Abstract
The neural crest arises at the border between the neural plate and the adjacent non-neural ectoderm. It has been suggested that both neural and non-neural ectoderm can contribute to the neural crest. Several studies have examined the molecular mechanisms that regulate neural crest induction in neuralized tissues or the neural plate border. Here, using the chick as a model system, we address the molecular mechanisms by which non-neural ectoderm generates neural crest. We report that in response to FGF the non-neural ectoderm can ectopically express several early neural crest markers (Pax7, Msx1, Dlx5, Sox9, FoxD3, Snail2, and Sox10). Importantly this response to FGF signaling can occur without inducing ectopic mesodermal tissues. Furthermore, the non-neural ectoderm responds to FGF by expressing the prospective neural marker Sox3, but it does not express definitive markers of neural or anterior neural (Sox2 and Otx2) tissues. These results suggest that the non-neural ectoderm can launch the neural crest program in the absence of mesoderm, without acquiring definitive neural character. Finally, we report that prior to the upregulation of these neural crest markers, the non-neural ectoderm upregulates both BMP and Wnt molecules in response to FGF. Our results provide the first effort to understand the molecular events leading to neural crest development via the non-neural ectoderm in amniotes and present a distinct response to FGF signaling.
Keywords: Neural crest, FGF, non-neural ectoderm, induction, Pax7
Introduction
The neural crest (NC) is a multipotent cell population that arises at the border of the neural plate (NP) and non-neural ectoderm (NNE) during early vertebrate development. NC cells delaminate from the NP border (NPB) after undergoing an epithelial-to-mesenchymal transition and migrate along stereotypic pathways throughout the whole vertebrate body, contributing to a varied array of derivates. NC cells produce the majority of the neurons and glia of the peripheral nervous system and melanocytes of the skin. Additionally, the cranial NC contributes to craniofacial bone and cartilage, smooth muscle, adipose tissue, and tooth-forming odontoblasts (as well as other derivatives) (Le Douarin and Kalcheim, 1999). NC occupy a prominent place in human biology because defects in their development lead to a wide range of health conditions and diseases such as cleft palate, Waardenburg's syndrome, and neuroblastoma, amongst many others (Bolande, 1997; Etchevers et al., 2006; Farlie et al., 2004)
In the last 20 years considerable progress has been gained regarding the molecular mechanisms underlying NC development. Interactions among signaling pathways, are thought to launch NC development via the induction of specific transcription factors at the border of the NP, referred to as border specifiers (Msx1/2, Dlx3/5, Pax3, and Pax7). The border specifiers, along with additional signaling, trigger the expression of later transcription factors known as NC specifiers (FoxD3, Snail2, Sox9, and Sox10) which further control the expression of NC-effector genes responsible for the migration and differentiation of NC cells (reviewed by Betancur et al., 2010; and Meulemans and Bronner-Fraser, 2005; and Stuhlmiller and García-Castro, 2012a).
In terms of inductive signaling, Bone Morphogenetic Proteins (BMPs), Fibroblast Growth Factors (FGFs) and Wnt molecules have dominated the focus of NC induction studies. A requirement for BMP molecules in this process has been supported through expression and functional studies in avian embryos (Liem et al., 1995; Selleck et al., 1998; Streit and Stern, 1999). Instead in Xenopus, assays of NC induction required BMP inhibition, and a threshold of BMP signaling was suggested to control NC development (Marchant et al., 1998; Tribulo et al., 2003). In zebrafish, however, loss of three BMP inhibitors does not prevent NC formation (Ragland and Raible, 2004). Wnt molecules are also thought to be critical for NC formation. In frogs, neuralized animal caps will express NC markers when treated with Wnts, and ectopic application of Wnts enhances NC marker expression in the NPB (Chang and HemmatiBrivanlou, 1998; Hong et al., 2008; Hong and Saint-Jeannet, 2007; LaBonne and Bronner-Fraser, 1998; Monsoro-Burq et al., 2005; Saint-Jeannet et al., 1997; Sasai et al., 2001). Conversely, inactivation of Wnt signaling prevents NC formation at the NPB in Xenopus (Abu-Elmagd et al., 2006; Carmona-Fontaine et al., 2007; Deardorff et al., 2001; Heeg-Truesdell and LaBonne, 2006; Hong et al., 2008; LaBonne and Bronner-Fraser, 1998; Monsoro-Burq et al., 2005; Steventon et al., 2009). In chick, Wnts are sufficient to induce NC formation (García-Castro et al., 2002; Patthey et al., 2009), and they have been proposed to be necessary, as Wnt antagonists applied to the prospective NPB (in vivo and in vitro) prevent expression of NC markers such as Snail2 (Basch et al., 2006; García-Castro et al., 2002; Litsiou et al., 2005; Patthey et al., 2009; Patthey et al., 2008; Schmidt et al., 2007). While most evidence suggests an involvement of the Wnt/β-catenin pathway on NC induction, reports from both Xenopus and chick embryos also support the participations of non-canonical Wnts (Ossipova and Sokol, 2011; Schmidt et al., 2007).
FGFs are also important during NC induction, although the exact mechanism appears to differ between model organisms. Several studies in Xenopus have shown that FGFs, specifically Fgf8, can trigger NC induction (Hong and Saint-Jeannet, 2007; LaBonne and Bronner-Fraser, 1998; Mayor et al., 1997; Mayor et al., 1995; Monsoro-Burq et al., 2003; Monsoro-Burq et al., 2005; Villanueva et al., 2002). It has been suggested that Fgf8 acts indirectly, by activating expression of Wnt8 in the mesoderm, which then induces NC in the overlaying ectoderm (Hong et al., 2008). However, recent evidence from Xenopus suggests that FGFs can act directly on NC formation through activation of the Stat pathway (Nichane et al., 2010). In chick, recent evidence shows that FGF activity is required in the ectoderm during gastrulation, as a targeted blockade of FGF signaling at the NPB prevents expression of NC markers (Stuhlmiller and García-Castro, 2012b).
Our current understanding of the relationship between these three pathways in chick can be summarized in a two-step process characterized by an initial phase of FGF and Wnt signaling and low or negative BMP signaling, followed by a second phase where Wnt and BMP activation are required to sustain NC development (Patthey et al., 2009; Stuhlmiller and García-Castro, 2012a ; Stuhlmiller and García-Castro, 2012b).
NC precursors appear in the region between the NP and the NNE, underlain by mesoderm, and these tissues have been proposed to be the source of inductive signals for NC formation. Studies in axolotl, Xenopus, and chick embryos have shown that juxtaposition of mesoderm and ectoderm induces NC (Mitani and Okamoto, 1991; Raven and Kloos, 1945; Selleck and Bronner-Fraser, 1995), yet evidence from zebrafish and Xenopus suggest mesoderm is dispensable for NC formation (Ragland and Raible, 2004; Wu et al., 2011). Experiments involving the juxtaposition of the NP and NNE have highlighted the relevance of interactions between these two during NC induction in amphibians (Mancilla and Mayor, 1996; Moury and Jacobson, 1990; Rollhäuser-ter Horst, 1979, 1980) and avians alike (Basch et al., 2000; Dickinson et al., 1995; Liem et al., 1995; Ruffins and Bronner-Fraser, 2000; Selleck and Bronner-Fraser, 1995; Streit and Stern, 1999). Collectively these studies support a model of classic induction in which the NNE signals to the NP which responds by generating NC.
Importantly, results from both Xenopus and chick suggest that the NNE is capable of producing NC. Experiments in Xenopus using animal caps show that NC are induced in response to BMP inhibition (Marchant et al., 1998; Tribulo et al., 2003), FGF activation (Monsoro-Burq et al., 2003), or the combination of BMP inhibition with Wnt or FGF activation (Chang and Hemmati-Brivanlou, 1998; Hong et al., 2008; Hong and Saint-Jeannet, 2007; LaBonne and Bronner-Fraser, 1998; Monsoro-Burq et al., 2005; Saint-Jeannet et al., 1997; Sasai et al., 2001). However some controversy exists over whether animal caps truly represent naïve ectoderm (Knecht et al., 1995; Lamb et al., 1993; Wills et al., 2010) or whether they actually represent a pro-neural state (Dale and Slack, 1987; Gamse and Sive, 2001; Linker et al., 2009; Sagerström et al., 2005). Experiments using fluorescently labeled grafts of NP into NNE of Xenopus embryos suggest that both NP and NNE can contribute to NC (Mancilla and Mayor, 1996). In the chick, NNE-NP juxtapositions have shown that NC markers and migratory NC can be found in cells derived from both NP and NNE tissue (Ruffins and Bronner-Fraser, 2000; Selleck and Bronner-Fraser, 1995; Streit and Stern, 1999). It was suggested that after contact with the NP, the NNE could directly form NC, or become neuralized first and then respond to adjacent NNE to make NC, or that perhaps the NP responded to NNE by generating cells of undefined identity which secondarily induced NNE to form NC (Selleck and Bronner-Fraser, 1995). Yet to date these possibilities have remained untested, and we do not know how the avian NNE generates NC.
To address the molecular mechanisms by which NNE generates NC in the chick we applied signaling molecules associated with NC development via bead implantation in the anterior NNE of early chick embryos and monitored NC induction. We report that FGF4 is sufficient to induce ectopic expression of early NPB markers (Pax7, Msx1, and Dlx5) as well as later NC markers (Sox9, FoxD3, Snail2, and Sox10), indicating a recapitulation of the NC developmental program in the NNE. While FGF4 is able to induce mesoderm, which could cause a secondary, indirect induction of NC in the NNE, we consistently observed ectopic NC independent of mesoderm formation. Furthermore, Sox2 (a definitive neural marker in chick) was not induced; instead the prospective neural maker Sox3 was often induced, suggesting that an incomplete neuralization of the ectopic tissue may accompany NC induction. Finally, we demonstrate that NNE tissue upregulates both Bmp4 and Wnt8c expression in response to FGF4 application prior to launching the expression of NC markers. Our work demonstrates the capacity of NNE to generate NC in the absence of mesoderm induction and without acquiring a definitive neural character. Furthermore, the FGF-induced upregulation of both BMP and Wnt ligands suggests a putative role for FGF in coordinating both pathways during NC formation.
Materials and Methods
Embryos and Bead Implantation
Fertile hen eggs were obtained from Hardy’s Hatchery (Massachusetts, USA). Embryos were staged according to Hamburger and Hamilton (1951). Approximately 50 to 100 Affi-Gel Blue beads (Bio-Rad) were coated with a mixture of molecules suspended in 1x PBS and 0.1% BSA (w/v). Beads were incubated in the mixture for one hour at room temperature, and subsequently kept at 4°C or on ice. Prior to implantation, beads were rinsed briefly in 1x PBS and 0.1% BSA. Beads were then implanted under the anterior hypoblast of St. 3–4 chick embryos, at the border of the area pellucida (Figure 1A). Embryos were cultured at 38°C for 16–20 hours in EC culture (Chapman et al., 2001). Recombinant proteins were used to coat beads; concentrations and sources are as follows: mouse Chordin (5µg/ml, R&D); mouse Noggin (5µg/ml, R&D); human WIF (10µg/ml, R&D); human Frizzle-5/FC (10µg/ml, R&D); human FGF12 (25µg/ml, R&D); human FGF4 (0.125, 0.625, 1.25 or 2.5µg/ml, R&D); mouse Wnt3a (10µg/ml, R&D); and Wnt agonist (5µM, Calbiochem #681665). Bioactivity of mouse Wnt3a and Wnt agonist were demonstrated by their ability to promote survival and proliferation in human embryonic stem cells and their ability to regulate gene expression (data not shown. N2 supplement (Gibco) was also included within certain mixes (see below).
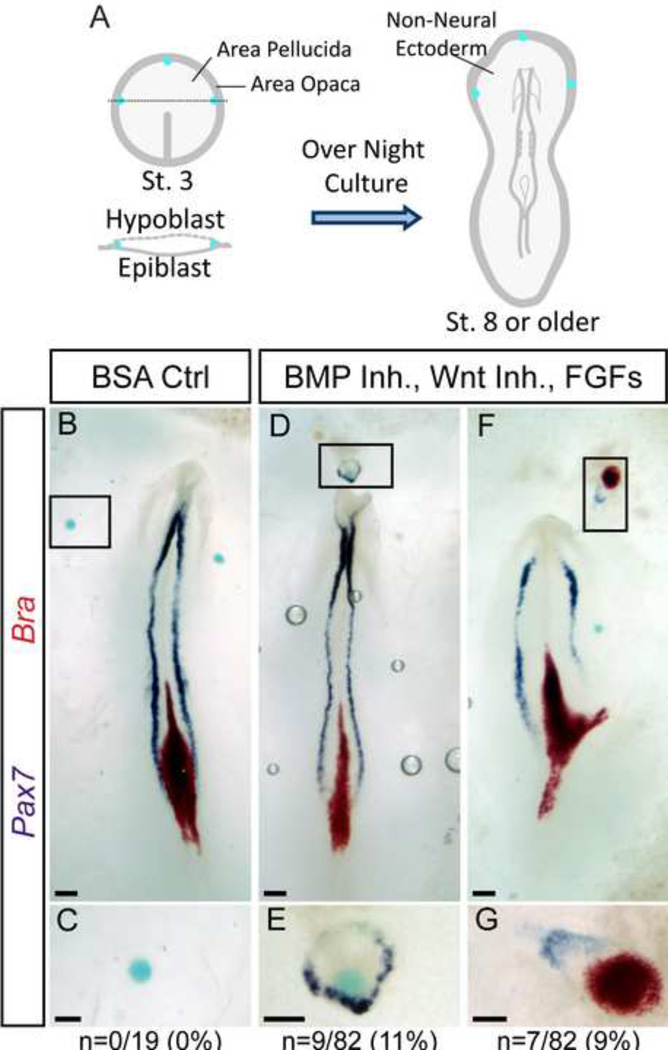
Figure 1. A mix of BMP inhibitors, Wnt inhibitors, and FGFs induce the expression of Pax7.
A) Affigel blue beads coated with molecules are inserted under the hypoblast at the area pellucida/area opaca border of gastrula stage embryos. After overnight culture, embryos are assessed for marker expression. B–C) Control, BSA-coated beads do not induce expression of Pax7 or Brachyury (Bra). D–G) Beads coated with Chordin, Noggin, WIF, Fz5-Fc, FGF12, FGF4 and N2 media supplement induce ectopic expression of Pax7 independent of Bra (D–E) or in combination with Bra in the non-neural ectoderm (F–G). Boxed regions in B, D, and F indicate magnified regions shown in C, E, and G. Total number of samples showing any ectopic expression of Pax7 alone (C, E) or combined Pax7 and Bra (G) are indicated (“n”). Scale bars are 200µm (B, D, F) and 100µm (C, E, G).
In Situ Hybridization and Immunofluorescence
Whole-mount in situ hybridization and immunofluorescence was performed as previously described (Basch et al., 2006). The in situ probe for cBrachyury (bp707–1139, NCBI Reference Sequence: NM_204940) was cloned into pBlu2SK+ through the addition of exogenous 5’ XhoI and 3’ XbaI sites. The in situ probe for cMsx1 (bp639–1228, NCBI Reference Sequence: NM_205488.2) was cloned into pBlu2SK+ through the addition of exogenous 5’ BamHI and 3’ PstI sites. The in situ probes for cDlx5 (bp582–1175, NCBI Reference Sequence: NM_204159.1) and cFoxD3 (bp955–1406, NCBI Reference Sequence: NM_204951.1) were cloned into pBlu2SK+ through the addition of exogenous 5’ EcoRI and 3’ BamHI sites. The cPax7 and cSox9 probe have been previously described (Basch et al., 2006). The probe for cOtx2 (chEST291J16) was obtained from ARK-Genomics. Other probes were gifts from the following sources: cSox2 (A. Groves), cBmp4 (O. Pourquie), cWnt8c (J. Dodd), and cSox3 (H. Kondoh).
Primary antibodies were diluted as follows: 1:25 Pax7 (mIgG1, Developmental Studies Hybridoma Bank), 1:100 Bra (gtIgG, R&D #AF2085), 1:1500 Snail2 (rIgG, Cell Signaling #C19G7), 1:2000 Sox10 (rbIgG, a generous gifts from the laboratory of Vivian Lee, Medical College of Wisconsin). All secondary antibodies (Alexa 488 or 568, Invitrogen) were used at 1:2000.
Images for in situ hybridization or immunofluorescence were acquired using a SPOT SE camera and SPOT software (version 4.5) and either a Nikon Eclipse 80i or Nikon SMZ1500 microscope; images were processed in Adobe Photoshop. Exposure time for Figure 6I and 6L were the same, no subsequent adjustments were made. Exposure time for Figure 7I and 7L were the same, levels in both images were adjusted exactly the same using Adobe Photoshop.
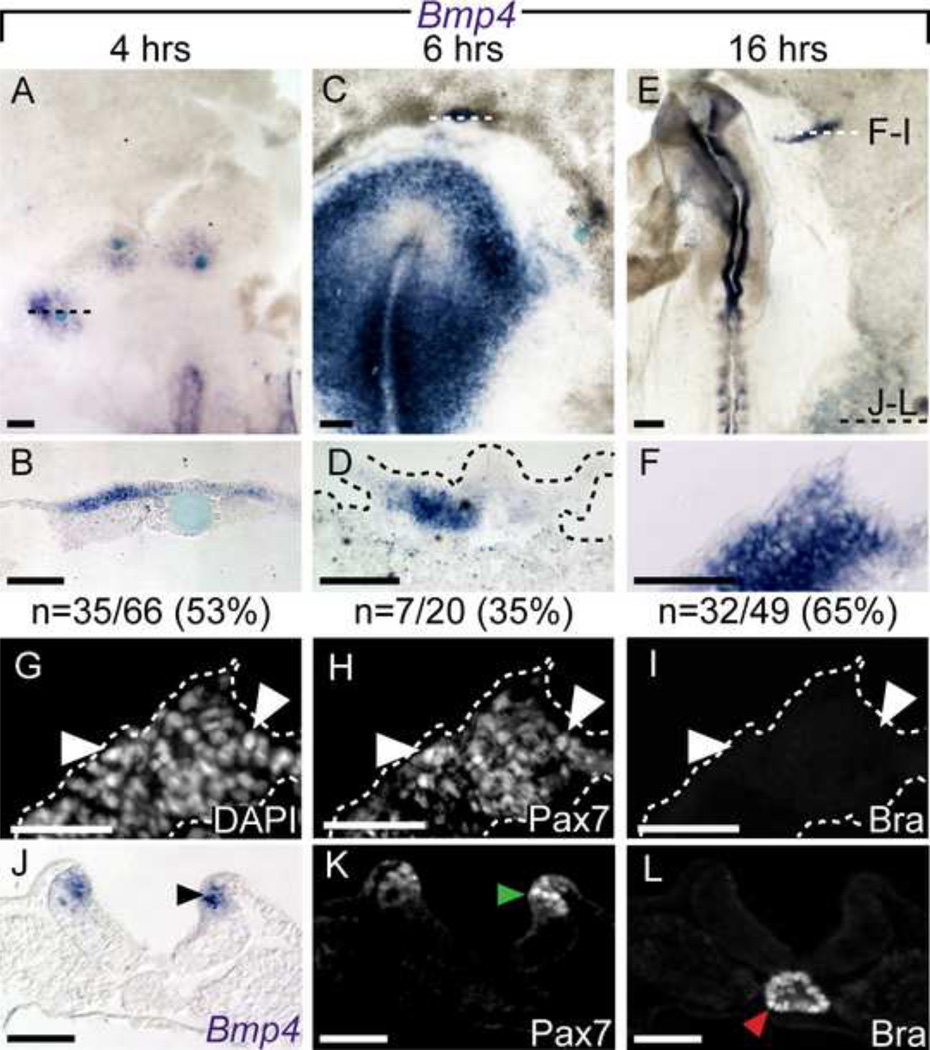
Figure 6. Bmp4 expression is upregulated by FGF4.
Using in situ hybridization, Bmp4 can be detected as early at 4 hours (A, B), 6 hours (C, D) and 16 hours (E, F) after FGF4 application. Dashed lines in A, C, and D indicate the plane of sections shown below. Further analysis of panel F displaying DAPI nuclear staining (G), Pax7+ (H arrowheads) and Bra− (I) immunofluorescence. A section from caudal region of embryo E displays endogenous signal for BMP4 (J, black arrowhead) and Pax7 (K, green arrowhead) in the neural folds, and Bra (L, red arrowhead) in the notochord. Scale bars are 200µm (A, C, E), 100µm (B, D, F) and 50µm (G–L).
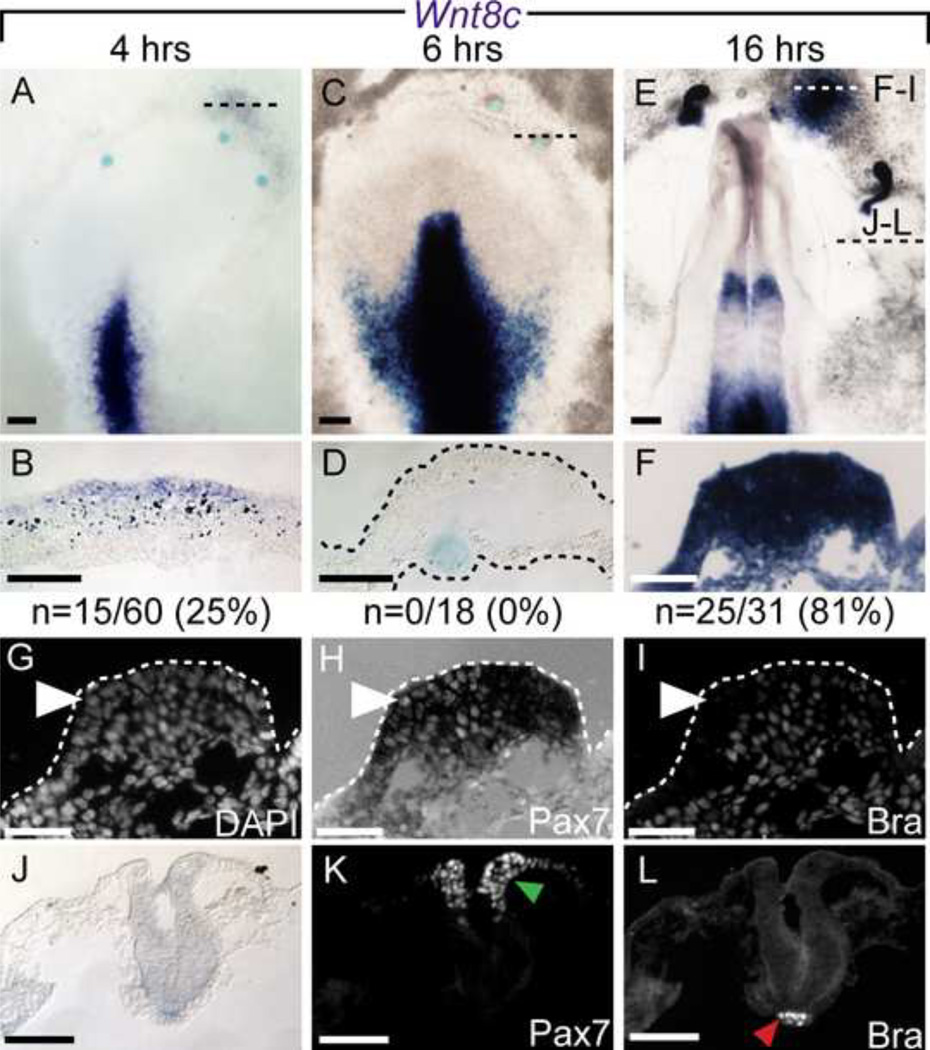
Figure 7. Wnt8c expression is upregulated by FGF4.
Wnt8c mRNA dynamic expression after FGF4 induction was detected 4 hours (A–B), and 16 hours (E–F) after FGF4 application, but not after 6 hours of application (C–D). Dashed lines in A, C and E indicate the plane of section shown below. Further analysis of panel F displaying DAPI (G), Pax7 (H), and Bra (I) immunofluorescent signal. While both markers were induced, instances of Pax7+/Bra-negative cells are indicated (arrowhead). J–L) Midbrain level section of E, showing endogenous Pax7 (K green arrowhead) and Bra (L, red arrowhead) signal. Scale bars are 200µm (A, C, E), 100µm (B, D) and 50µm (F–L).
For sectioning, embryos were mounted in gelatin and sectioned at 12µm using a Leica CM1900 Cryostat. Sections were mounted with Permaflour (Thermo Scientific), with or without the addition of DAPI (10µg/ml).
Results
Combined BMP inhibition, Wnt inhibition and FGF signaling induce Pax7 in the NNE
To better understand what signals are sufficient to convert the NNE to NC, an in vivo bead implantation strategy was employed. Beads coated with a mixture of signaling molecules were implanted under the hypoblast at the anterior border of the area pellucida of gastrulating chick embryos (St. 3–4, (Hamburger and Hamilton, 1951)). Embryos were cultured overnight (16–20 hours) and subsequently analyzed for markers via in situ hybridization (ISH) (Figure 1A).
Previous experiments have shown that grafts of Hensen’s node are capable of inducing a secondary axis and ectopic NP and NPB markers when placed in the area opaca which normally gives rise to extraembryonic tissue (Litsiou et al., 2005; Storey et al., 1992; Streit and Stern, 1999). Work in our laboratory has shown that Pax7 expression can be induced by grafts of quail node into chick area opaca (García-Castro, unpublished observations). Further investigations have shown that emulating the signals emanating from the node using a combination of BMP inhibition (Smad6), Wnt inhibitors (Dkk, NFz8, Crescent, and Cerberus), and FGF (FGF2, FGF3, FGF4 or FGF8) was not sufficient to induce neural tissue. These studies did not investigate the potential of such combinations to induce NC (Linker and Stern, 2004). To test this possibility, we implanted beads coated with a similar cocktail containing BMP inhibitors (Chordin and Noggin), Wnt inhibitors (WIF and Frizzled5/Fc), and FGFs (FGF12 and FGF4). Additionally, we included the cell-culture media supplement N2 – which contains insulin, progesterone, putrescine, transferrin and selenium – intended to promote neural differentiation; this supplement has been widely used in chick explant assays for neural and NC development (Basch et al., 2006; Bottenstein and Sato, 1979; García-Castro et al., 2002; Linker et al., 2009; Patthey et al., 2009; Selleck et al., 1998; Wilson et al., 2000; Wilson et al., 2001), and we reason that it could be involved in disparate results between reports of induction using coculture experiments in vitro and in vivo approaches.
We performed double ISH and analyzed the expression of the transcription factor Pax7, given its restricted expression and associated role with early NC development (Basch et al., 2006; Khudyakov and Bronner-Fraser, 2009; Otto et al., 2006), and the expression of the mesodermal marker Brachyury (Bra). Neither Pax7 nor Bra was induced by control beads (0.1% BSA) (0/19, Figure 1B–C). The mix of BMP inhibitors, Wnt inhibitors, and FGFs induced Bra in 22/82 beads (27%) and Pax7 in 20% of the beads implanted (16/82; Figure 1D–G). Pax7 was accompanied by Bra in 7 out of 16 instances (44%), and the remaining 56% of Pax7 expression occurred in the absence of Bra. While expression of Pax7 and Bra tends to occur in distinct domains (see Figure 1F–G) instances of dual expression within the same cell do occur, which are reminiscent of the endogenous patterns of expression for both molecules at the border of the caudal most portion of the open neural plate (see Figure 1B, 2G, 2M). These results suggest that these node-like signals are sufficient to trigger Pax7 expression in the NNE in the absence of mesoderm.
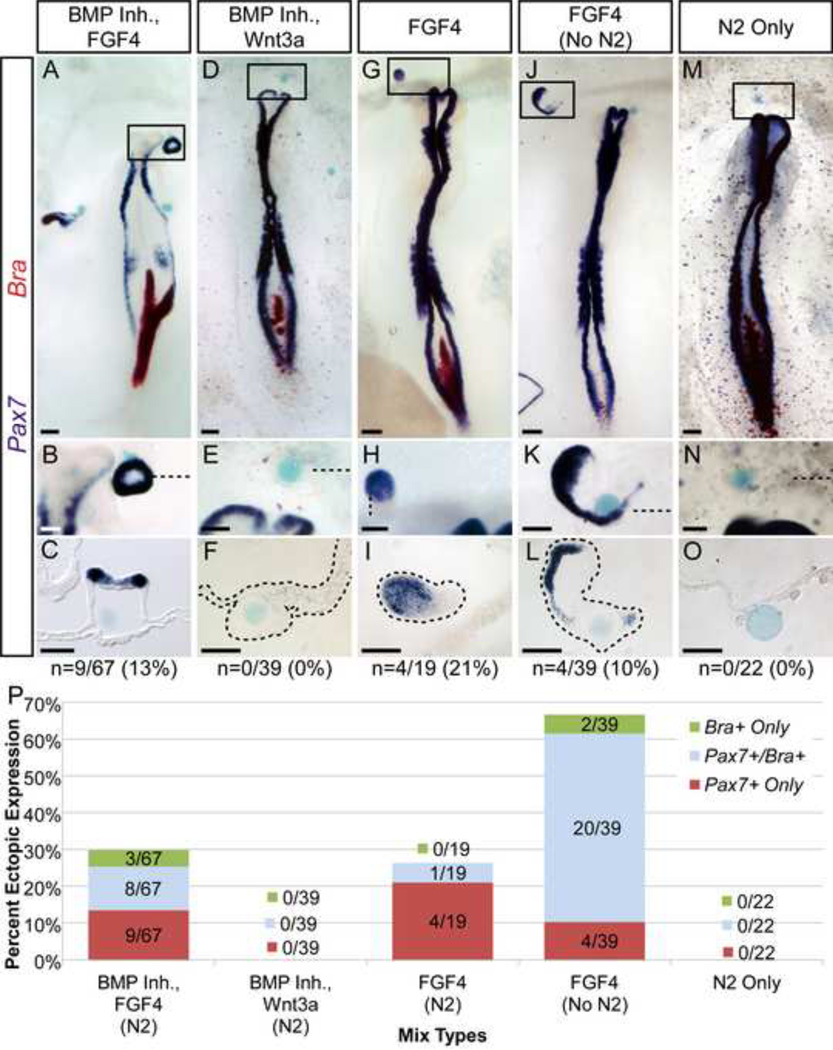
Figure 2. FGF4 is sufficient to induce ectopic expression of Pax7 in the non-neural ectoderm (NNE).
A–O) Mixes containing FGF4 induce ectopic expression of Pax7 (A, G, J), while those that do not contain FGF4 do not induce Pax7 (D, M). B, H, K) Ectopic Pax7 expression can occur in the absence of mesoderm (Bra). J–L) Ectopic Pax7 is induced by FGF4 in the absence of N2 supplement. M–O) N2 supplement alone does not induce either marker. Boxed areas in whole mounts indicate magnified regions shown below each sample, and dashed lines indicate plane of corresponding sections. Samples showing ectopic Pax7+/Bra− expression are indicated (“n”). P) In mixes that contain FGF4, 10% or more of beads induce ectopic expression of Pax7 in the absence of Bra (red bars). Using FGF4 alone (without inclusion of N2) greatly increased the frequency of total Pax7 expression (red and blue bars combined), while Pax7 alone (red bar only) is seen in 10% of beads. Scale bars are 200µm (A, D, G, J, M) and 100µm (B, C, E, F, H, I, K, L, N, O).
BMP Inhibition and FGF4 are sufficient to induce Pax7 in NNE
Given the complexity of the node-like cocktail, we aimed to determine the minimal signals that could still induce ectopic Pax7 in the NNE. Wnts are required for NC formation at the NPB, however the anterior of the embryo endogenously expresses Wnt inhibitors such as Dkk1, Crescent, and Sizzled (Chapman et al., 2004; GEISHA database; Patthey et al., 2009). In an effort to enhance NC formation, we eliminated Wnt inhibitiors from the cocktail. Additionally, as FGF is a potent inducer of mesoderm, we eliminated FGF12 and reduced the concentration of FGF4 used in our mix from 2.5 µg/ml to 1.25 µg/ml. This new mix, containing Chordin, Noggin, FGF4 and N2, lead to an overall reduction in Bra expression (16% vs. 27% seen with the full mix) without affecting the previously observed ectopic Pax7 (with or without Bra) expression in the NNE (25%, 17/67 beads; Figure 2A–C, P). Importantly, sectioned Pax7+ Bra-induced tissue displays no underlying mesenchymal cells, further supporting the absence of mesoderm (Figure 2B–C). While we had anticipated that removing Wnt inhibitors from the original cocktail would lead to an increase in the proportion of Pax7 induction, these results suggest that the additional Wnt inhibitiors had little (or no) effect on Pax7 induction in the NNE (16/82 beads, 20% vs. 17/67 beads, 25%).
FGF4 is sufficient for ectopic Pax7 induction in the NNE
To test if FGF was required to induce NC in NNE, we removed FGF4 and tested a mixture containing only Chordin and Noggin, however this mix did not produce any ectopic Pax7 (0/19 beads; data not shown). These results suggested that BMP inhibitors alone were not sufficient to induce NC in the NNE. Because Wnt signaling is required for avian NC induction and can induce NC in naïve neural plate (García-Castro et al., 2002; Patthey et al., 2009), we tested the capacity of Wnt3a or a Wnt agonist (whose bioactivity was verified in parallel experiments; see materials and methods) to induce Pax7 in the NNE. However, a mix containing Wnt3a combined with Chordin and Noggin was unable to trigger ectopic expression of Pax7 or Bra (0/39 beads, Figure 2D–F, P). Similarly, no Pax7 induction was observed when embryos were incubated with beads containing a Wnt agonist (0/12 beads, data not shown).
Next we tested if FGF4, without Wnt and BMP inhibitors, was sufficient for the ectopic expression of Pax7 in the NNE. Mixes containing 0.125 or 0.625 µg/ml of FGF4 did not yield any ectopic Pax7 (0/6 beads for each concentration, data not shown). However, implanted beads containing FGF4 at 1.25 µg/ml had a higher incidence of Bra-independent Pax7 expression than other mixes tested (21%, 4/19 beads; Figure 2G–I, P). Overall Bra expression was reduced compared to previous mixes tested (1/19 beads; Figure 2P).
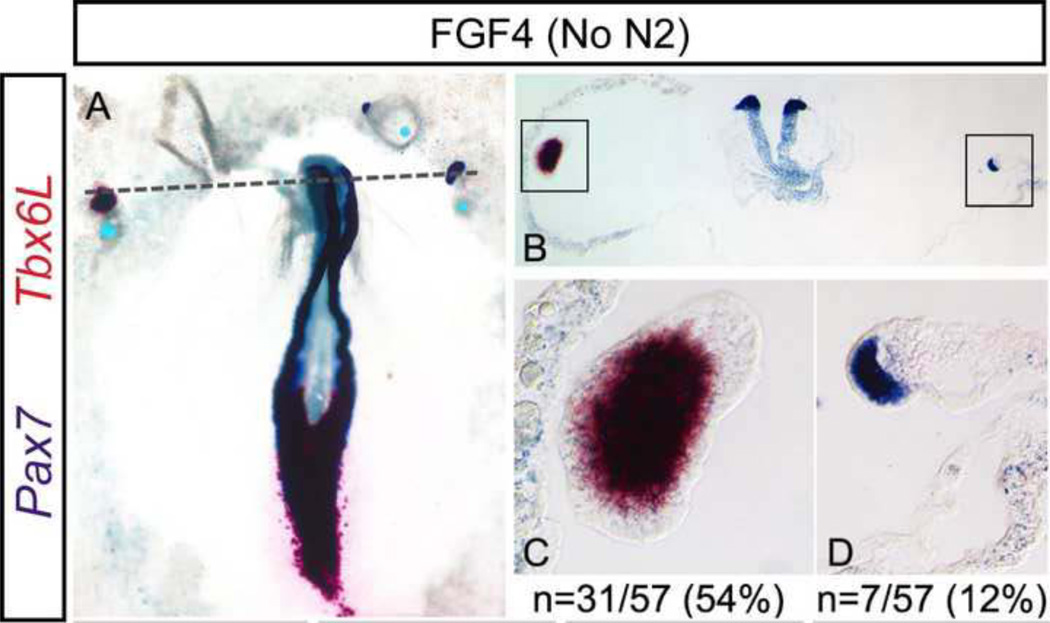
Importantly, all of the above mixes contained the N2 media supplement which could potentially influence the formation of ectopic tissue. To address this, we monitored expression of Bra and Pax7 in response to beads coated in N2 alone. Neither marker was induced (0/22 beads; Figure 2M–O, P). While this strongly suggests that N2 is not sufficient to trigger ectopic induction of these markers, its ingredients may still modify the response of the NNE to other signaling molecules; therefore, we tested FGF4 in the absence of N2, addressing directly if FGF4 is sufficient to induce Pax7 in NNE. Implanted beads containing FGF4 (1.25 µg/ml) without N2 lead to an increased ectopic induction of Bra (56%, 22/39 beads; Figure 2P), the majority of which co-express Pax7 (20/22; Figure 2P). Most importantly however, Braindependent expression of Pax7 is still observed (10%, 4/39 beads; Figure 2J–L, P). The absence of mesenchymal tissue was again confirmed in sectioned tissues, where ectopic Pax7+ tissue exhibits a thickened ectoderm surrounding the bead (Figure 2L). Additional evidence of mesoderm independent induction of Pax7 in the NNE by FGF was obtained by monitoring the expression of the paraxial mesoderm marker Tbx6L. Control beads soaked in BSA elicited no ectopic expression (0/8 beads, data not shown), instead FGF4 (without N2) beads induced Tbx6L in 31 out of 57 cases (54%). However, Pax7 was induced in the absence of Tbx6L in 12% of cases (7/57 beads, Figure 3), which is comparable to the 10% (4/39 beads) of Pax7+/Bra− ectopic tissue. This data further supports the notion that Pax7 expression can be induced in the absence of mesodermal markers. While FGF4 with N2 lead to less Bra, the overall Pax7 induction observed in the absence of N2 was far greater, therefore all further experiments were performed using FGF4 (1.25 µg/ml) only.
Figure 3. Pax7 is induced in the absence of Paraxial Mesoderm.
Beads coated with FGF4 induce ectopic expression of Pax7 and the paraxial mesoderm marker Tbx6L in the non-neural ectoderm A–D. Examples of independent expression of Tbx6L (C) or of Pax7 (D) are shown. Dashed line in A indicates the plane of section shown in B. Boxed regions in B indicate magnified regions shown in C and D. Total incidence of ectopic Tbx6L expression (C) and of Pax7+/Tbx6L− tissue (D) are indicated (“n”). Scale bars are 200µm (A, B) and 50µm (C, D).
FGF4 upregulates Neural Plate Border Specifiers and Neural Crest Specifiers
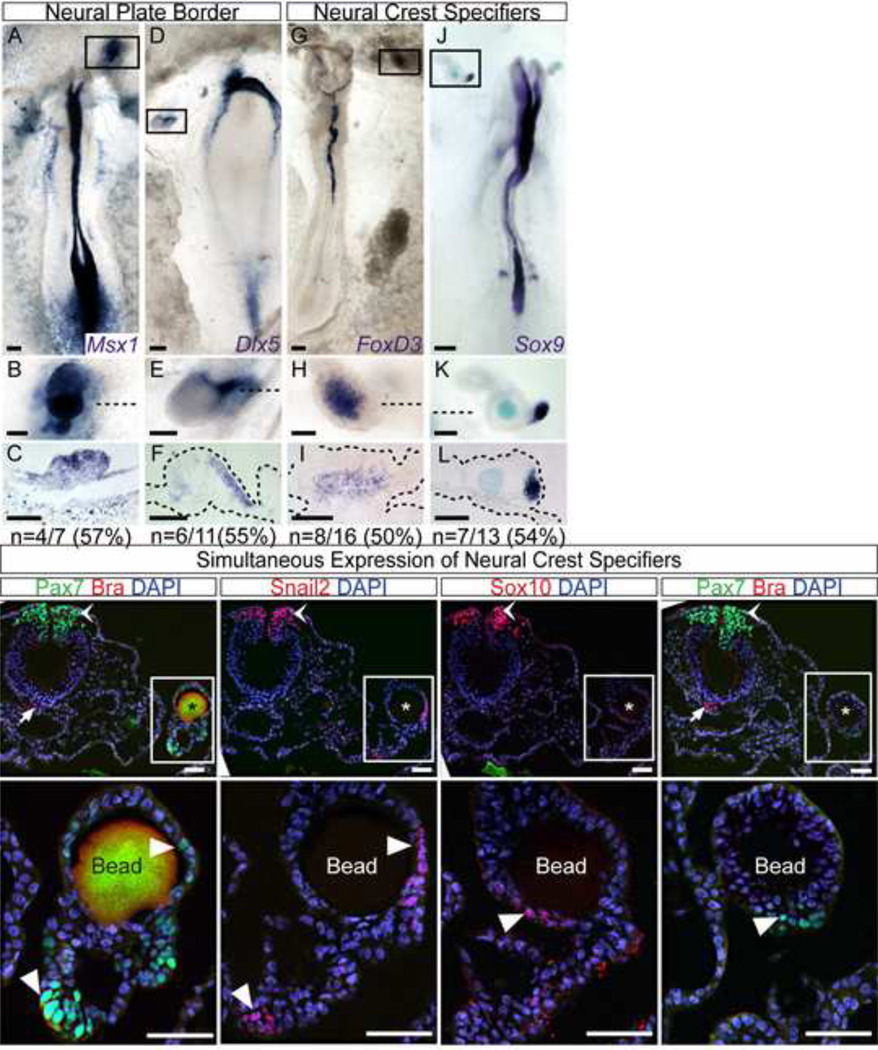
Our findings indicate that FGF4 is sufficient to trigger ectopic Pax7 expression in the NNE. We have previously shown that Pax7 function is critical for NC development and that its early expression marks the NPB (Basch et al., 2006). At early stages the NPB contains placodal and neural crest progenitors. Slightly later however, only NC precursors, but not placodal precursors, express the set of transcription factors known as NC specifiers and are found in the neural folds, separated from the placodal precursors (Bailey and Streit, 2005; Litsiou et al., 2005; Schlosser, 2008; Streit, 2007). To better establish the character of the ectopic tissue induced by FGF4 we analyzed by in situ hybridization the expression of NPB and NC specifiers. As expected, FGF4 coated beads induced ectopic expression of the NPB specifiers Msx1 (4/7 beads) and Dlx5 (6/11 beads) (Figure 4A–F). Of note, NC specifiers FoxD3 (8/16 beads), and Sox9 (7/13 beads) were also induced (Figure 4G–L). Additionally we analyzed by immunofluorescence the expression of other NC specifiers. In multiple examples we identified Snail2 and Pax7 coexpression (not shown). Importantly, analysis of ectopic tissue adjacent to an FGF bead in consecutive serial sections revealed expression of Pax7, Snail2, and Sox10 (Figure 4M–T). Furthermore, the upregulation of these NC specifiers can occur in the absence of mesoderm as indicated by the lack of Bra, which is clearly identified in endogenous locations but was not found in the ectopic tissue (Figure 4M, S). Thus, FGF4 is capable of inducing the NC regulatory program upregulating NPB and NC specifiers in the NNE in the absence of mesodermal markers.
Figure 4. FGF4 induces Neural Plate Border and Neural Crest Markers.
A–F) FGF4 is sufficient to induce ectopic expression of the neural plate border markers Msx1 (A–C) and Dlx5 (D–F). G–L) Neural crest specifiers FoxD3 (G–I) and Sox9 (J–L) are also ectopically induced. Boxed areas in whole mounts indicate magnified regions shown below each sample, and dashed lines indicate plane of corresponding sections. Incidence of ectopic gene expression is indicated (“n”). Immunofluorescence in consecutive sections of FGF4-induced ectopic tissue reveals additional crest markers in the absence of mesoderm. Pax7 (M, N, S, T), Snail2 (O, P) and Sox10 (Q, R) are detected in FGF4-induced ectopic tissue (white arrowheads) in addition to their endogenous NF location (chevron). Instead Bra is only detected in the notochord, its endogenous location (M, S; white arrows). Boxed areas indicate magnified regions shown below each sample. Location of the bead is indicated by an asterisk in M, O, Q, and S. Scale bars are 200 mm (A, D, G, J), 100 mm (B, C, E, F, H, I, K, L), and 50 mm (M–T).
NNE exhibits a prospective, but not definitive, neural character in association with ectopic NC induction
Previous reports have suggested that upon NP-NNE juxtaposition, the NNE could generate NC cells through different mechanisms. NNE could respond directly to signals from the NP, or alternatively the NNE could be first neuralized, and then in a secondary inductive interaction the newly neuralized tissue could respond to signals from the NNE to form NC (Baker and Bronner-Fraser, 1997; Selleck and Bronner-Fraser, 1995). To address the possibility that the ectopic tissue had been neuralized, we monitored the expression of the prospective neural marker Sox3, the definitive neural marker Sox2, and the anterior neural marker Otx2.
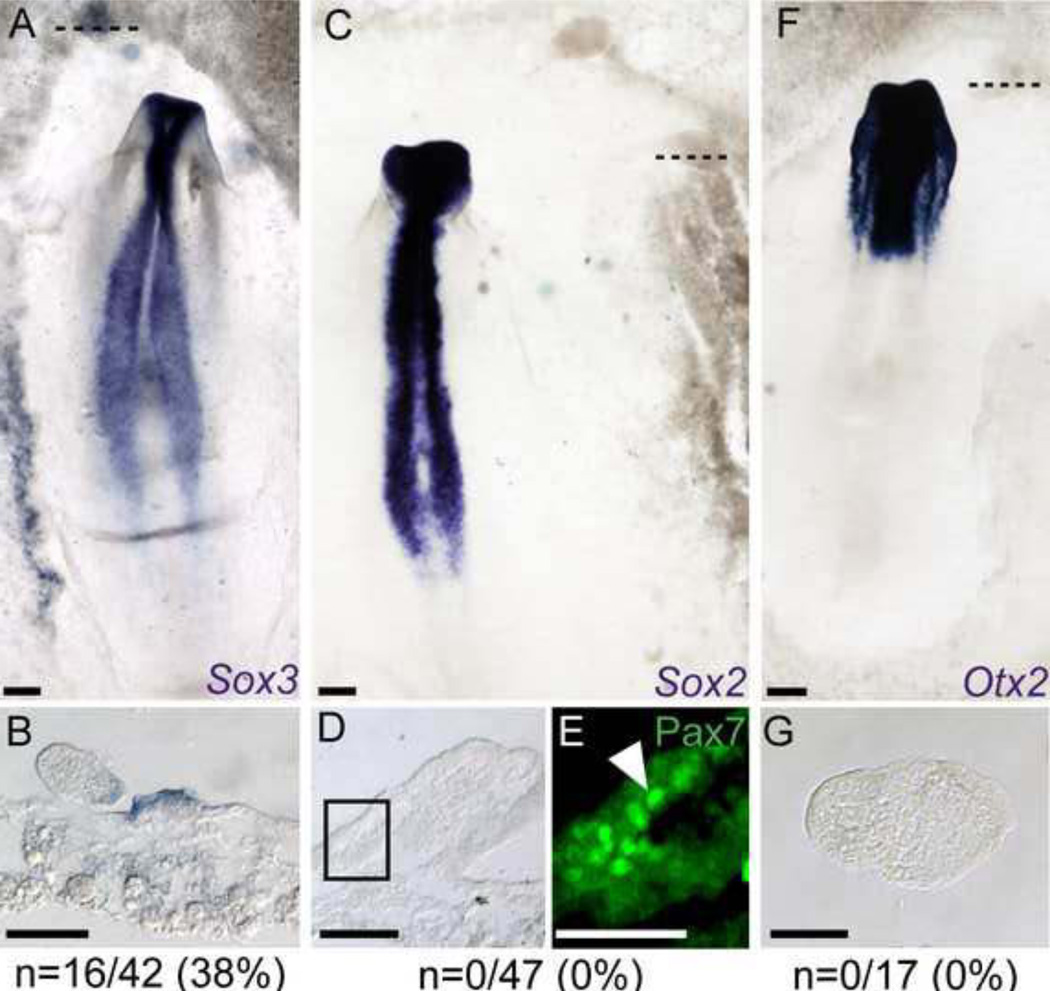
FGFs induce early neural markers such as Sox3 and ERNI (Albazerchi and Stern, 2007; Pinho et al., 2011; Streit et al., 2000), and in agreement, we observed robust expression of Sox3 in the NNE after 16 hours of incubation with FGF4 coated beads (38%, 16/42 beads, Figure 5A, B). Consistent with previous reports, FGF4-coated beads implanted in the NNE did not induce ectopic Sox2 expression (0/47 beads, Figure 5C, D). Ectopic tissue morphologies similar to those in which Pax7 was induced are readily observed (Figure 5D). Furthermore, immunostaining of Pax7 protein after sectioning these ectopic regions reveal the presence of Pax7 positive cells in the Sox2-negative tissue (Figure 5E). We also tested the expression of Otx2, which marks anterior neural tissue at later stages, and in agreement with the lack of Sox2, no Otx2 was observed after 16 hours of FGF4 treatment (0/17 beads, Figure 5F–G). Our results agree with other data showing that FGFs have the potential to initiate neural induction but are unable to lead to definitive neural character (Streit et al., 2000; Wilson et al., 2000). Thus we propose that after application of FGF4, appearance of ectopic NC coincides with a partial neural induction that does not lead to a full-fledged neural character.
Figure 5. FGF4 induces prospective neural, but not definitive neural tissue.
A–B) FGF4 coated beads induce the prospective neural marker Sox3. C–E) FGF4 does not induce definitive neural (Sox2), F–G) or anterior neural (Otx2) markers in the NNE. Dashed lines indicate the plane of section shown below. E) Magnified region of boxed area in D displays Pax7 immunofluorescence (arrowhead) in a Sox2-negative tissue. Scale bars are 200µm (A, C, F), 50µm (B, D, G), and 25µm (E).
BMP upregulation precedes NC induction by FGF in the NNE
In an effort to understand how FGF4 was affecting the NNE, we investigated the expression of other signaling molecules known to be involved in NC formation. During chick gastrulation, Bmp4 is expressed in the prospective epidermis and becomes restricted to the neural folds at later stages (Liem et al., 1995; Streit and Stern, 1999; Watanabe and Le Douarin, 1996); furthermore, Bmp4 can trigger the formation of NC when added to NP explants, mimicking the results observed using ectoderm (Liem et al., 1995). To determine if FGF4 altered Bmp4 expression in the NNE, we examined its temporal expression via ISH after bead implantation (Figure 6). FGFs have been reported to inhibit BMP expression in the prospective NP and NPB (Stuhlmiller and García-Castro, 2012b; Wilson et al., 2001), in contrast, our findings in the NNE revealed ectopic Bmp4 expression 4 hours after bead implantation in over 50% of the beads (35/66 beads; Figure 6A, B). Ectopic Bmp4 expression is maintained at 6 hours (7/20 beads) and at 16 hours (32/49 beads; Figure 6C–F). To assess the formation of NC in regions ectopically expressing Bmp4, we sectioned embryos and found that Pax7 is co-expressed with Bmp4 (Figure 6H). This embryo simultaneously provides an example of Bmp4 and Pax7 induction in the absence of Brachyury (Figure 6I). These results provide evidence for a novel role of FGF signaling in upregulation of BMP expression in the NNE.
NC induction in the NNE by FGF is accompanied by Wnt8c expression
Wnts are another crucial player in NC development. It has been suggested that Wnt6 could induce NC in chick embryos (García-Castro et al., 2002; Schmidt et al., 2007), however, its expression has only been reported after gastrulation (stage 6 onwards), and we have not been able to detect Wnt6 expression at earlier stages. Instead, FGF signaling has been shown to regulate Wnt8c expression in the primitive streak and in the caudal NP (Olivera-Martinez and Storey, 2007; Stuhlmiller and García-Castro, 2012b), and therefore we sought to determine if FGF application modulated its expression in the NNE.
Similar to the BMP analysis, we examined Wnt8c expression via ISH after bead implantation (Figure 7). Unlike Bmp4, Wnt8c was infrequently induced at 4 hours (25%, 15/60 beads; Figure 7A, B) and absent at 6 hours (0/18 beads; Figure 7C, D) after bead implantation. However, a large proportion of implanted beads analyzed at 16 hours after treatment display ectopic Wnt8c expression (81% 25/31 beads, Figure 7E–F). Sections through ectopic tissue at 16 hours show that Pax7 is co-expressed with Wnt8c+ tissue (Figure 7H). In this example, the ectopic tissue also expresses Brachyury (Figure 7I). These results suggest that FGF4 initiates a complex pattern of Wnt expression in conjunction with ectopic NC formation in the NNE.
Discussion
FGF signaling can trigger the generation of NC from NNE
Previous work on amniote or chick NC induction has focused on signals required to produce NC from neural/neuralized tissue (Basch et al., 2006; Basler et al., 1993; García-Castro et al., 2002; Liem et al., 1995; Patthey et al., 2009; Patthey et al., 2008; Streit and Stern, 1999; Stuhlmiller and García-Castro, 2012b). Our study is the first dedicated to examining the inductive signals that enable the NNE to contribute to NC formation. In vivo, we show that addition of FGF4 to the NNE leads to distinct patterns of BMP and Wnt expression, and ultimately leads to the expression of NPB specifiers (Pax7, Msx1, Dlx5) and NC specifiers (FoxD3, Sox9, Snail2, Sox10). In Xenopus, FGFs, and particularly mesodermal Fgf8a, have been proposed to participate in NC induction (Hong et al., 2008; Hong and Saint-Jeannet, 2007; LaBonne and Bronner-Fraser, 1998; Mayor et al., 1997; Mayor et al., 1995; Monsoro-Burq et al., 2003; Monsoro-Burq et al., 2005; Villanueva et al., 2002). In chick, no mesodermal FGF has been identified with an expression profile suggestive of a role similar to that seen for Fgf8a in Xenopus. During chick gastrulation Fgf2, 3, 4, 8, 13, 18, and 19 are expressed in the primitive streak, while Fgf12 is distributed throughout the epiblast (Karabagli et al., 2002; Kurose et al., 2004). Fgf4 and Fgf8 have been previously associated with avian neural induction (Huang et al., 2010; Streit et al., 2000; Streit and Stern, 1999); however, no evidence has been provided to identify the possible participation of these or any other FGF ligands in NC induction. Future work should aim to resolve this pending issue. Several FGF receptors (FGFRs) are expressed in the ectoderm and could mediate a response to FGF ligands. Both Fgfr1 and Fgfr4 are expressed broadly in the early ectoderm of St. 3 gastrula embryos encompassing the prospective NP and epidermis. Towards the end of gastrulation (St. 4+) their expression becomes progressively more restricted from the lateral edges of the embryo. By the end of gastrulation, Fgfr2 and Fgfr3, which are not expressed initially, can be detected in the lateral edges of the embryo (Lunn et al., 2007; Stuhlmiller and García-Castro, 2012b).
NNE, prospective neural tissue, and the generation of NC
NC appear at the border between neural and NNE and tissue juxtaposition assays from different laboratories have suggested that in addition to the NP, NNE tissues also generate NC derivatives (Mancilla and Mayor, 1996; Mitani and Okamoto, 1991; Moury and Jacobson, 1990; Ruffins and Bronner-Fraser, 2000; Selleck and Bronner-Fraser, 1995; Streit and Stern, 1999). It has been argued, that data supporting the NC induction on the NNE were inconclusive based on lack of proper molecular markers for NC tissue, improper labeling of grafts, or failure to determine if NNE was neuralized prior to NC induction (Schlosser, 2006, 2008); yet a recent study from the same author demonstrates that Xenopus NNE can respond to inductive NPB signals by expressing NC markers (Pieper et al., 2012). Furthermore, in chick embryos it has been demonstrated that NNE transplanted to the lumen of the neural tube, is induced to express Snail2 and generate migratory HNK1+ cells, strongly supporting the NC forming capacity of the NNE (Ruffins and Bronner-Fraser, 2000). Here we have provided further evidence by showing that NNE can be induced to form NC, demonstrating the expression of early NPB specifiers(Pax7, Msx1, and Dlx5), early NC specifiers (Sox9, FoxD3, Snail2), and a later NC specifier (Sox10) (Figures 1–4). In particular, the expression of the NC specifiers distinctly identifies NC and not placode precursors in endogenous tissue (Litsiou et al., 2005), and thus observation of these markers in FGF4-induced ectopic tissue in the NNE offers strong support for a NC character. However, to our knowledge no definitive evidence in model organisms has demonstrated absolute lineage-restricted compartments associated with these gene expression domains (Bailey and Streit, 2005). Yet, work focused on crest contributions made to the nasal epithelium using murine cre lines (Pax7 and Wnt1) and quail chick grafts (Barraud et al., 2010; Forni et al., 2011; Murdoch et al., 2010), have clearly established NC contributions in territories previously thought to be made by placodes alone. These studies support our interpretation that the FGF4-induced ectopic tissue adopts a NC character.
It has been proposed that during NP-NNE juxtapositions, NNE could be neuralized prior to forming NC (Selleck and Bronner-Fraser, 1995). Signals from the surrounding NNE (such as BMPs or Wnts), could then launch a second inductive event upon the neuralized NNE triggering NC formation. Alternatively, NC induction may occur directly in the NNE as a result of the combination of signals emanating from the NP (e.g. FGF, BMP antagonists) and the NNE (e.g. BMP, Wnt). Here we show that NC and NPB markers are induced in the absence of the definitive neural marker Sox2 (Figure 5); however, ectopic application of FGF4 does induce the prospective neural marker Sox3. Sox3 is thought to mark ectoderm that is competent to form neural tissue after receiving additional signals from the node or mesoderm (Albazerchi and Stern, 2007; Rex et al., 1997). Thus it may be that priming the ectoderm towards a neural fate is a prerequisite to the normal formation of NC; this is supported by the fact that, in chick, the expression domain of Sox3 extends more laterally than prospective NP, encompassing the adjacent NPB (Rex et al., 1997). Recent work in Xenopus further supports the contribution of NC from NNE and suggests that neural competence precedes NC formation (Pieper et al., 2012). Pieper et al. also show that loss of the transcription factors Dlx3 and GATA2, which confer non-neural competence to ectodermal cells, prevents proper NC formation in a non-cell autonomous manner. It will be interesting to determine the requirement of Sox3, and other early neural induction molecules that respond to FGF signaling like ChCh and ERNI, for NC formation in the NPB and from the NNE.
Mesoderm is dispensable during induction of NC from NNE
In the developing embryo, lateral mesoderm underlies the NPB, and studies in Xenopus suggest that signals from the lateral mesoderm play a crucial role in NC formation (Hong et al., 2008; Mitani and Okamoto, 1991; Steventon et al., 2009). In the chick, however, there is little evidence in support of a mesodermal role during NC induction (Basch et al., 2006; Patthey et al., 2009; Patthey et al., 2008; Selleck and Bronner-Fraser, 1995; Stuhlmiller and García-Castro, 2012b). FGF is known to be a potent inducer of mesoderm (Linker et al., 2009; Linker and Stern, 2004; Slack et al., 1987; Storey et al., 1998; Streit and Stern, 1999), however, our experiments used much lower concentrations than previous reports, and only induced Bra expression in half of our treatments (Figure 2P). Therefore we suggest that at the doses we used here, only a fraction of the mesoderm inducing capacity is elicited. Yet, this lower dose robustly induced Pax7, and in as much as 20% of the treated samples, Pax7 expression appears independently of underlying mesoderm and Bra expression. Additionally, Pax7 expression is induced in the absence of the paraxial mesodermal marker Tbx6L (12%). These results agree with others demonstrating that in avians, NC induction can proceed in vitro in the absence of mesoderm (Basch et al., 2006; Patthey et al., 2009; Stuhlmiller and García-Castro, 2012b).
Importantly, in Xenopus Fgf8, expressed by the mesoderm, has been identified as an indirect NC inducer, mediating its effect through the induction of Wnt ligands in the mesoderm. It has been postulated that the Wnt signal travels to the overlaying ectoderm, and is in effect, the NC inducer (Hong et al., 2008; Steventon et al., 2009). Instead, we previously reported that FGF/ERK signaling at the NPB is required during avian gastrulation to maintain the expression of Pax7 and other NPB markers (Stuhlmiller and García-Castro, 2012b). Furthermore, in chick, it is the epiblast, and not the mesoderm at this stage, which expresses FGF receptors (Lunn et al., 2007; Stuhlmiller and García-Castro, 2012b), making a mesodermal FGF-mediated signal unlikely in avians.
Perhaps during normal development at the NPB both neural and mesodermal tissues provide a supportive role for endogenous NC induction through spatial and temporal control of requisite signaling molecules. For instance, FGF and BMP antagonists emanate from the node and act to reduce BMP signals (from the ectoderm) in the initial stages of NC development. And though the exact Wnt ligand acting during the early stages of NC has not yet been identified, several Wnts (Wnt3a, Wnt5b, and Wnt8c) are expressed in the mesoderm (especially the PS) (Chapman et al., 2004), and may serve to fulfill this role.
N2 and mesoderm induction in the NNE
In our assays, N2 supplement, which is commonly added to explant and neural cultures, appears to have an inhibitory effect on mesoderm formation. Mixes which contain N2 exhibit less Bra expression compared to a mix containing FGF4 without N2 (see Figure 2P). This effect could be due to insulin, an important component of N2 supplement, as insulin-like growth factors (IGFs) have been shown to be required for anterior neural formation and to inhibit expression of mesodermal markers in Xenopus explants (Pera et al., 2003; Pera et al., 2001). It seems relevant in this context to further examine the role of insulin and other N2 components (like progesterone) during NC induction.
Ectopic NC formation in the NNE occurs in the presence of BMP and Wnt
Interestingly, in response to FGF4, we observed early ectopic Bmp4 and Wnt8c expression in the NNE, and robust signal for both ligands was identified concomitant with NC markers at 16 hrs (Figures 6 and 7). Interestingly, Litsiou et al. (2005), reported that after a common phase for border fates requiring FGF signaling, Wnt and BMP activation favors NC formation, while inhibition of these pathways leads to placodal derivatives. It is therefore possible that our FGF modulation in the NNE triggers both Wnt and BMP establishing the required conditions for NC development. The FGF, BMP and Wnt pathways have been shown to extensively cross-regulate one another. FGFs are known to inhibit BMPs (Branney et al., 2009; Fletcher and Harland, 2008; Kretzschmar et al., 1997; Pera et al., 2003; Sapkota et al., 2007; Wilson et al., 2001), and have also been shown to upregulate Wnt8c in Xenopus and chick (Hong et al., 2008; Olivera-Martinez and Storey, 2007). Wnts can inhibit FGF activity in the NP (Wilson et al., 2001), and have also been shown to upregulate BMP expression in the NP (Patthey et al., 2009).
During early chick development, FGF signaling in the prospective neural plate has been shown to inhibit BMP expression (Stuhlmiller and García-Castro, 2012b; Wilson et al., 2000). Instead FGF activity in more lateral NNE seems necessary for Bmp4 expression (Stuhlmiller and García-Castro, 2012b). In agreement with this, we report here that FGF beads induce Bmp4 in the NNE, and together would suggest that FGF is necessary and sufficient to trigger Bmp4 expression in NNE. Our results also indicate that BMP signaling is important for NC formation in the NNE. Ectopic Pax7 expression by FGF4 is reduced by 60% in the presence of Chordin and Noggin, from a total of 24/39 beads (62%) for FGF4 alone to 17/67 beads (25%) using FGF4, Chordin, and Noggin (Figure 2P). Similarly, ectopic expression of NPB and NC specifiers is reduced between 17% and 46% when Chordin and Noggin are included in addition to FGF4 (data not shown). A lack of total abrogation of Pax7 expression may be explained by insufficient BMP inhibition by Chordin and Noggin alone. It is possible that Bmp4 expression is induced indirectly, downstream of FGF4-induced Wnt expression (this work, Olivera-Martinez and Storey, 2007). It has been reported that explants of prospective NP increase Bmp4 expression in the presence of Wnt3a, while Bmp4 expression is reduced in prospective NPB explants in the presence of a Wnt antagonist (Patthey et al., 2009).
Given that the current model of NC induction proposes an early phase of FGF/ Wnt followed by a later phase of Wnt/BMP activation (Patthey et al., 2009; Steventon et al., 2009; Stuhlmiller and García-Castro, 2012b), it seems important to determine if the ectopic expression of these ligands is matched by activation of their pathways. It also remains to be established if the induction of BMP and Wnt expression by FGF in our experiments is direct or mediated indirectly.
Is FGF-mediated NC induction in the NNE equivalent to that occurring in the NPB?
Early in development the epiblast gives rise to the primitive ectoderm from which neural and NNE are derived. The border between them is composed of cells from both territories. NC cells are thought to arise at the border through a classic induction mechanism, and it is clear that both neural and NNE cells can contribute to NC. It has been proposed that the NNE must be neuralized to be able to respond to NC inductive signals and generate NC (Pieper et al., 2012; Selleck and Bronner-Fraser, 1995). Here we have shown that the NC developmental program can be elicited by FGF signals delivered to the NNE, and that the expression of NC related markers arises in the absence of mesoderm and the definitive neural marker Sox2. Our results suggest two possible mechanisms by which FGF4 causes the NNE to adopt a NC fate. One possibility is that the NNE responded to FGF by launching a prospective neural state (marked by the expression of Sox3) which was then able to respond to signals from adjacent non-transformed NNE to form NC in a similar fashion to what occurs to lateral prospective NP cells expressing Sox3 at the NPB. Alternatively, it is plausible that the NC program could be launched directly in the NNE in response to FGF signaling without further involvement from adjacent NNE. FGF signaling may have triggered expression of Wnt activating the NC program while simultaneously quenching BMP signaling through at least two know mechanisms, phosphorylating the linker region of R-SMADs (Kretzschmar et al., 1997; Pera et al., 2003; Sapkota et al., 2007), and increasing the expression of TGF-β inhibitors (Branney et al., 2009; Fletcher and Harland, 2008; Kudoh et al., 2004). While our current results cannot definitively distinguish between these two possibilities, further examination of the requirement of Sox3 (and other prospective neural makers) as well as investigations on the activation status of the Wnt, BMP, and FGF pathways (and the cross-talk that exists between their downstream effectors), will clarify the mechanism by which NC arise in the NNE in response to FGF signaling. This system offers novel opportunities to explore the requirements and interactions at play during NC development.
Highlights.
FGF signaling in the non-neural ectoderm induces neural crest cells
FGF induces neural crest without mesodermal or definitive neural markers
Non-neural ectoderm induced neural crest exhibit prospective neural markers
FGF induced Bmp4 and Wnt8c expression precedes neural crest formation.
Acknowledgements
The authors would like to thank the members of the García-Castro lab for their comments and discussion. The Pax7 antibody obtained from the Developmental Studies Hybridoma Bank (Department of Biology, University of Iowa; NICHD) was generated by Atsushi Kawakami (Tokyo Institute of Technology). This work was supported by Yale University Cell and Molecular Biology Training Grant (GM007223, N.Y.) and NIH (RO1DE017914, M.I.G-C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu-Elmagd M, Garcia-Morales C, Wheeler GN. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev Biol. 2006;298:285–298. doi: 10.1016/j.ydbio.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Albazerchi A, Stern CD. A role for the hypoblast (AVE) in the initiation of neural induction, independent of its ability to position the primitive streak. Dev Biol. 2007;301:489–503. doi: 10.1016/j.ydbio.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory Organs: Making and Breaking the Pre-Placodal Region. In: Gerald PS, editor. Current Topics in Developmental Biology. Academic Press; 2005. pp. 167–204. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech Dev. 1997;69:3–11. doi: 10.1016/s0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, Liu KJ, Baker CVH. Neural crest origin of olfactory ensheathing glia. Proceedings of the National Academy of Sciences. 2010;107:21040–21045. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, García-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Basch ML, Selleck MA, Bronner-Fraser M. Timing and competence of neural crest formation. Dev Neurosci. 2000;22:217–227. doi: 10.1159/000017444. [DOI] [PubMed] [Google Scholar]
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolande RP. Neurocristopathy: its growth and development in 20 years. Pediatr Pathol Lab Med. 1997;17:1–25. [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branney PA, Faas L, Steane SE, Pownall ME, Isaacs HV. Characterisation of the Fibroblast Growth Factor Dependent Transcriptome in Early Development. PLoS ONE. 2009;4:e4951. doi: 10.1371/journal.pone.0004951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Acuña G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Developmental Biology. 2007;309:208. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. Neural Crest Induction by Xwnt7B in Xenopus. Developmental Biology. 1998;194:129. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental Dynamics. 2001;220:284. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Saint-Jeannet J-P, Klein PS. A role for frizzled 3 in neural crest development. Development. 2001;128:3655–3663. doi: 10.1242/dev.128.19.3655. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, Selleck MA, McMahon AP, Bronner-Fraser M. Dorsalization of the neural tube by the non-neural ectoderm. Development. 1995;121:2099–2106. doi: 10.1242/dev.121.7.2099. [DOI] [PubMed] [Google Scholar]
- Etchevers H, Amiel J, Lyonnet S. Molecular bases of human neurocristopathies. Adv Exp Med Bio. 2006;589:213–234. doi: 10.1007/978-0-387-46954-6_14. [DOI] [PubMed] [Google Scholar]
- Farlie PG, McKeown SJ, Newgreen DF. The neural crest: Basic biology and clinical relationships in the craniofacial and enteric nervous systems. Birth Defects Research Part C: Embryo Today: Reviews. 2004;72:173. doi: 10.1002/bdrc.20013. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Harland RM. The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Dev Dyn. 2008;237:1243–1254. doi: 10.1002/dvdy.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural Crest and Ectodermal Cells Intermix in the Nasal Placode to Give Rise to GnRH-1 Neurons, Sensory Neurons, and Olfactory Ensheathing Cells. The Journal of Neuroscience. 2011;31:6915–6927. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse JT, Sive H. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mechanisms of Development. 2001;104:21. doi: 10.1016/s0925-4773(01)00358-6. [DOI] [PubMed] [Google Scholar]
- García-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- GEISHA database. Tuscon, AZ 85724: University of Arizona; [January 12, 2012]. Availiable from: http://geisha.arizona.edu; [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C. Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling. Developmental Biology. 2006;298:71. doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hong C-S, Park B-Y, Saint-Jeannet J-P. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development. 2008;135:3903–3910. doi: 10.1242/dev.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol Biol Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chen J, Zhang T, Zhu Q, Xiang Y, Chen CD, Jing N. The dual histone demethylase KDM7A promotes neural induction in early chick embryos. Developmental Dynamics. 2010;239:3350. doi: 10.1002/dvdy.22465. [DOI] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher R, Schoenwolf G. Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anatomy and Embryology. 2002;205:365. doi: 10.1007/s00429-002-0264-7. [DOI] [PubMed] [Google Scholar]
- Khudyakov J, Bronner-Fraser M. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Developmental Dynamics. 2009;238:716. doi: 10.1002/dvdy.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AK, Good PJ, Dawid IB, Harland RM. Dorsal-ventral patterning and differentiation of noggin-induced neural tissue in the absence of mesoderm. Development. 1995;121:1927–1935. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagu J. Opposing BMP and EGF signalling pathways converge on the TGF-[beta] family mediator Smad1. Nature. 1997;389:618. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expression Patterns. 2004;4:687. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The neural crest. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Linker C, De Almeida I, Papanayotou C, Stower M, Sabado V, Ghorani E, Streit A, Mayor R, Stern CD. Cell communication with the neural plate is required for induction of neural markers by BMP inhibition: evidence for homeogenetic induction and implications for Xenopus animal cap and chick explant assays. Dev Biol. 2009 doi: 10.1016/j.ydbio.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Fishwick KJ, Halley PA, Storey KG. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Developmental Biology. 2007;302:536. doi: 10.1016/j.ydbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. [DOI] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Mayor R, Guerrero N, Martinez C. Role of FGF and noggin in neural crest induction. Dev Biol. 1997;189:1–12. doi: 10.1006/dbio.1997.8634. [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Central role of gene cooption in neural crest evolution. J Exp Zoolog B Mol Dev Evol. 2005;304:298–303. doi: 10.1002/jez.b.21047. [DOI] [PubMed] [Google Scholar]
- Mitani S, Okamoto H. Inductive differentiation of two neural lineages reconstituted in a microculture system from Xenopus early gastrula cells. Development. 1991;112:21–31. doi: 10.1242/dev.112.1.21. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Moury JD, Jacobson AG. The origins of neural crest cells in the axolotl. Dev Biol. 1990;141:243–253. doi: 10.1016/0012-1606(90)90380-2. [DOI] [PubMed] [Google Scholar]
- Murdoch B, DelConte C, García-Castro MI. Embryonic Pax7-expressing progenitors contribute multiple cell types to the postnatal olfactory epithelium. J Neurosci. 2010;30:9523–9532. doi: 10.1523/JNEUROSCI.0867-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichane M, Ren X, Bellefroid EJ. Self-regulation of Stat3 activity coordinates cell-cycle progression and neural crest specification. EMBO J. 2010;29:55. doi: 10.1038/emboj.2009.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Martinez I, Storey KG. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 2007;134:2125–2135. doi: 10.1242/dev.000216. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Sokol SY. Neural crest specification by noncanonical Wnt signaling and PAR-1. Development. 2011;138:5441–5450. doi: 10.1242/dev.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73–83. doi: 10.1242/dev.025890. [DOI] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L, Edlund T. Early Development of the Central and Peripheral Nervous Systems Is Coordinated by Wnt and BMP Signals. PLoS ONE. 2008;3:e1625. doi: 10.1371/journal.pone.0001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- Pieper M, Ahrens K, Rink E, Peter A, Schlosser G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development. 2012;139:1175–1187. doi: 10.1242/dev.074468. [DOI] [PubMed] [Google Scholar]
- Pinho S, Simonsson PR, Trevers KE, Stower MJ, Sherlock WT, Khan M, Streit A, Sheng G, Stern CD. Distinct Steps of Neural Induction Revealed by Asterix, Obelix and TrkC, Genes Induced by Different Signals from the Organizer. PLoS ONE. 2011;6:e19157. doi: 10.1371/journal.pone.0019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JW, Raible DW. Signals derived from the underlying mesoderm are dispensable for zebrafish neural crest induction. Dev Biol. 2004;276:16–30. doi: 10.1016/j.ydbio.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Raven CP, Kloos J. Induction by medial and lateral pieces of the archenteron roof, with special reference to the determination of the neural crest. Acta. Morphol. 1945;5:348–362. [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rollhäuser-ter Horst J. Artificial neural crest formation in amphibia. Anat Embryol (Berl) 1979;157:113–120. doi: 10.1007/BF00315644. [DOI] [PubMed] [Google Scholar]
- Rollhäuser-ter Horst J. Neural crest replaced by gastrula ectoderm in amphibia. Effect on neurulation, CNS, gills and limbs. Anat Embryol (Berl) 1980;160:203–211. doi: 10.1007/BF00301861. [DOI] [PubMed] [Google Scholar]
- Ruffins S, Bronner-Fraser M. A Critical Period for Conversion of Ectodermal Cells to a Neural Crest Fate. Developmental Biology. 2000;218:13. doi: 10.1006/dbio.1999.9555. [DOI] [PubMed] [Google Scholar]
- Sagerström CG, Gammill LS, Veale R, Sive H. Specification of the enveloping layer and lack of autoneuralization in zebrafish embryonic explants. Developmental Dynamics. 2005;232:85. doi: 10.1002/dvdy.20198. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci U S A. 1997;94:13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota G, Alarcón C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP Signaling through Integrated Inputs into the Smad1 Linker. Molecular Cell. 2007;25:441. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Do vertebrate neural crest and cranial placodes have a common evolutionary origin? Bioessays. 2008;30:659–672. doi: 10.1002/bies.20775. [DOI] [PubMed] [Google Scholar]
- Schmidt C, McGonnell IM, Allen S, Otto A, Patel K. Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Dev Dyn. 2007;236:2502–2511. doi: 10.1002/dvdy.21260. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Selleck MA, García-Castro MI, Artinger KB, Bronner-Fraser M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development. 1998;125:4919–4930. doi: 10.1242/dev.125.24.4919. [DOI] [PubMed] [Google Scholar]
- Slack JMW, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KG, Crossley JM, De Robertis EM, Norris WE, Stern CD. Neural induction and regionalisation in the chick embryo. Development. 1992;114:729–741. doi: 10.1242/dev.114.3.729. [DOI] [PubMed] [Google Scholar]
- Storey KG, Goriely A, Sargent CM, Brown JM, Burns HD, Abud HM, Heath JK. Early posterior neural tissue is induced by FGF in the chick embryo. Development. 1998;125:473–484. doi: 10.1242/dev.125.3.473. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Stuhlmiller TJ, García-Castro MI. Current Perspectives of the Signaling Pathways Directing Neural Crest Induction. Cellular and Molecular Life Sciences. 2012a doi: 10.1007/s00018-012-0991-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller TJ, García-Castro MI. FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development. 2012b;139:289–300. doi: 10.1242/dev.070276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, Mayor R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev Biol. 2002;241:289–301. doi: 10.1006/dbio.2001.0485. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Le Douarin NM. A role for BMP-4 in the development of subcutaneous cartilage. Mech Dev. 1996;57:69–78. doi: 10.1016/0925-4773(96)00534-5. [DOI] [PubMed] [Google Scholar]
- Wills AE, Choi VM, Bennett MJ, Khokha MK, Harland RM. BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Developmental Biology. 2010;337:335. doi: 10.1016/j.ydbio.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Wu MY, Ramel M-C, Howell M, Hill CS. SNW1 Is a Critical Regulator of Spatial BMP Activity, Neural Plate Border Formation, and Neural Crest Specification in Vertebrate Embryos. Plos Biology. 2011;9 doi: 10.1371/journal.pbio.1000593. e1000593. [DOI] [PMC free article] [PubMed] [Google Scholar]