Abstract
Previous evidence has suggested both preserved emotional function in aging and age-related differences in emotional processing, but the neural networks underlying such processing alterations in the context of preserved affective function are not clear. Using event-related fMRI, we scanned young and older adults while they made valence ratings for emotional pictures. Behavioral results showed a similar pattern of emotional evaluation, but older adults experienced negatively valenced pictures as being less negative. Consistent with behavioral findings, we identified common activity in the right amygdala, but age-related differences in the functional connectivity of this region with the rest of the brain. Compared to young adults, older adults had greater functional connectivity between the right amygdala and ventral anterior cingulate cortex, possibly reflecting increased emotional regulation. Conversely, older adults showed decreased functional connectivity with posterior brain regions, likely reflecting decreased perceptual processing. Thus, age-related differences in evaluating negatively valenced stimuli might reflect decreased perceptual processing of these stimuli, as well as the engagement of control processes that inhibit the response to negative emotion.
Keywords: Aging, Emotion, fMRI
Emotional processing is relatively well preserved in healthy older adults, despite changes in other cognitive domains (for a review see Reuter-Lorenz and Lustig, 2005). This is an idea mainly supported by behavioral and anatomical evidence. For example, compared to young adults, older adults are just as likely to perceive and detect emotional stimuli (Keightley et al., 2006; LaBar et al., 2000; Mather and Knight, 2006; Phillips et al., 2002) and to report intense emotional experiences (Levine and Bluck, 1997). Supporting this behavioral evidence of preserved emotional function during aging, there is anatomical evidence that healthy aging does not markedly impair the structural integrity of the amygdala (Good et al., 2001; Grieve et al., 2005; Soininen et al., 1994), a region importantly involved in the processing of emotional stimuli (Anderson and Phelps, 2001; Hamann et al., 2002; Murphy et al., 2003; Phillips et al., 2003; Zald, 2003). Moreover, there is also evidence that physiological responses to emotional stimuli are similar in young and older adults, although somewhat reduced in older adults (Denburg et al., 2003; Levenson et al., 1991; Tsai et al., 2000). Consistent with a possible reduction in physiological response, functional neuroimaging evidence suggests that aging may alter amygdala responses towards emotional stimuli (Mather et al., 2004; although see Wright et al., 2006). For example, some functional MRI (fMRI) studies have found reduced activity in the amygdala during the perception of negative stimuli (Iidaka et al., 2002; Mather et al., 2004), and one study failed to find amygdala activation in older adults during the perception of emotional stimuli containing a greater number of negatively valenced faces (Gunning-Dixon et al., 2003). Thus, whereas behavioral and anatomical evidence suggest a preservation of emotional responses and amygdalar structure in older adults, respectively, functional neuroimaging evidence suggests an age-related attenuation in amygdala response to emotional stimuli, especially for negative stimuli.
A possible solution of this apparent inconsistency is that, although emotional processes and the underlying amygdalar functions are preserved in older adults they recruit additional emotional regulatory processes that sometimes lead to attenuated amygdalar responses to negative stimuli. Emotional processing involves the perception and production of affect, as well as the regulation of emotional responses (Phillips et al., 2003) via suppression or reappraisal (Ochsner and Gross, 2005). Emotional regulation is initiated via cortical structures in the prefrontal cortex (PFC) including the anterior cingulate (ACC), whereas emotional perception and response involve subcortical regions such as the amygdala (Ochsner and Gross, 2005; Phillips et al., 2003). For example, Ochsner et al. (2004) asked young adults to up- or down-regulate emotional responses while viewing negative and neutral pictures. They found that activity in ACC and PFC was recruited during both types of regulation, and that activity in the amygdala was modulated according to regulatory goals. Similarly, Phan et al. (2005) scanned young adult participants viewing negative pictures while they voluntarily suppressed emotional responses via reappraisal. They found that activity in ACC increased as emotional responses decreased, whereas amygdala activity increased as emotional responses increased. Emotional regulation processes can also occur incidental to task instructions. For example, in a positron emission tomography (PET) study Taylor et al. (2003) compared activity in the amygdala when participants were asked to subjectively rate versus passively view negative pictures. The rating task resulted in attenuation of amygdala activity coupled with greater activity in the ACC (although see Hutcherson et al., 2005). Taylor et al. (2003) suggest that these results indicate that participants are more likely to regulate emotions during cognitive tasks such as when making subjective ratings (also see Hariri et al., 2000; for review, see Phan et al., 2002). However, the developmental trajectory of the recruitment of regulatory processes during emotional tasks is currently not well understood. One idea is that older adults might engage regulatory emotional responses more than young adults (cf. Mather, 2006), which consequently engages cortical regions that alter the response of subcortical regions to a greater extent than in young adults.
Supporting this hypothesis, older adults have been shown to have an increased focus on emotions (Hashtroudi et al., 1990), to report fewer negative experiences and greater emotional control (Gross et al., 1997), to experience decreased negative affect coupled with increased positive affect over time (Mroczek, 2001), and to advance emotional goals by choosing to spend time with emotionally close people rather than acquaintances (Carstensen et al., 2003), when compared to young adults. Socioemotional selective theory (SST) postulates that aging is associated with motivational differences in allocating attention to emotional information because of a limited perspective on time (Carstensen et al., 2003; Mather and Carstensen, 2005). Specifically, the theory has two predictions: (1) aging involves the greater allocation of cognitive resources to emotional stimuli, and (2) that older adults are more likely to allocate these limited resources to information, activities and pursuits that maximize emotional well being. Thus, SST suggests that older adults will be more likely to attend to positively valenced stimuli and less likely to attend to negatively valenced stimuli than young adults (i.e., the so-called positivity effect, Carstensen and Mikels, 2005). Consistent with SST there is evidence for preserved working memory for emotional stimuli (Mikels et al., 2005), and a reduction in the positivity effect during divided attention (Mather and Knight, 2005). In sum, older adults are more likely to allocate cognitive resources and control over emotion, possibly because of an increased motivation to regulate emotions.
The idea of an age-related increase in emotional regulation is supported by functional neuroimaging studies that have found decreased amygdala activity coupled with increased activity in cortical control regions during the perception and evaluation of negative stimuli. For example, some fMRI studies have found that older adults recruit the anterior cingulate cortex (ACC) in addition to (Iidaka et al., 2002) or instead of (Gunning-Dixon et al., 2003) the amygdala when viewing negative stimuli, consistent with the role of this region in emotional regulation (Bush et al., 2000; Ochsner and Gross, 2005; Ochsner and Schacter, 2003), whereas young adults do not. Indeed, the ACC might be more generally involved in subcortical regulation, with a recent study finding reduced activity in striatal regions coupled with increased ACC activity in older adults, but not in young adults, during the anticipation of monetary loss (Larkin et al., 2007). Similarly, Fischer et al. (2005) found an age-related reduction in amygdala activity during the perception of angry faces, coupled with an age-related increase in anterior-ventral insula cortex. Gunning-Dixon et al. (2003) also found that compared to young adults, older adults recruited greater activity in the left lateral PFC when viewing negative stimuli, a region involved in cognitive control (see also Tessitore et al., 2005). Using both fMRI and event-related potentials to investigate the emotional evaluation of faces across the lifespan, Williams et al. (2006) demonstrated that emotional well being in aging was predicted by greater activity in the medial PFC related to controlled processing for negatively valenced stimuli, but with a decrease in controlled processes mediated by the ventromedial PFC for positively valenced stimuli. Williams et al. (2006) suggest that aging results in greater plasticity of medial PFC regions that regulate emotional function, possibly as the result of greater motivation to maximize emotional well-being. Consistent with these ideas, Urry et al. (2006) found that the amygdala and ventromedial PFC are inversely coupled when older adults voluntarily decreased emotional responses to negative stimuli. In sum, these results suggest that older adults might engage the emotional network (i.e., the brain regions involved in processing emotional information) differently, because they are motivated to regulate affect, which leads to a reduced amygdala response for negatively valenced stimuli (Gunning-Dixon et al., 2003; Iidaka et al., 2002). However, previous studies have not directly examined the idea that age-related functional changes (e.g., positivity effect), in the context of relative preservation of overall emotional function, might be coupled with age-related differences in the emotional network.
The pattern of brain activity observed in fMRI studies investigating the evaluation of emotional stimuli in aging are generally consistent with the Posterior–Anterior Shift in Aging (PASA) of age-related changes in brain activity (for review, see Dennis and Cabeza, 2008), where healthy aging is associated with increased activity in anterior brain regions coupled with decreased activity in posterior brain regions during cognitive tasks (e.g. Grady et al., 1994). For example, in a blocked fMRI study Tessitore et al. (2005) asked young and older adult participants to match the facial expression of negative faces. They found reduced activity in fusiform gyrus coupled with increased activity in frontal regions for older adults when compared to young adults. Similarly, compared to young adults, Gunning-Dixon et al. (2003) found that older adults had increased activity in frontal cortices when judging emotional expressions, and Iidaka et al. (2002) found reduced activity in the parieto-occipital cortex during an emotional discrimination task in older adults. However, in these studies it is difficult to determine whether the attenuation of activity in posterior regions reflects a more general pattern of activity related to aging (i.e. PASA) or is the result of the reduced amygdala activity and its modulatory influence on posterior regions (cf. Tessitore et al., 2005). Previous studies have not directly examined whether the PASA pattern of activation might also contribute to age-related differences in the emotional network in the context of preserved amygdala function.
Thus, the main goals of the present study were two-fold: (1) to identify activity related to preserved emotional function in aging that was common in both young and older adults, and (2) to investigate age-related differences in the emotional network. Additionally, a secondary goal of the study was to examine the PASA pattern of activity in the context of preserved emotional response to negative pictures in aging. In the present study, we compared brain activity when young and older participants viewed negative and neutral pictures while making concurrent valence ratings. An important feature was that we examined brain activity based on participants’ own perception and evaluation of negative and neutral stimuli, whereas previous fMRI studies might have confounded age-related reductions in amygdala activity with age-related differences in emotional ratings (Gunning-Dixon et al., 2003; Iidaka et al., 2002; Wright et al., 2006). We made the following two main predictions. First, given evidence of preserved anatomical integrity and function of the amygdala in aging (Good et al., 2001; Grieve et al., 2005; Soininen et al., 1994) and evidence supporting its main involvement in processing negative valence (e.g. Zald, 2003), we predicted that the amygdala would be commonly engaged by negative emotions in both young and older adults. To investigate this issue, we used a conjunction approach coupled with a region of interest (ROI) approach to examine brain activity in the amygdala that was commonly engaged during the perception and evaluation of negative versus neutral pictures in both age groups. To confirm the common engagement of the amygdala and to investigate the PASA pattern, we used an ANOVA approach to examine the Emotion × Age Group interaction. Given previous evidence using neutral stimuli (for review, see Dennis and Cabeza, 2008), we also predicted a PASA pattern of activity during the perception of negative pictures in the context of preserved emotional response in aging. Second, given evidence of enhanced emotional control in aging (Mather and Carstensen, 2005), along with the evidence suggesting an overall reduction in perceptual processing (Dennis and Cabeza, 2008), possibly coupled with reduced likelihood to attend to negatively valenced stimuli (Carstensen and Mikels, 2005), we predicted age-related differences in the emotional network. Specifically, we predicted that older adults would show enhanced functional connectivity between the amygdala and the frontal regions involved in emotional regulation, such as the ACC, but reduced functional connectivity with posterior regions involved in perceptual processes.
1. Methods
1.1. Participants
Fifteen young adults (mean age = 24.80, S.D. = 4.71) and 15 older adults (mean age = 70.23, S.D. = 5.31) participated in the study. Participants were female, healthy, right-handed, native English speakers, with no history of neurological or psychiatric episodes. Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board.
1.2. Materials
Stimuli consisted of 180 pictures selected from the International Affective Picture System (Lang et al., 1997). An equal number of positive, negative, and neutral pictures were selected on the basis of normative arousal and valence scores in young adults. The mean arousal scores (1 = calm, 9 = excited) were similar for positive (M = 6.0, S.D. = 2.2) and negative pictures (M = 6.15, S.D. = 2.2), whereas neutral pictures had lower arousal scores (M = 3.15, S.D. = 2.0). The mean valence score (1 = negative, 5 = neutral, 9 = positive) was 7.1 for positive (S.D. = 1.7), 2.3 for negative (S.D. = 1.5), and 5.2 for neutral (S.D. = 1.4). Additional neutral pictures were selected from other sources (Yamasaki et al., 2002), which had similar ratings with the neutral IAPs pictures, to equate the pictures for visual complexity and content (e.g., human presence). The exact number of pictures per valence category differed depending upon participants’ own ratings (young adults: positive = 61.80, S.D. = 12.38, negative = 60.23, S.D. = 10.60, neutral = 57.93, S.D. = 20.31; older adults: positive = 58.13, S.D. = 20.14, negative = 53.00, S.D. = 7.99, neutral = 66.53, S.D. = 19.77). Positive pictures were included for separate analyses conducted in young adults, which are published elsewhere (Dolcos et al., 2004a,b, 2005), but were excluded in the present age-group analysis on the basis of two factors. First, the positive stimuli contained a large number of pictures with radical sport and erotic content, which are processed differently in older adults (see Backs et al., 2005). Second, the behavioral results for the positive stimuli were difficult to interpret, because there were no clear differences in the consistency of subjective ratings for positive and neutral pictures with the normative ratings in the older participants. Specifically, while the young adults were more consistent in rating positive pictures as positive, there were no significant biases in how older adults were subjectively rating the positive pictures (i.e., it was not clear why older adults were less consistent in positive ratings); this could be due to the limited 3-point rating scale, which might not have allowed for fine dissociation of responses for positive valence. Thus, it would be difficult to meaningfully interpret the fMRI data based on these behavioral results.
1.3. Procedure
The pool of 180 pictures was divided into six sets of 30 pictures (10 positive, 10 negative, and 10 neutral), which were randomly assigned to six study blocks. Six different block orders were randomly assigned to the participants. To avoid the induction of long-lasting mood states, the pictures within each block where pseudo-randomized so that no more than two pictures of the same valence were consecutively presented, and there was an inter-trial interval of 12 s. Functional MR images were recorded while participants viewed emotional and neutral pictures. The pictures were presented for 3 s, using an LCD projector, to a screen located behind the participants’ crown so that they could see via an angled mirror. Participants were instructed to experience any feelings or thoughts the pictures might elicit in them, and to rate each picture on a 3-point pleasantness scale (1 = negative, 2 = neutral, 3 = positive) concurrently with picture presentation. The baseline used for the tasks is the implicit baseline as calculated by SPM.
1.4. Behavioral methods
Behavioral results were based on the proportion of match between the standard ratings and subjective ratings in each group. We calculated the average number of pictures with subjective ratings that matched each standard valence category (i.e. the amount of overlap or consistency between subjective ratings and the standard ratings). In order to determine discrepancies between subjective and standard ratings, we also calculated the average number of subjective ratings that did not match the standard ratings (i.e. the shift in subjective ratings with respect to the standard ratings). Separate t-tests were then calculated to determine the proportion of match between subject and standard ratings on negative and neutral pictures within each group, as well as to determine age-related differences in the proportion of match between negative pictures based on the standard ratings that were subjectively rated as negative and neutral. An alpha level of .05 was used for the behavioral statistical tests.
1.5. fMRI methods
1.5.1. Scanning
Scanning was conducted using a 1.5 T GE magnet. Stimuli were presented using an LCD projector and an angled mirror and behavioral responses were recorded using a four-button fiber optic response box (Resonance Technology, Northridge, CA). Head motion was minimized using foam pads and a headband. Anatomical scanning started with a T1-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC–PC plane. High-resolution T1-weighted structural images were acquired with a 450 ms repetition time (TR), a 9 ms echo time (TE), 24 cm field of view (FOV), 3.75 mm slice thickness, and a 2562 matrix. Functional scanning employed an echoplanar image sequence with a 3000 ms TR, 40 ms TE, 24 cm FOV, a 642 image matrix, and a 90° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels.
1.5.2. fMRI analyses
Image processing and analyses were performed using Statistical Parameter Mapping software implemented in Matlab (SPM99 and SPM2/SPM5, respectively; Wellcome Department of Cognitive Neurology, London, UK). Functional images were corrected for slice acquisition order, realigned to correct for motion artifacts, and then spatially normalized to a standard stereotactic space, using the template implemented in SPM99. Subsequently, the functional images were spatially smoothed using an 8 mm isotropic Gaussian kernel. For each subject, evoked hemodynamic responses to event types were modeled with a delta (stick) function corresponding to stimulus presentation convolved with a canonical hemodynamic response function (HRF) within the context of the general linear model (GLM). Since the global mean did not correlate with task-related activity, we were able to use proportional scaling to remove individual differences in the global signal (Aguirre et al., 1998; Junghofer et al., 2005).
1.5.3. Common activity in the amygdala
To examine activity in the brain that was commonly activated in both young and older adults during emotional evaluation, we employed the GLM to generate contrasts for the evaluation of negative versus neutral pictures based on participants’ own ratings. It is important to note that although there are potential age-related differences in the hemodynamic response (for review, see Dennis and Cabeza, 2008), these differences were not problematic in the present study because we examined the effects of relative activity between task conditions (Buckner et al., 2000; Huettel et al., 2001). For assessing common areas of activation associated with negative versus neutral evaluation across age groups, a conjunction map was created thresholding each age group’s random effects of the negative versus neutral contrast at P = .05. Thus, the conjoint probability of the conjunction map was P = .0025 (Fisher, 1950). A region of interest (ROI) approach, using the Talaraich Daemon Atlas (Lancaster et al., 1997, 2000) implemented with PickAtlas software (Maldjian et al., 2003), was used to examine our a priori hypothesis regarding the amygdala (cluster size ≥5 voxels).
In order to confirm that the amygdala was commonly engaged by both age groups, as well as to examine the PASA pattern, we performed an additional analysis to examine the overall Emotion (Negative and Neutral) × Age Group (Young and Old) interaction by employing the GLM to generate a mixed design ANOVA in SPM5 (P = .05, uncorrected, with a cluster size ≥5 voxels). In order to confirm that this interaction was driven by differences in young and older groups, we inclusively masked this interaction image with the corresponding statistical maps showing greater effects for the negative versus neutral stimuli and greater effects for the negative versus implicit baseline (both at P = .05, uncorrected, with a cluster size ≥5 voxels). Thus, the resulting pattern of activity also had to be confirmed by real differences observed in individual groups, which should show not only greater effects in the comparison with the corresponding control condition (negative > neutral), but also in the comparison with the implicit baseline (negative > baseline); the conjoint probability following inclusive masking approaches P = .000125 (Fisher, 1950).
1.5.4. Age-related differences in the emotional network for negative pictures
Seed voxels in the amygdala that were identified by our ROI analysis, which were commonly active in both age-groups, were further interrogated via individual trial analysis to examine the functional network of brain regions correlated with activity in this region. To find these functional connectivity maps, we employed a second analysis based on individual trial activity. We created a GLM in which each individual trial was modeled by a separate covariate, yielding different parameter estimates for each individual trial and for each individual subject. The validity of the use of this design has been confirmed in previous studies (e.g., Daselaar et al., 2006a,b; Rissman et al., 2004). As a second step, we employed the GLM to generate a mixed design ANOVA in SPM5 using the individual correlation maps associated with the seed voxel. To examine age-related differences in functional connectivity with the amygdala we focused on the Emotion (Negative and Neutral) × Age Group (Young and Old) interaction (P = .05, uncorrected, with a cluster size ≥5 voxels). Similar to above, in order to confirm that this interaction was driven by the expected differences in young and older groups, we inclusively masked this interaction image with the corresponding statistical maps showing greater effects for the negative versus neutral stimuli and with the correlation map showing greater effects for the negative versus baseline (both at P = .05, uncorrected, with a cluster size ≥5 voxels). Thus, the resulting activity showing age-related differences in the functional connectivity with the amygdala also had to be confirmed by real differences observed in individual groups, which should show not only greater effects in the comparison with the corresponding control condition (negative > neutral), but also in the comparison with the implicit baseline (negative > baseline); the conjoint probability following inclusive masking approaches P = .000125 (Fisher, 1950).
2. Results
2.1. Behavioral results
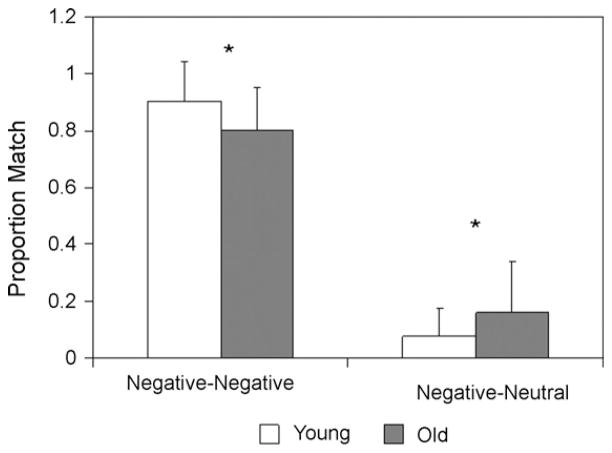
Analysis of the behavioral data revealed both a common pattern in young and older adults’ ratings and age-related differences in the subjective ratings compared to the standard ratings (see Fig. 1). In young adults, there was greater overlap for negative pictures compared to neutral pictures (t(14) = 2.87, P < .05) and a similar trend in older adults, (t(14) = 2.08, P = .06). However, older adults also rated more negative pictures as neutral (i.e., negative-to-neutral shift; see Fig. 1). Specifically, the overlap between subjective ratings and the standard ratings of negative pictures was greater in young adults than older adults (t(28) = 2.36, P < .05), such that older adults were more likely to rate negative pictures as being neutral (t(28) = 2.07, P < .05). Thus, young adults were more consistent in subjective ratings of negative pictures, whereas older adults showed a shift in subjective ratings of negative pictures from negative-to-neutral. In sum, consistent with evidence that the overall emotional response is preserved with aging, we found a common pattern in young and older adults’ ratings of the pictures, and consistent with the positivity effect, older adults also tended to rate negative pictures as more neutral (i.e., a negative-to-neutral shift), which suggests that there are differences in how they process emotional valence. Because of this behavioral difference it was important to use participants’ own classification of negative and neutral stimuli in the fMRI data analyses.
Fig. 1. Negative-to-neutral shift.
Participant ratings for negative pictures. Bars represent the mean proportion of the overlap between participants’ ratings of negative and neutral that corresponds to the standardized ratings for negative pictures. Young adults rated a higher proportion of negative pictures (according to the standard) as negative, whereas older adults rated a higher proportion of negative pictures (according to the standard) as neutral. Error bars indicate the standard deviation.
2.2. fMRI results
2.2.1. Common activity in the amygdala
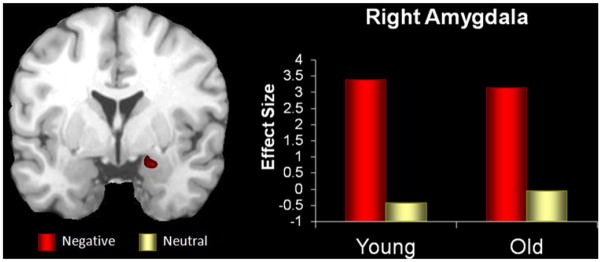
Confirming our first prediction, the conjunction analysis on the brain activity associated with evaluation of negative versus neutral pictures revealed a common pattern of activity in the right amygdala (see Table 1; Fig. 2). Visual inspection of the peak responses in each group revealed that the coordinates fell within the amygdala of each participant, which validates the basis of the functional connectivity analysis below.
Table 1.
Common activity for negative versus neutral stimuli
| Region | BA | H | Talairach coodinates
|
T score | P value | Voxels | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Young ∩ older amygdalaa | R | 19 | 3 | −16 | 17.43b | <.0001 | 6 | |
Note: BA, Brodmann’s area; H, Hemisphere.
Based on region of interest approach.
Multiplied score from the conjunction analysis of young (t(14) = 4.48) and older (t(14) = 3.89) groups.
Fig. 2. Common amygdala activation.
Common emotional evaluation in the right amygdala from the conjunction analysis based on a region of interest approach, where both young and older groups showed common activity for negative versus neutral stimuli (P = .0025). The y-axis represents the difference in activity between negative and neutral conditions and units are in effect size, the difference in the parameter estimates of the activation.
Also consistent with the prediction that amygdala activity was commonly engaged by both age-groups, we did not find significant differences in this region in the ANOVA examining Emotion (Negative and Neutral) × Age Group (Young and Old). Instead, these results were consistent with the PASA pattern of activity, with older adults showing reduced activity in the visual cortices, but greater activity in frontal regions (see Table 2). Furthermore, we found significant negative correlations between frontal and visual cortices in older adults, but not in young adults (also see Davis et al., 2007; Grady et al., 1994). In older adults, there was a significant negative correlation between right superior frontal and left visual cortices (r = −.55, P < .05), and between medial frontal and both left visual (r = −.55, P < .05) and right visual cortices (r = −.54, P < .05). No significant correlations between regions were found in young adults, or between performance and regional activity.
Table 2.
Emotion (negative, neutral) × Group (young, older)
| Region | BA | H | Talairach coodinates
|
F score | P value | Voxels | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Young > older | ||||||||
| Visual cortex | 19/37 | L | −45 | −69 | 0 | 8.32 | 0.006 | 34 |
| 19 | R | 45 | −70 | −6 | 7.98 | 0.007 | 45 | |
| 19/31 | R | 26 | −79 | 26 | 6.33 | 0.015 | 10 | |
| Older > young | ||||||||
| Superior frontal | 6 | R | 37 | 7 | 58 | 6.98 | 0.011 | 6 |
| Medial frontal | 9 | 4 | 35 | 36 | 8.81 | 0.004 | 7 | |
| Somatosensory cortex | 2 | L | −56 | −23 | 50 | 5.70 | 0.020 | 6 |
| Caudate | 11 | 8 | 10 | 1.81 | 0.035 | 5 | ||
| Thalamus | −4 | −22 | 1 | 6.45 | 0.014 | 20 | ||
Note: BA, Brodmann’s area; H, hemisphere.
2.2.2. Age-related differences in the emotional network for negative pictures
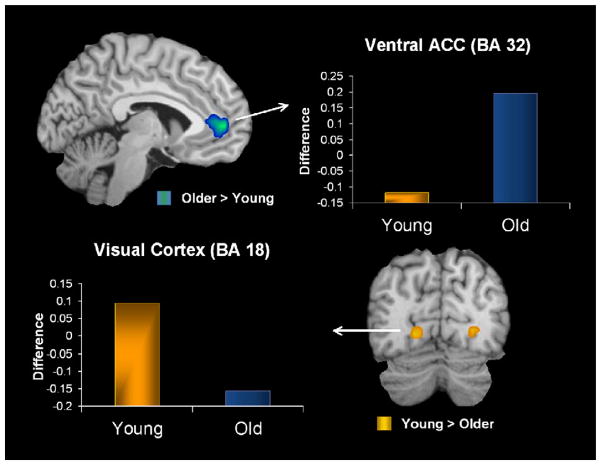
Confirming our second prediction, functional connectivity analysis revealed that in older adults the amygdala showed enhanced functional coupling with frontal cortical regions associated with emotional control, but reduced functional connectivity with posterior regions involved in perceptual processes. For these analyses, we used the peak voxel identified in the right amygdala region commonly active in both age groups as a seed voxel to examine possible age-related differences in functional connectivity in the emotional network. These analyses revealed an age-related decrease in the co-activation of the amygdala with posterior perceptual brain regions (i.e., bilateral visual, parahippocampal, and retrosplenial cortices), but an increased co-activity with frontal regions (i.e., ventral anterior cingulate cortex (ACC); see Table 3 and Fig. 3). Furthermore, we found that there was also a significant positive correlation between the activity in the ventral ACC and right visual cortex (r = 0.56, P < .005), suggesting a possible regulatory strategy. Thus, these results (see Fig. 3) are consistent with a shift in the processing of negative emotion in healthy aging, involving diminished perceptual processing and enhanced emotional regulation (e.g., Mather and Carstensen, 2005).
Table 3.
Age-related differences in the negative network
| Region | BA | H | Talairach coodinates
|
F score | P value | Voxels | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Young > older | ||||||||
| Parahippocampus | L | −30 | −33 | −11 | 9.93 | 0.003 | 23 | |
| R | 26 | −37 | −14 | 9.65 | 0.003 | 61 | ||
| Retrosplenial | 29 | −7 | −39 | 12 | 10.59 | 0.002 | 24 | |
| Posterior cingulate | 30 | R | 15 | −47 | 6 | 6.13 | 0.016 | 15 |
| Visual cortex | 18 | L | −19 | −77 | −3 | 11.85 | 0.001 | 8 |
| 18 | R | 22 | −73 | 0 | 8.35 | 0.006 | 8 | |
| 19 | R | 22 | −48 | −4 | 9.69 | 0.003 | 61 | |
| 19 | R | 26 | −58 | −3 | 5.9 | 0.018 | 6 | |
| Middle temporal | 21 | L | −52 | −22 | −8 | 7.8 | 0.007 | 8 |
| Cerebellum | R | 19 | −63 | −16 | 6.86 | 0.011 | 6 | |
| R | 4 | −60 | −35 | 10.97 | 0.002 | 13 | ||
| R | 26 | −49 | −30 | 5.91 | 0.018 | 5 | ||
| Older > young | ||||||||
| Ventral anterior cingulate | 32 | −4 | 44 | −2 | 23.67 | <.0001 | 33 | |
Note: BA, Brodmann’s area; H, hemisphere.
Fig. 3. Age-related differences in functional connectivity.
Age-related differences in the emotional network for negative pictures, showing a Posterior–Anterior Shift in Aging (approaches P = .000125). Older adults had enhanced functional connectivity between the right amygdala and anterior brain regions (ACC), and reduced functional connectivity between the right amygdala and posterior brain regions. The y-axis represents the difference in correlations between negative and neutral conditions. ACC: anterior cingulate cortex.
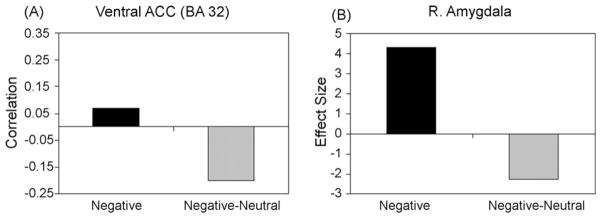
In order to further interrogate the age-related differences in the emotional network, we examined functional connectivity in a subset of older adults who had enough trials to investigate the negative-to-neutral shift (i.e. pictures subjectively rated as neutral, which are categorized as negative by the IAPs). There were 8 older adults with at least 10 pictures showing a negative-to-neutral shift. In these participants, we examined the functional connectivity between activity in the right amygdala and ventral ACC and found a negative correlation for the negative-to-neutral shift (see Fig. 4A). Thus, there was a decrease in activity in the amygdala coupled with an increase in activity in the ventral ACC on those trials in which older adults subjectively experienced negative pictures, according to the IAPs standardized norms, as neutral. Consistent with this result, right amgydala activity was reduced for the negative-to-neutral shift, but not for negative pictures subjectively rated as negative (see Fig. 4B). In this subset of older adults, we also examined the standardized IAPs intensity and valence ratings for the negative pictures subjectively rated as negative (negative–negative) or neutral (negative-to-neutral). We found that the standardized IAPs intensity ratings for the negative–negative pictures (M = 6.09, S.D. = 0.08) and the negative-to-neutral pictures (M = 6.16, S.D. = 0.03) did not significantly differ in intensity (P = .14). However, they differed in valence, such that the negative–negative pictures (M = 2.22, S.D. = 0.05) were slightly more negative (t(7) = 4.37, P < .005) than the negative-to-neutral pictures (M = 2.54, S.D. = 0.17), and thus, possibly more difficult to suppress.
Fig. 4. Amygdala and ventral ACC connectivity.
(A) Functional connectivity between the right amygdala and ventral ACC in older adults for the overall negative condition compared to a subset of older adults for the negative-to-neutral shift, negative pictures subjectively rated as neutral (negative-neutral). ACC: anterior cingulate cortex. (B) Reduction of amygdala activity in a subset of older adults for the negative-to-neutral shift (negative-neutral) compared to the overall negative condition.
2.3. Discussion
There were two main findings in the present study. First, we identified activity in the right amygdala that was commonly activated in both young and older adults and demonstrated a PASA pattern of activation in the context of this preserved emotional response. Second, functional connectivity of this region revealed age-related differences in the emotional network. Whereas in young adults the right amygdala was functionally connected to posterior regions involved in visual and perceptual processing, including bilateral visual, parahippocampal, and retrosplenial cortices, in older adults, right amygdala was functionally connected to the ventral ACC, a region involved in emotion regulation. Thus, we found preserved function in the amygdala in older adults, but differentiation in the emotional network; these findings are discussed below.
2.3.1. Preserved amygdala activity in aging
The results of the present study are consistent with evidence that aging is associated with structural preservation (Good et al., 2001; Grieve et al., 2005; Soininen et al., 1994) and robust functional activation (Wright et al., 2006) in the amygdala, and might account for some behavioral findings suggesting that the general ability to process emotion is not impaired in aging (Keightley et al., 2006; Mather and Knight, 2006; Phillips et al., 2002). For example, Mather and Knight (2006) tested older adults’ ability to quickly detect angry, happy, and sad schematic faces that were targets in an array of neutral faces and found that, although older adults were slower overall, both age groups were faster to detect angry faces. Keightley et al. (2006) found that young and older adults were equally able to categorize positive, negative, and neutral faces, although older adults were slower at recognizing negative faces. Furthermore, older adults were not impaired at categorizing the valence associated with emotional words.
There is evidence, however, that older adults are impaired when recognizing some discrete emotions, such as fear (for review, see Isaacowitz et al., 2007). For example, Calder et al. (2003) asked young and older adults to label faces that varied in emotional expression (happy, sad, angry, fear, disgust, surprise). They found that older adults were impaired at recognizing fearful and sad faces, compared to young adults, but there were no age-related differences in recall for happy faces. Similarly, Keightley et al. (2006) found that older adults were impaired at recognizing specific negative facial expressions such as fear. These results are consistent with some fMRI evidence for an age-related loss of function in the amygdala, especially for negative stimuli (Gunning-Dixon et al., 2003; Iidaka et al., 2002; Tessitore et al., 2005). For example, Iidaka et al. (2002) found greater left amygdala activity for negative faces in young adults compared to older adults, but there were no age-related differences in the amygdala for positive faces. Gunning-Dixon et al. (2003) found that older adults did not activate the amygdala when viewing emotional faces, which were predominantly negative, whereas young adults did. Nevertheless, these fMRI studies demonstrating age-related differences in activation in the amygdala are difficult to interpret, because the emotion condition in these studies was based on valence ratings that significantly differed between young and older adults (although see Fischer et al., 2005). Thus, the attenuation of amygdala activity reported in the literature could have reflected these differences, rather than older adults’ emotional response to stimuli they categorized as negative. Supporting this idea, the present findings demonstrate that when subjective ratings of emotion are used to separate negative and neutral categories, and thus the ratings are equated in young and older adults, a similar pattern of activation is found in the amygdala (see also Mather et al., 2004). Collectively, these findings suggest that older adults are just as likely to engage this region during the processing of negative stimuli as are young adults (Anderson and Phelps, 2001; Hamann et al., 2002; Murphy et al., 2003; Phillips et al., 2003; Zald, 2003).
In the present study, we found that right amygdala was commonly engaged by both young and older adults. There are several hypotheses regarding lateralization differences in amygdala function (e.g. Glascher and Adolphs, 2003; Markowitsch, 1998; Phelps et al., 2001), but there seem to be inconsistent findings with respect to the particular task conditions that mediate these differences in young adults (for review, see Baas et al., 2004). Indeed, previous fMRI studies of emotional perception in aging have been inconclusive with respect to potential age-related effects in the laterality of activation in the amygdala during emotional perception, with some studies finding greater left amygdala response in young adults (Iidaka et al., 2002), others greater right amygdala response (Gunning-Dixon et al., 2003; Tessitore et al., 2005), and one study, which directly tested laterality, found no age-related differences (Mather et al., 2004). Future research is needed to examine laterality differences in amygdala activity and how this might interact with age-related effects on emotional processing.
In the context of preserved amygdala function, we also observed a PASA pattern of activation during the perception of negative pictures. The amygdala has direct anatomical connections with posterior regions (LeDoux and Phelps, 2000; Price, 2003) and has been found to modulate visual attention to emotional stimuli in young adults (Adolphs, 2004; Anderson and Phelps, 2001; Vuilleumier et al., 2004). However, age-related findings regarding the modulation of amygdala activity on visual cortices are less clear. Previous studies of emotion and aging are consistent with the PASA pattern of activation (Fischer et al., 2005; Gunning-Dixon et al., 2003; Iidaka et al., 2002; Tessitore et al., 2005). In these studies, however, it is difficult to interpret reductions in posterior activity as evidence for PASA because this activity was coupled with an age-related reduction in amygdala activity that might also contribute to the attenuation of visual cortex activation. In the present study, we found that the PASA pattern holds even when the amygdala is commonly engaged by older adults. Furthermore, we also found a significant negative correlation between frontal and visual cortices in older adults, but not in young adults, such that older adults who had the greatest increase in frontal cortical activation also had a reduction in visual cortical activation. Previous studies using neutral stimuli have suggested that the PASA pattern is compensatory (e.g. Davis et al., 2007; Grady et al., 1994), but the PASA pattern could also be viewed as an emotional regulation strategy. Specifically, the increased activity in frontal cortices could reflect top-down controlled processes that modulate activity in the visual cortex and subsequently reallocate attention away from negative stimuli. Consistent with these ideas, Mather and Knight (2005) found that the positivity effect is reduced in older adults during a divided attention task, which suggests that cognitive resources are required to achieve these regulatory goals. It should be noted, however, that these two views (i.e., “compensation” and “emotion regulation”) are not mutually exclusive. Consistent with this idea, the positivity effect itself might be seen as a form of compensation in that it involves the recruitment of anterior regions to achieve a certain goal (i.e., to regulate emotional response), although achieving the regulatory goal might involve a reduction rather than an increase in “performance” (e.g., less consistent ratings for negative events compared to young adults). At any rate, the present findings provide evidence that a PASA pattern of activity is also observed with emotional stimulation, although the exact nature of this pattern awaits further confirmation from studies directly testing the “compensation” versus “emotion regulation” accounts.
2.3.2. Age-related differences in the emotional network for negative pictures
The findings from functional connectivity analyses suggest that, despite this common engagement of the amygdala, there are also age-related differences in the functional networks that are coactive with the amygdala during the processing of negative emotion in young and older adults. First, there was greater functional connectivity between the right amygdala and the ventral anterior cingulate (ACC) in older than in young adults. The ACC is part of the emotional network and the ventral division of the ACC has anatomical connections to amygdala and other regions implicated in emotional processing (Devinsky et al., 1995). On the bases of these anatomical connections, as well as empirical evidence, it has been suggested that the ventral ACC is involved in the processing of emotional information and the automatic regulation of emotional responses (for reviews see Bush et al., 2000; Phillips et al., 2003; Vogt et al., 1992). For example, the ventral ACC is activated during an emotional version of the stroop task in which participants must regulate responses to distracting negative information (Whalen et al., 1998). Moreover, this region malfunctions in depressed patients who are characterized by impaired ability to regulate emotions, and activity in this region is reversed back to normal in patients who respond to treatment (Mayberg, 1997; Mayberg et al., 1999).
Given this evidence, along with the evidence that healthy aging is characterized by enhanced ability to regulate emotions (Carstensen et al., 2003; Mather and Carstensen, 2005), the present finding concerning the ventral ACC suggests that this region comes online in synchrony with the amygdala during emotional processing of negative pictures as older adults’ automatically regulate emotional responses. We found an overall positive correlation between the right amygdala and ventral ACC, suggesting that the ventral ACC might need to work harder as activity in the amygdala increases, whereas there was a negative correlation between these same regions for the negative-to-neutral shift, suggesting that the ventral ACC might dampen activity in the amygdala when regulation is successful. Furthermore, there was a reduction in amygdala activity for the negative-to-neutral shift pictures. Consistent with the idea that some of the negative pictures were more emotionally charged and were more difficult to suppress, the negative IAPs pictures that were subjectively experienced as negative were also slightly more negatively valenced when compared to the pictures in the negative-to-neutral shift. The idea of ACC–amygdala interaction during emotion regulation is also supported by evidence from fMRI studies reporting opposing patterns of activity in the amygdala (reduced) and ACC (enhanced) in older adults (Gunning-Dixon et al., 2003; Iidaka et al., 2002), which supports the idea that older adults were probably regulating their emotions (cf. Mather, 2006). However, to our knowledge, previous studies have not directly examined age-related differences in the emotional network linked to behavioral changes using functional connectivity methods. Thus, the present study provides initial evidence supporting the idea that the negative-to-neutral shift observed behaviorally in the picture ratings may be the consequence of greater engagement of regulatory effects in the emotional network in healthy aging, which involves functional coupling between the amygdala and the ACC.
Second, aging was associated with less functional connectivity between the right amygdala and posterior brain regions, including posterior parahippocampus, visual and retrosplenial cortices. Posterior parahippocampal cortex is involved with spatial perception (Epstein and Kanwisher, 1998), and likely reflects better processing of the visuospatial aspects of the negative pictures by young adults in the present study. Similarly, the visual cortex is well known to be involved in processing specific visual detail, and is frequently found during tasks of emotional processing and evaluation (Murphy et al., 2003; Phan et al., 2002). In young adults, emotion influences perceptual processes (Adolphs, 2004; Anderson and Phelps, 2001; Vuilleumier et al., 2004) via direct connections with the amygdala (LeDoux and Phelps, 2000; Price, 2003). Our results suggest that the fluent processing of emotional stimuli via amygdala modulation of perception, which is found in young adults, is attenuated in older adults during the evaluation of negative pictures (also see Iidaka et al., 2002; Tessitore et al., 2005). The attenuation of visual cortex activity might also be partly mediated by the increased functional connectivity between the ventral ACC and the amygdala, because we also found a significant positive correlation between ventral ACC activity and a region in the right visual cortex. This idea is supported by some studies of visual attention in aging, which report biases in attention away from negative stimuli (Isaacowitz et al., 2006; Mather and Carstensen, 2003), possibly as a regulatory strategy. Finally, the retrosplenial cortex has been implicated in emotional evaluation, especially as emotion interacts with episodic memory, and there are indirect connections between the amygdala and retrosplenial cortices via parahippocampal and visual cortices, as well as other brain regions (for review, see Maddock, 1999). The diminished connectivity between amygdala and retrosplenial cortices in older adults during emotional evaluation of negative pictures might lead to poorer episodic memory for this stimuli type when compared to young adults (for review, see Mather, 2006).
The findings of the present study fit well with theories of emotional aging, which suggest that aging is associated with attenuation in response to negatively valenced stimuli. Socioemotional selectivity theory (SST Carstensen et al., 2003) posits that aging is associated with an increased motivation to regulate emotions, which leads to both increased attention to positively valenced stimuli (i.e., positivity effect) and a diminished response to negatively valenced stimuli (Mather, 2006). For example, in an event-related fMRI study, Mather et al. (2004) asked young and older adults to make arousal ratings while viewing positive, negative and neutral faces. Similar to previous studies using the standardized valence ratings (e.g., Gunning-Dixon et al., 2003; Iidaka et al., 2002), older adults had a diminished response in the amygdala during emotional evaluation of negative pictures. However, the amygdala response during emotional evaluation of positively valenced pictures was preserved in aging. Mather et al. (2004) suggested that with aging there is a shift in how the amygdala responds to emotional stimuli from negative to positive valence, which corresponded with a reduction in older adults’ arousal ratings for negatively valenced stimuli, when compared to young adults’ ratings. The present findings also suggest that there is a shift in the processing of negative emotion in healthy aging, which involves diminished perceptual processing and enhanced emotional regulation consistent with SST. This shift might be the result of age-related differences in the engagement of the emotional network, with older adults recruiting to a lesser extent regions associated with increased visual attention, such as visual cortices, and to a greater extent regions associated with emotional regulation, such as the ventral ACC. To our knowledge, this is the first study showing that age-related behavioral changes observed in the context of preservation of overall emotional function is coupled with age-related differences in the emotional network.
3. Conclusions
In the present study, we examined the functional connectivity of the amygdala with the emotional network associated with the evaluation of negative pictures in aging. We found two main results: (1) activity in the right amygdala was commonly active in young and older adults during the evaluation of negative pictures when valence ratings where based on participants’ own responses, and (2) there were age-related differences in the engagement of the emotional network, which suggested that older adults had greater functional connectivity between the right amygdala and ventral ACC, possibly reflecting increased emotional regulation of negative pictures, but decreased functional connectivity with posterior brain regions, possibly reflecting decreased perceptual and evaluative processing of negative pictures. These findings advance our understanding concerning the age-related alterations in the neural networks underlying processing of negative emotions in the context of preserved affective function and they suggest that older adults might be more likely to regulate negative responses. Future investigations should examine the possibility of age-related differences in the connectivity of other subcortical regions involved in emotional network, such as the insula and ventral striatal regions, and whether these age-related differences will emerge on tasks that explicitly instruct participants to regulate.
Acknowledgments
This study was supported by NIH grants RO1 AG19731 and RO1 AG023123. FD was supported by a NSERC Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada, a CPRF Award from the Canadian Psychiatric Research Foundation, and by a NARSAD Young Investigator Award from the US National Alliance for Research on Schizophrenia and Depression.
Footnotes
Disclosure statement
The authors certify that they have no actual or potential conflicts of interest regarding the research reported in this paper. The experimental protocol employed in the present study was approved for ethical treatment of human participants by the Institutional Review Board at Duke University Medical Center, and the experimental data were collected with the understanding and written consent of each participant.
Conflict of interest
There are no actual or potential conflicts of interest.
Contributor Information
Peggy St. Jacques, Email: peggy.st.jacques@duke.edu.
Florin Dolcos, Email: fdolcos@ualberta.ca.
References
- Adolphs R. Emotional vision. Nat Neurosci. 2004;7 (11):1167–1168. doi: 10.1038/nn1104-1167. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8 (3):302–306. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411 (6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45 (2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Backs RW, da Silva SP, Han K. A comparison of younger and older adults’ self-assessment manikin ratings of affective pictures. Exp Aging Res. 2005;31 (4):421–440. doi: 10.1080/03610730500206808. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12 (Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4 (6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, Young AW. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41 (2):195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Fung HH, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation Emotion. 2003;27 (2):103–123. [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: aging and the positivity effect. Curr Directions Psychol Sci. 2005;14:117–121. [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006a;96 (4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006b;16 (12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm155. advanced acess published on October 8, 2007. doi: 2010.1093/cercor/bhm2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3 (3):239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3. Lawrence Erlbaum Associates; Mahwah, NJ: 2008. pp. 1–54. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004a;23 (1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42 (5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA. 2005;102 (7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392 (6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett. 2005;386 (2):99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd; London: 1950. [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23 (32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14 (1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14 (3 Pt 2):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Map. 2005;25 (4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Skorpen CG, Hsu AY. Emotion and aging: experience, expression, and control. Psychol Aging. 1997;12 (4):590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24 (2):285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13 (2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11 (1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychol Aging. 1990;5 (1):119–126. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13 (1):161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27 (3):656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12 (3):352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Lockenhoff CE, Lane RD, Wright R, Sechrest L, Riedel R, Costa PT. Age differences in recognition of emotion in lexical stimuli and facial expressions. Psychol Aging. 2007;22 (1):147–159. doi: 10.1037/0882-7974.22.1.147. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychol Aging. 2006;21 (1):40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Schupp HT, Stark R, Vaitl D. Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. Neuroimage. 2005;25 (2):520–526. doi: 10.1016/j.neuroimage.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Burianova H, Hongwanishkul D, Grady CL. Age effects on social cognition: faces tell a different story. Psychol Aging. 2006;21 (3):558–572. doi: 10.1037/0882-7974.21.3.558. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam M, Gitelman DR, Weintraub S. Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2000;38 (13):1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5(4):S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talaraich atlas labels for functional brain mapping. Hum Brain Map. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System. NIMH Center for the Study of Emotion and Attention; Gainesville, Fl: 1997. [Google Scholar]
- Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10 (6):787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Phelps EA. Emotional networks in the brain. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. The Guilford Press; New York: 2000. pp. 157–171. [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychol Aging. 1991;6 (1):28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Levine LJ, Bluck S. Experienced and remembered emotional intensity in older adults. Psychol Aging. 1997;12 (3):514–523. doi: 10.1037//0882-7974.12.3.514. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22 (7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroantomic and cytoarchitectonic Atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 1232.1233) [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Differential contribution of right and left amygdala to affective information processing. Behav Neurol. 1998;11 (4):233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Mather M. Why memories may become more positive as people age. In: Uttl B, Ohta AL, editors. Memory and Emotion: Interdisciplinary Perspectives. Blackwell; Malden, MA: 2006. pp. 135–157. [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci. 2004;15 (4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychol Sci. 2003;14 (5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9 (10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging. 2005;20 (4):554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: threat detection is not impaired among older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61 (1):54–57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9 (3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156 (5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Larkin GR, Reuter-Lorenz PA, Cartensen LL. Divergent trajectories in the aging mind: changes in working memory for affective versus visual information with age. Psychol Aging. 2005;20 (4):542–553. doi: 10.1037/0882-7974.20.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK. Age and emotion in adulthood. Curr Directions Psychol Sci. 2001;10 (3):87–90. [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3 (3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9 (5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23 (2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Schacter DL. Remembering emotional events: a social cognitive neuroscience approach. In: Davidson RJ, Scherer K, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford UP; NY: 2003. pp. 643–659. [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57 (3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16 (2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4 (4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips LH, MacLean RD, Allen R. Age and the understanding of emotions: neuropsychological and sociocognitive perspectives. J Gerontol B Psychol Sci Soc Sci. 2002;57 (6):526–530. doi: 10.1093/geronb/57.6.p526. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I. The neural basis of normal emotion perception. Biol Psychiatry. 2003;54 (5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15 (2):245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23 (2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen M, Koivisto K, Riekkinen PJ., Sr Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44 (9):1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18 (3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, Mattay VS. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139 (1):9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW, Carstensen LL. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychol Aging. 2000;15 (4):684–693. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26 (16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2 (6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7 (11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44 (12):1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: neural basis of improving emotional stability over age. J Neurosci. 2006;26 (24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol Aging. 2006;27 (2):361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA. 2002;99 (17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41 (1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]