology Transfer Grant 08–081 from the Ohio Department of Development, the Ellison Medical Foundation, and the Musculoskeletal Transplant Foundation.
References
- 1.Chen MC, Tsai HW, Liu CT, Peng SF, Lai WY, Chen SJ, Chang Y, Sung HW. A nanoscale drug-entrapment strategy for hydrogel-based systems for the delivery of poorly soluble drugs. Biomaterials. 2009;30:2102–2111. doi: 10.1016/j.biomaterials.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–2232. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 3.des Rieux A, Ucakar B, Mupendwa BP, Colau D, Feron O, Carmeliet P, Preat V. 3D systems delivering VEGF to promote angiogenesis for tissue engineering. J Control Release. 2011;150:272–278. doi: 10.1016/j.jconrel.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Lee WY, Chang YH, Yeh YC, Chen CH, Lin KM, Huang CC, Chang Y, Sung HW. The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials. 2009;30:5505–5513. doi: 10.1016/j.biomaterials.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Jeon O, Kang SW, Lim HW, Choi D, Kim DI, Lee SH, Chung JH, Kim BS. Synergistic effect of sustained delivery of basic fibroblast growth factor and bone marrow mononuclear cell transplantation on angiogenesis in mouse ischemic limbs. Biomaterials. 2006;27:1617–1625. doi: 10.1016/j.biomaterials.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Orive G, De Castro M, Kong HJ, Hernandez RM, Ponce S, Mooney DJ, Pedraz JL. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J Control Release. 2009;135:203–210. doi: 10.1016/j.jconrel.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Jukes JM, van der Aa LJ, Hiemstra C, van Veen T, Dijkstra PJ, Zhong Z, Feijen J, van Blitterswijk CA, de Boer J. A newly developed chemically crosslinked dextran-poly(ethylene glycol) hydrogel for cartilage tissue engineering. Tissue Eng Part A. 2010;16:565–573. doi: 10.1089/ten.TEA.2009.0173. [DOI] [PubMed] [Google Scholar]
- 8.Kim HK, Shim WS, Kim SE, Lee KH, Kang E, Kim JH, Kim K, Kwon IC, Lee DS. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A. 2009;15:923–933. doi: 10.1089/ten.tea.2007.0407. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JS, Yang HJ, Kim BS, Kim JD, Kim JY, Yoo B, Park K, Lee HY, Cho YW. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2–7. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54:13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 12.Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355:1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Leach JB, Bivens KA, Patrick CW, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 15.Pitarresi G, Casadei MA, Mandracchia D, Paolicelli P, Palumbo FS, Giammona G. Photocrosslinking of dextran and polyaspartamide derivatives: a combination suitable for colon-specific drug delivery. J Control Release. 2007;119:328–338. doi: 10.1016/j.jconrel.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Jeon O, Powell C, Ahmed SM, Alsberg E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng Part A. 2010;16:2915–2925. doi: 10.1089/ten.TEA.2010.0096. [DOI] [PubMed] [Google Scholar]
- 17.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105:249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 19.Zawko SA, Truong Q, Schmidt CE. Drug-binding hydrogels of hyaluronic acid functionalized with beta-cyclodextrin. J Biomed Mater Res A. 2008;87A:1044–1052. doi: 10.1002/jbm.a.31845. [DOI] [PubMed] [Google Scholar]
- 20.Obara K, Ishihara M, Ozeki Y, Ishizuka T, Hayashi T, Nakamura S, Saito Y, Yura H, Matsui T, Hattori H, Takase B, Ishihara M, Kikuchi M, Maehara T. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J Control Release. 2005;110:79–89. doi: 10.1016/j.jconrel.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–S15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 23.Uebersax L, Merkle HP, Meinel L. Biopolymer-based growth factor delivery for tissue repair: from natural concepts to engineered systems. Tissue Eng Part B Rev. 2009;15:263–289. doi: 10.1089/ten.TEB.2008.0668. [DOI] [PubMed] [Google Scholar]
- 24.Maynard HD, Hubbell JA. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 2005;1:451–459. doi: 10.1016/j.actbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang AY, Leong S, Liang YC, Huang RC, Chen CS, Yu SM. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell DJ, Hicks BC, Parsons S, Sakiyama-Elbert SE. Development of rationally designed affinity-based drug delivery systems. Acta Biomater. 2005;1:101–113. doi: 10.1016/j.actbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin Drug Deliv. 2008;5:1173–1184. doi: 10.1517/17425240802431811. [DOI] [PubMed] [Google Scholar]
- 28.Wu JM, Xu YY, Li ZH, Yuan XY, Wang PF, Zhang XZ, Liu YQ, Guan J, Guo Y, Li RX, Zhang H. Heparin-functionalized collagen matrices with controlled release of basic fibroblast growth factor. J Mater Sci Mater Med. 2011;22:107–114. doi: 10.1007/s10856-010-4176-4. [DOI] [PubMed] [Google Scholar]
- 29.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216–221. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie T, Akins RE, Jr, Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomaterialia. 2009;5:865–875. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CC, Boyer PD, Aimetti AA, Anseth KS. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J Control Release. 2010;142:384–391. doi: 10.1016/j.jconrel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon JJ, Chung HJ, Park TG. Photo-crosslinkable and biodegradable pluronic/heparin hydrogels for local and sustained delivery of angiogenic growth factor. J Biomed Mater Res A. 2007;83:597–605. doi: 10.1002/jbm.a.31271. [DOI] [PubMed] [Google Scholar]
- 34.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 35.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci U S A. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92:1131–1138. doi: 10.1002/jbm.a.32441. [DOI] [PubMed] [Google Scholar]
- 37.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25:1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Lee WR, Park JH, Kim KH, Kim SJ, Park DH, Chae MH, Suh SH, Jeong SW, Park KK. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen. 2009;17:505–510. doi: 10.1111/j.1524-475X.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 40.Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 41.Mosahebi A, Simon M, Wiberg M, Terenghi G. A novel use of alginate hydrogel as Schwann cell matrix. Tissue Eng. 2001;7:525–534. doi: 10.1089/107632701753213156. [DOI] [PubMed] [Google Scholar]
- 42.Noushi F, Richardson RT, Hardman J, Clark G, O’Leary S. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol. 2005;26:528–533. doi: 10.1097/01.mao.0000169780.84588.a5. [DOI] [PubMed] [Google Scholar]
- 43.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Kim JH, Jeon O, Kwon IC, Park K. Engineered polymers for advanced drug delivery. Eur J Pharm Biopharm. 2009;71:420–430. doi: 10.1016/j.ejpb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 47.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–43235. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 48.Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, Chung UI, Koike T, Takaoka K, Kamijo R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281:23246–23253. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 49.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci USA. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]