Abstract
Objective
To assess the relationship between statins and prognosis in ischemic and nonischemic patients with heart failure (HF) in a real-life cohort followed up for a long period.
Patients and Methods
This prospective study included 960 patients with HF with preserved or depressed left ventricular ejection fraction (LVEF), irrespective of HF etiology, who were referred to the HF clinic of a university hospital between August 1, 2001, and December 31, 2008. The patients were followed up for a maximum of 9.1 years (median, 3.7 years), and survival in ischemic and nonischemic patients was determined.
Results
Median age was 69 years, and median LVEF was 31%. Of the 960 patients, 532 (55.4%) had ischemic HF etiology, and most received angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (846; 88.1%) and β-blockers (776; 80.8%). Patients with HF of ischemic origin were more often treated with statins (P<.001). During follow-up, 440 patients (45.8%) died. Statin therapy was associated with significantly improved survival (hazard ratio, 0.45 [95% confidence interval, 0.37-0.54]; P<.001). After adjustment for HF prognostic factors (age, sex, cholesterol level, New York Heart Association class, HF etiology, LVEF, body mass index, HF duration, atrial fibrillation, implantable cardioverter-defibrillator therapy, and medicines), statins remained significantly associated with lower mortality risk in both ischemic (P=.007) and nonischemic (P=.002) patients.
Conclusion
In contrast to results of large randomized trials, statins were independently and significantly associated with lower mortality risk in our real-life HF cohort, including patients with nonischemic HF etiology.
Abbreviations and Acronyms: CI, confidence interval; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association
Small prospective trials have suggested that statin treatment in patients with heart failure (HF) have a positive impact that is pleiotropic and irrespective of atherosclerotic burden control.1,2 Moreover, observational studies and post hoc analyses of randomized trials designed to test drugs other than statins suggest that this therapy could have a positive influence on the prognosis of patients with HF.3-6 On the basis of these promising findings, large randomized trials with rosuvastatin, the GISSI HF trial (GISSI-HF) and the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA), were undertaken. The results of these trials were opposite to what was expected, showing that rosuvastatin did not reduce the number of deaths in patients with HF.7,8 Of note, both studies were performed with the same drug and dose. Because of the controversy generated by the results of these randomized trials, we analyzed the relationship between the use of statins and the prognosis of ischemic and nonischemic patients with HF in a real-life cohort over a long follow-up period.
Patients and Methods
The study population consisted of 960 consecutive patients referred to the HF clinic of a university hospital between August 1, 2001, and December 31, 2008. Our HF unit is an ambulatory clinic integrated into a tertiary care hospital. Most patients were referred from cardiology (678; 71%) and internal medicine (145; 15%) wards; 47 (5%) came from the emergency department or short-stay unit, and 90 (9%) from other hospital wards (eg, oncology, nephrology, respiratory). The principal referral criterion was HF irrespective of etiology (at least 1 HF hospitalization and/or reduced left ventricular ejection fraction [LVEF]). Of the 960 patients, 623 (65%) had at least 1 hospital admission for HF in the previous year. All patients were followed up regularly at the HF clinic according to their clinical status. Follow-up visits took place every 3 months with a nurse and every 6 months with a physician (cardiologist, internist, or family physician); there also were optional visits from specialists in geriatrics, psychiatry, and rehabilitation. At first visit, all patients gave written consent for obtaining analytical samples and using their clinical data for research purposes. The study was performed in compliance with the law protecting personal data in accordance with the international guidelines on clinical investigation of the World Medical Association Declaration of Helsinki.
Patients were followed up for a maximum of 9.1 years for living patients (median, 3.7 years [interquartile range, 2.1-6.3 years]).
Data Collection
Patients' clinical status, treatment, and biochemical data were prospectively obtained at baseline. Patients were classified as ischemic or nonischemic according to the baseline etiology of their HF syndrome. Patients were considered to have HF of ischemic etiology when their ventricular dysfunction was secondary to myocardial infarction or to diffuse severe coronary artery disease. In the ischemic etiology group, 83% of patients had a previous myocardial infarction. Nonischemic HF was caused by idiopathic dilated cardiomyopathy (90 patients; 21%), hypertensive heart disease (93; 22%), alcoholic cardiomyopathy (50; 12%), drug-related cardiomyopathy (18; 4%), valvular disease (101; 24%), and other causes (76; 18%). The vital status and cause of death were checked every 3 months. If a patient did not come to a scheduled visit, telephone contact with the patient or patient's relatives was attempted. If contact was not possible and death was not certified by clinical records from other hospital wards, the emergency department, or general practitioners, vital status was checked from registries of the Catalan and Spanish Health Systems.
Statistical Analyses
Descriptive analyses were performed at the first step. Categorical variables were described by frequencies and percentages. Continuous variables were described by means and standard deviations or medians and interquartile ranges in case of skewed distribution. Baseline characteristics of ischemic and nonischemic patients were examined by the χ2 test for categorical variables. The comparison of continuous variables between groups was performed using analysis of variance for unpaired data once normality was demonstrated (Kolmogorov-Smirnov test); otherwise, a nonparametric test (Mann-Whitney or Kruskal-Wallis test) was used. To identify independent predictors of death, a multivariate Cox proportional hazards model (Enter Method with all covariates together) was performed, adjusting for classic covariates and including the covariates statistically significant at the univariate analysis. Significant predictors of mortality were expressed in terms of hazard ratios (HRs) and 95% confidence intervals (CIs). Kaplan-Meier survival curves were plotted, and the groups were compared using the log-rank test. Statistical analyses were performed using SPSS 11 statistical package (SPSS Inc, Chicago, IL). A 2-sided P<.05 was considered significant.
Results
Of the 960 patients with HF enrolled in the present study, 681 (70.9%) were men and 279 (29.1%) were women. Table 1 displays demographic, clinical, and biochemical data at enrollment, as well as the pharmacological treatment of patients during follow-up. The median patient age was 69 years (interquartile range, 59-76 years). Most patients were in New York Heart Association (NYHA) functional classes II (528; 55.0%) and III (353; 36.8%) at inclusion and were treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and β-blockers. The median LVEF was 31%. Systolic function was preserved (LVEF, ≥40%) in 236 patients (24.6%) vs 724 (75.4%) with systolic dysfunction, and 532 patients (55.4%) had ischemic etiology. Heart failure etiology and statin use are shown in Figure 1. Patients with an ischemic etiology were more often treated with statins (P<.001). The main statins used were simvastatin and atorvastatin (573 of 591 patients, 97.0%). More patients in the statin treatment group had diabetes, renal failure, and anemia; they had less atrial fibrillation and were treated more intensely for HF.
TABLE 1.
Demographic, Clinical, Biochemical, and Pharmacological Treatment Data of Study Populationa-c
| Characteristic | Total cohort N=960 | Statin treatment n=591 | No statin treatment n=369 | P value |
|---|---|---|---|---|
| Age (y) | 69 (59-76) | 67 (57-73) | 72 (62-79) | .001 |
| Female sex | 279 (29.1) | 143 (24.2) | 136 (36.9) | .001 |
| Ischemic etiology | 532 (55.4) | 413 (69.9) | 119 (32.2) | .001 |
| Heart failure duration (mo) | 12 (2-48) | 12 (1-50) | 10 (2-48) | .55 |
| LVEF (%) | 31 (24-39) | 31 (24-37) | 30 (23-44) | .11 |
| Preserved LVEF (≥40%) | 236 (24.6) | 120 (20.3) | 116 (31.4) | .001 |
| NYHA functional class | .001 | |||
| I | 55 (5.7) | 39 (6.6) | 16 (4.3) | |
| II | 528 (55.0) | 354 (59.9) | 174 (47.2) | |
| III | 353 (36.8) | 188 (31.8) | 165 (44.7) | |
| IV | 24 (2.5) | 10 (1.7) | 14 (3.8) | |
| Comorbidities | ||||
| Hypertension | 563 (58.6) | 352 (59.6) | 211 (57.2) | .47 |
| Diabetes mellitus | 377 (39.3) | 252 (42.6) | 125 (33.9) | .007 |
| Hypercholesterolemia | 411 (42.8) | 346 (58.5) | 65 (17.6) | .001 |
| COPD | 200 (20.8) | 113 (19.1) | 87 (23.6) | .09 |
| Renal failure (CrCl, <60 mL/min) | 512 (53.3) | 291 (49.2) | 221 (59.9) | .001 |
| Anemia (Hb, <12 g/dL) | 317 (33.0) | 168 (28.4) | 149 (40.4) | .001 |
| Peripheral vascular disease | 171 (17.8) | 106 (17.9) | 65 (17.6) | .90 |
| OSAS | 40 (4.2) | 23 (3.9) | 17 (4.6) | .59 |
| Atrial fibrillation | 162 (16.9) | 73 (12.4) | 89 (24.1) | .001 |
| Left atrium diameter (mm/m2) | 25.6 (22.7-29.0) | 24.9 (22.4-28.0) | 27.2 (23.8-30.8) | .001 |
| LBBB | 129 (13.4) | 82 (13.9) | 47 (12.7) | .62 |
| Serum urea (mg/dL) | 57 (43-82) | 55 (42-78) | 61 (44-86) | .009 |
| Serum sodium (mmol/L) | 139 (137-141) | 139 (137-141) | 139 (136-141) | .99 |
| Serum total cholesterol (mg/dL) | 170 (143-201) | 174 (151-205) | 163 (135-194) | .001 |
| Systolic BP (mm Hg) | 120 (110-140) | 120 (110-140) | 120 (110-140) | .99 |
| Heart rate (beats/min) | 72 (63-81) | 70 (62-80) | 75 (67-85) | .001 |
| BMI (kg/m2) | 27.1 (24.1-30.5) | 27.5 (24.6-30.5) | 26.5 (22.7-30.4) | .001 |
| Treatments (follow-up) | ||||
| ACEI or ARB | 846 (88.1) | 547 (92.6) | 299 (81.0) | .001 |
| β-Blockers | 776 (80.8) | 529 (89.5) | 247 (66.9) | .001 |
| Antialdosterone | 343 (35.7) | 200 (33.8) | 143 (38.8) | .12 |
| Loop diuretics | 803 (83.6) | 473 (80.0) | 330 (89.4) | .001 |
| Digoxin | 292 (30.4) | 152 (25.7) | 140 (37.9) | .001 |
| Amiodarone | 211 (22.0) | 121 (20.5) | 90 (24.4) | .15 |
| Hydralazine | 316 (32.9) | 187 (31.6) | 129 (35.0) | .29 |
| Nitrates | 517 (53.9) | 362 (61.3) | 155 (42.0) | .001 |
| Anticoagulants | 406 (42.3) | 256 (43.3) | 150 (40.7) | .42 |
| Antiplatelet | 602 (62.7) | 430 (72.8) | 172 (46.6) | .001 |
| ICD | 80 (8.3) | 67 (11.3) | 13 (3.5) | .001 |
| CRT | 44 (4.6) | 33 (5.6) | 11 (3.0) | .06 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; BP = blood pressure; COPD = chronic obstructive pulmonary disease; CrCl = creatinine clearance; CRT = cardiac resynchronization therapy; Hb = hemoglobin; ICD = implantable cardioverter-defibrillator; LA = left auricle; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; OSAS = obstructive sleep apnea syndrome.
SI conversion factors: To convert creatinine clearance values to mL/sec, multiply by 0.0167; to convert hemoglobin values to g/L, multiply by 10.0; to convert urea values from mg/dl to mmol/L, multiply by 0.16585; to convert urea into blood urea nitrogen, multiply urea values by 0.466; to convert cholesterol values to mmol/L, multiply by 0.0259.
Data are expressed as median (interquartile range) or absolute number (percentage).
FIGURE 1.
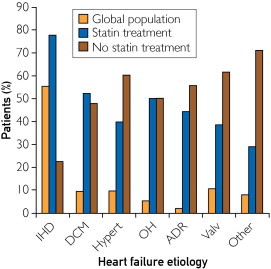
Heart failure etiology of the study population. Difference between patients treated with statins and patients not treated with statins was P<.001. ADR = drug-related cardiomyopathy; DCM = idiopathic dilated cardiomyopathy; Hypert = hypertensive cardiomyopathy; IHD = ischemic heart disease; OH = alcoholic cardiomyopathy; Valv = valular cardiomyopathy.
During the follow-up period, 440 patients (45.8%) died. The causes of death, both cardiovascular and noncardiovascular, are shown in Figure 2, and no differences were found for the statin and no-statin treatment groups (P=.34). Cardiovascular death was registered for a total of 272 patients (28.3%), including 148 (15.4%) in the statin treatment group and 124 (12.9%) in the no-statin treatment group (P=.09). However, statin therapy was associated with significantly improved survival in patients with HF of both ischemic and nonischemic etiology as observed in Cox regression analysis (HR, 0.45 [95% CI, 0.37-0.54]; P<.001). Figure 3 shows Kaplan-Meier survival curves for patients with HF of both ischemic and nonischemic etiology with or without statin treatment. In analyses of causes of death, the Cox HRs of statin treatment for cardiovascular death were as follows: HR, 0.52 (95% CI, 0.41-0.65); P<.001 for total population; HR, 0.41 (95% CI, 0.30-0.55); P<.001 for ischemic patients; and HR, 0.39 (95% CI, 0.26-0.59); P<.001 for nonischemic patients. The Cox HRs of statin treatment for noncardiovascular death were as follows: HR, 0.33 (95% CI, 0.23-0.46); P<.001 for total population; HR, 0.24 (95% CI, 0.14-0.39); P<.001 for ischemic patients; and HR, 0.37 (95% CI, 0.22-0.64); P<.001 for nonischemic patients. After adjustment for demographic and HF prognostic factors, including age, sex, cholesterol levels, NYHA functional class, HF etiology, LVEF, comorbidities, body mass index, hemoglobin level, creatinine clearance, HF duration, atrial fibrillation, implantable cardioverter-defibrillator therapy, and pharmacological treatment, statin therapy remained significantly associated with a lower risk of all-cause death in both ischemic and nonischemic patients (Table 2).
FIGURE 2.
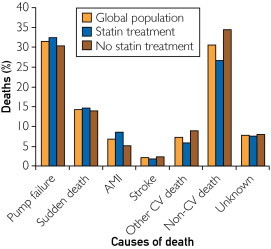
Causes of death reported in the study population. Difference between patients treated with statins and patients not treated with statins was P=.34. AMI = acute myocardial infarction; CV = cardiovascular.
FIGURE 3.
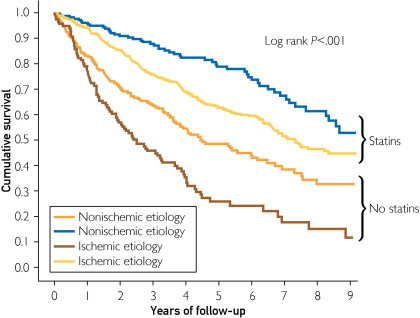
Kaplan-Meier survival curves for ischemic and nonischemic patients with heart failure according to statin use.
TABLE 2.
Multivariate Cox Regression Analysis for Ischemic vs Nonischemic Patients With Heart Failure
| Ischemic etiology |
Nonischemic etiology |
All patients |
||||
|---|---|---|---|---|---|---|
| Characteristic | Cox HR (95% CI) | P value | Cox HR (95% CI) | P value | Cox HR (95% CI) | P value |
| Age | 1.04 (1.02-1.05) | <.001 | 1.03 (1.01-1.05) | .003 | 1.03 (1.02-1.05) | <.001 |
| Female | 0.79 (0.55-1.15) | .23 | 0.61 (0.41-0.91) | .02 | 0.72 (0.55-0.93) | .01 |
| NYHA | 1.23 (0.97-1.57) | .09 | 1.53 (1.16-2.01) | .003 | 1.36 (1.14-1.63) | .001 |
| LVEF | 0.99 (0.98-1.00) | .04 | 0.99 (0.99-1.01) | .62 | 0.99 (0.98-0.99) | .02 |
| Diabetes | 1.98 (1.51-2.59) | <.001 | 1.02 (0.69-1.51) | .91 | 1.50 (1.22-1.85) | <.001 |
| Cholest | 1.00 (0.99-1.00) | .78 | 0.99 (0.99-1.00) | .05 | 0.99 (0.99-1.00) | .17 |
| COPD | 1.05 (0.75-1.46) | .79 | 1.21 (0.82-1.79) | .34 | 1.18 (0.92-1.49) | .19 |
| PVD | 1.71 (1.26-2.31) | .001 | 1.59 (0.98-2.59) | .06 | 1.62 (1.26-2.09) | <.001 |
| BMI | 1.04 (1.01-1.08) | .02 | 0.96 (0.92-0.99) | .03 | 0.99 (0.97-1.02) | .83 |
| Hb | 0.96 (0.88-1.05) | .41 | 0.93 (0.85-1.03) | .15 | 0.95 (0.89-1.01) | .13 |
| CrCl | 0.99 (0.98-0.99) | .004 | 0.99 (0.99-1.01) | .76 | 0.99 (0.99-0.99) | .03 |
| HF t | 1.00 (0.99-1.01) | .26 | 1.00 (1.00-1.01) | .03 | 1.00 (1.00-1.00) | .006 |
| AF | 1.07 (0.66-1.74) | .78 | 1.08 (0.72-1.61) | .71 | 0.99 (0.74-1.34) | .98 |
| Statins | 0.66 (0.49-0.89) | .007 | 0.54 (0.37-0.79) | .002 | 0.66 (0.53-0.83) | <.001 |
| ACEI/ARB | 0.52 (0.36-0.76) | .001 | 0.61 (0.37-1.00) | .05 | 0.52 (0.39-0.69) | <.001 |
| β-Blockers | 0.38 (0.26-0.55) | <.001 | 0.57 (0.39-0.84) | .004 | 0.51 (0.39-0.66) | <.001 |
| Antipl | 0.73 (0.51-1.05) | .09 | 0.77 (0.54-1.11) | .16 | 0.85 (0.67-1.07) | .16 |
| ICD | 0.71 (0.42-1.18) | .19 | 0.76 (0.33-1.78) | .53 | 0.77 (0.49-1.18) | .23 |
ACEI = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; Antipl = antiplatelet therapy; ARB = angiotensin receptor blocker; BMI = body mass index; Cholest = cholesterol; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CrCl = creatinine clearance; Hb = hemoglobin; HF t = heart failure duration from onset; HR = hazard ratio; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; PVD = peripheral vascular disease.
When analyzing the effect of statin treatment across NYHA functional classes, we found that the positive effects were maintained in both groups I-II (HR, 0.60 [95% CI, 0.43-0.84]; P=.003) and III-IV (HR, 0.53 [95% CI, 0.38-0.74]; P<.001).
Discussion
Although large randomized trials found that statin treatment did not reduce the number of deaths in patients with HF,7,8 our study suggests that “real-life” patients taking statins have better survival than patients with HF who are not treated with them. Our results concur with previous data reported before the GISSI-HF and CORONA trials era.3-6
These 2 large, randomized, placebo-controlled trials were designed to evaluate the role of statins in the prognosis of HF. However, both trials have issues worthy of clinical interpretation.9 For example, the CORONA trial enrolled mostly an old cohort (mean age, 73 years), with all patients older than 60 years. In the GISSI-HF trial, patients already taking statins were not included, which may have resulted in more patients with severe ischemia being excluded from the trial (HF of ischemic etiology represented only 40% of patients). In addition, patients receiving cardiac resynchronization therapy were either excluded or represented a small percentage of the studied population, and a recent retrospective analysis of the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial found that statin use is associated with improved survival in patients with advanced HF receiving resynchronization therapy.10 An editorial accompanying the CORONA study already points out that “trials simply must focus more attention on including patients who are representative of those seen in clinical practice.”11
Another issue is that both trials were conducted using the same statin, rosuvastatin, at the same dose (10 mg). First, regarding the dose, other trials have shown more beneficial results with higher doses.12 Second, rosuvastatin is a hydrophilic statin, which relies on active transport into hepatocytes to exert its effect and has poor penetration into extrahepatic tissues; thus, it has less risk of adverse effects but also very low uptake by cardiac muscle. By contrast, simvastatin and other lipophilic statins (most commonly used in this cohort) tend to achieve higher levels of exposure in nonhepatic tissues and have very high cardiac muscle uptake.13,14 In a recent meta-analysis of randomized controlled trials of statins in HF that included the GISSI-HF and CORONA trials, it was observed that randomization to lipophilic statins showed a significant benefit not observed in patients randomized to rosuvastatin.15 The authors discussed that benefits of statins in patients with HF should not be considered a class effect. They did not find any correlation between statin dose equivalence and outcome, suggesting that the type of statin used has a greater impact on outcome than the statin dosage in patients with HF.15 In real life, most patients take lipophilic statins.
An alternative theory has been raised to explain the controversial results between real-life cohorts and the large randomized trials: if patients with ischemic heart disease typically derive substantial benefit from statin therapy,16 at some point after the development of HF their cardiovascular disease is too advanced to be modified by statin therapy.17 In fact, in the CORONA trial the lowest N-terminal pro-B-type natriuretic peptide tertile did benefit from rosuvastatin therapy, with a significant reduction in the primary end point.18 It has been suggested that in milder HF, coronary events can be modified by statins, whereas in severe HF, progressive loss of pump function is not substantially improved by statin treatment.19 In our population, all NYHA functional classes benefited from statin treatment, including those patients with more advanced functional impairment. We have N-terminal pro-B-type natriuretic peptide data for a limited sample of patients, and thus we could not perform a similar analysis to that of the CORONA study.
Furthermore, the benefit obtained in noncardiovascular deaths is remarkable and was more important in patients with ischemic etiology (HR, 0.24 [95% CI, 0.14-0.39]; P<.001). There has been much debate and discussion about the role of non–lipid-lowering benefits of statins, and their pleiotropic effects have been proposed to contribute to protection against deaths due to infections and other respiratory illnesses.20,21
The limitations of our study include, first, the fact that it is an observational study. Second, the differences between statin and no-statin groups could act as confounders. However, a very comprehensive multivariate analysis was performed to minimize the influence of such differences, and statins remained highly significant in all the analyses, with HRs changing from 0.45 in the univariate analysis to 0.66 in the multivariate analysis. Nevertheless, although multivariate analysis can eliminate many covariates, it cannot eliminate the factor causally related to the decision to prescribe statins in patients who are likely to benefit from the drug, which can be considered as “indication bias.”
Conclusion
Several issues regarding the large randomized trials performed to assess the prognostic benefit of statins in patients with HF may explain the differences between their results and those in our and other real-life cohorts. The results derived from our population of prospectively enrolled patients who attended an HF clinic suggest that additional randomized trials with longer follow-up periods should be performed to assess the effect of statins other than rosuvastatin in patients with HF who are representative of those in clinical practice.
Acknowledgments
We would like to thank all the nurses of the HF unit, especially Beatriz González, Lucía Cano, and Roser Cabanes, for the collection of data for this study and their invaluable work.
Supplemental Online Material
Author Interview Video
References
- 1.Sola S., Mir M.Q., Lerakis S., Tandon N., Khan B.V. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47(2):332–337. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 2.Node K., Fujita M., Kitakaze M., Hori M., Liao J.K. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108(7):839–843. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Nye R., Levy W.C. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol. 2004;93(9):1124–1129. doi: 10.1016/j.amjcard.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Horwich T.B., MacLellan W.R., Fonarow G.C. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43(4):642–648. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Krum H., Latini R., Maggioni A.P. Statins and symptomatic chronic systolic heart failure: a post-hoc analysis of 5010 patients enrolled in Val-HeFT. Int J Cardiol. 2007;119(1):48–53. doi: 10.1016/j.ijcard.2006.07.106. [DOI] [PubMed] [Google Scholar]
- 6.Go A.S., Lee W.Y., Yang J., Lo J.C., Gurwitz J.H. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 7.GISSI-HF Investigators. Tavazzi L., Maggioni A.P., Marchioli R. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 8.Kjekshus J., Apetrei E., Barrios V., CORONA Group Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 9.Lavie C.J., Mehra M.R. Statins and advanced heart failure—alive but barely breathing after CORONA and GISSI-HF. Congest Heart Fail. 2009;15(4):157–158. doi: 10.1111/j.1751-7133.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- 10.Sumner A.D., Boehmer J.P., Saxon L.A. Statin use is associated with improved survival in patients with advanced heart failure receiving resynchronization therapy. Congest Heart Fail. 2009;15(4):159–164. doi: 10.1111/j.1751-7133.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- 11.Masoudi F.A. Statins for ischemic systolic heart failure. N Engl J Med. 2007;357(22):2301–2304. doi: 10.1056/NEJMe0707221. [DOI] [PubMed] [Google Scholar]
- 12.Khush K.K., Waters D.D., Bittner V. Effect of high-dose atorvastatin on hospitalizations for heart failure: subgroup analysis of the Treating to New Targets (TNT) study. Circulation. 2007;115(5):576–583. doi: 10.1161/CIRCULATIONAHA.106.625574. [DOI] [PubMed] [Google Scholar]
- 13.Nezasa K., Higaki K., Matsumura T. Liver-specific distribution of rosuvastatin in rats: comparison with pravastatin and simvastatin. Drug Metab Dispos. 2002;30(11):1158–1163. doi: 10.1124/dmd.30.11.1158. [DOI] [PubMed] [Google Scholar]
- 14.White C.M. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42(9):963–970. [PubMed] [Google Scholar]
- 15.Lipinski M.J., Cauthen C., Biondi-Zoccai G.L. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am J Cardiol. 2009;104(12):1708–1716. doi: 10.1016/j.amjcard.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 17.Daniels L.B., Barrett-Connor E. Can natriuretic peptides help identify heart failure patients for whom statins are beneficial? J Am Coll Cardiol. 2009;54(20):1860–1861. doi: 10.1016/j.jacc.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Cleland J.G., McMurray J.J., Kjekshus J. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Am Coll Cardiol. 2009;54(20):1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 19.Athyros V.G., Karagiannis A., Mikhailidis D.P. Statins and heart failure. J Am Coll Cardiol. 2010;55(15):1644–1645. doi: 10.1016/j.jacc.2009.11.071. [DOI] [PubMed] [Google Scholar]
- 20.Sever P.S., Chang C.L., Gupta A.K., Whitehouse A., Poulter N.R., ASCOT Investigators The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32(20):2525–2532. doi: 10.1093/eurheartj/ehr333. [DOI] [PubMed] [Google Scholar]
- 21.Tleyjeh I.M., Kashour T., Hakim F.A. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169(18):1658–1667. doi: 10.1001/archinternmed.2009.286. [published correction appears in Arch Intern Med 2010;170(1):42] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


