Abstract
Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme mediating triglyceride (TG) hydrolysis. The lack of ATGL results in TG accumulation in multiple tissues, underscoring the critical role of ATGL in maintaining lipid homeostasis. Recent evidence suggests that ATGL affects TG metabolism via activation of peroxisome proliferator-activated receptor α (PPARα). To investigate specific effects of intestinal ATGL on lipid metabolism we generated mice lacking ATGL exclusively in the intestine (ATGLiKO). We found decreased TG hydrolase activity and increased intracellular TG content in ATGLiKO small intestines. Intragastric administration of [3H]trioleate resulted in the accumulation of radioactive TG in the intestine, whereas absorption into the systemic circulation was unchanged. Intraperitoneally injected [3H]oleate also accumulated within TG in ATGLiKO intestines, indicating that ATGL mobilizes fatty acids from the systemic circulation absorbed by the basolateral side from the blood. Down-regulation of PPARα target genes suggested modulation of cholesterol absorption by intestinal ATGL. Accordingly, ATGL deficiency in the intestine resulted in delayed cholesterol absorption. Importantly, this study provides evidence that ATGL has no impact on intestinal TG absorption but hydrolyzes TGs taken up from the intestinal lumen and systemic circulation. Our data support the role of ATGL in modulating PPARα-dependent processes also in the small intestine.
Keywords: triglyceride absorption, cholesterol absorption, enterocytes, peroxisome proliferator-activated receptor alpha target genes
Absorption of fat takes place within the epithelial cells of the small intestine. The uptake of dietary triglycerides (TGs) is very effective: 95% of TG is absorbed by enterocytes. TGs are cleaved by pancreatic lipase in the lumen of the gut, resulting in the release of FFAs and monoglycerides, which are taken up by absorptive cells (1). Once inside the enterocytes, FFAs become activated and esterified by acyl-CoA:monoacylglycerol acyltransferase-2 and acyl-CoA:diacylglycerol acyltransferase-1. Reassembled TGs are packed into chylomicrons, which are transported via the lymphatic system and released into the circulation (2).
Enterocytes also store TGs within cytosolic lipid droplets (CLDs) (3). TGs found within enterocytes are derived partly from dietary sources (absorbed by the apical membrane from the gastrointestinal lumen) and partly from the systemic circulation (absorbed by the basolateral membrane from the blood) (4). Niot et al. (5) suggested that, during the postprandial period, TGs generated by acyl-CoA:diacylglycerol acyltransferase-1 are immediately available for lipoprotein synthesis, whereas TGs produced by acyl-CoA:diacylglycerol acyltransferase-2 are mainly stored as CLDs. FFAs absorbed from the apical side (intestinal lumen) are mainly used for production of acylglycerols, which are then packed into chylomicrons, whereas FFAs taken up from the basolateral side (circulation) are mainly oxidized or incorporated into phospholipids (PLs) (4). Thus, evidence supports the presence of distinct pools of neutral lipids within enterocytes.
Enterocytes exhibit acylglycerol hydrolase activity, which might be responsible for mobilizing FFAs from CLDs (6, 7). Several cytosolic and microsomal lipases, including hormone-sensitive lipase (HSL), intestinal pancreatic lipase (iPTL), arylacetamide deacetylase (AADA), and adipose triglyceride lipase (ATGL), have been identified in enterocytes. HSL has been shown to have acylglycerol and cholesteryl ester hydrolase activity in the small intestine (6). Indeed, we have recently shown that deficiency of intestinal HSL modulates cholesterol but not TG metabolism in the small intestine (8). Rat iPTL is regulated by dietary fat and has been suggested to mobilize FFAs for the transport to the liver via the portal vein (7). AADA mRNA is expressed in intestinal mucosal cells (probably enterocytes) (9); its role has not been studied. ATGL, a member of the patatin domain-containing protein A family, selectively performs the first step in TG catabolism, resulting in the formation of diglycerides (DGs) and FFAs (10). ATGL is expressed and active in most tissues and cells, such as white and brown adipose tissue, liver, brain, heart, skeletal muscle, and macrophages (11–16). Consequently, the lack of ATGL results in profound lipid accumulation in essentially all tissues, including the ileum (13). ATGL deficiency leads to down-regulation of peroxisome proliferator-activated receptor α (PPARα) target genes in certain tissues such as heart, liver, and brown adipose tissue (11, 14, 15). In the small intestine, PPARα regulates several important processes such as β-oxidation, defense against oxidative stress, and cholesterol absorption (17–19). However, the physiological relevance of ATGL in the intestine and its impact on PPARα signaling remain unknown.
To investigate the independent effect of intestinal ATGL on lipid metabolism, we generated mice lacking ATGL exclusively in the intestine (ATGLiKO), thereby excluding systemic effects of whole-body ATGL deficiency on the gut. In this study, we have determined the tissue-specific and systemic impact of intestine-specific ATGL deficiency on lipid homeostasis.
MATERIALS AND METHODS
Animals and diets
Mice carrying a LoxP-modifed Atgl allele (B6.129-Pnpla2tm1Eek mice; backcrossed onto C57BL/6 × N3; herein designated as Atgl-flox mice) were generated in the laboratory of Erin Kershaw. Briefly, LoxP sites were inserted into introns 1 and 7 of the Atgl gene using BAC recombineering and ET cloning technologies. Mice carrying the Lox-P modified Atgl allele were identified by PCR using the following primers: forward: 5′-cggtgagggtggggaacggagtc-3′ reverse: 5′-cagggggccaggcggtcaga-3′ wild-type allele 343 bp, LoxP-modified allele 497 bp. Subsequent Cre-mediated recombination of the above Atgl-flox allele results in deletion of exons 2 through 7, thereby preventing expression of a functional ATGL protein.
To produce ATGLiKO mice, Atgl-flox mice were interbred with transgenic mice expressing Cre recombinase under the control of the intestinal epithelial cell-specific Villin (Vil1) promoter B6;SJL-Tg(Vil-cre)997Gum (20) backcrossed on to C57Bl/6 × N3 (herein designated as Villin-Cre mice). ATGLflox/flox/Villin-Cre mice were then mated to ATGLflox/flox mice to generate the following experimental groups: ATGLflox/flox/Villin-Cre (ATGLiKO) and ATGLflox/flox (control) mice. All experiments were performed using male ATGLiKO mice and their corresponding control littermates at 12 to 16 weeks of age. Mice had free access to food and water under a 12-h light/12-h dark cycle in a temperature-controlled environment. ATGLiKO and control mice were fed chow diet (11.9% caloric intake from fat; Ssniff®, Soest, Germany) or challenged with a high-fat diet (HFD) for 3 or 6 weeks at the age of 7 to 10 weeks. The HFD contained 30% (wt/wt) crude fat (Ssniff®, Soest, Germany). HFD-fed mice were housed individually, and food intake was monitored over a period of 3 days. Food intake was calculated as g/day/mouse. All experiments were conducted in conformity with the Public Health Service on Human Care and Use of laboratory Animals, approved by the Division of Genetic Engineering and Animal Experiments, Austrian Federal Ministry of Science and Research (Vienna, Austria).
Plasma lipid analysis
TG, total cholesterol (TC), and nonesterified fatty acid (NEFA) concentrations were assayed in plasma from 4 h-fasted mice using enzymatic kits according to manufacturer's protocols (DiaSys, Holzheim, Germany; Wako Chemicals GmbH, Neuss, Germany).
Western blotting analysis
Mucosal scrapings were sonicated (Labsonic B. Braun, Melsungen, Germany) in RIPA buffer, and protein concentrations were determined by Bradford assay. Tissue lysates (pools of three mice) were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane. For detection of ATGL protein, anti-ATGL polyclonal antibody (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:200. Monoclonal anti-mouse β-actin (1:5,000) (Santa Cruz Biotechnology, Heidelberg, Germany) was used as loading control.
RNA isolation and quantitative real-time PCR
Total RNA from tissues was extracted using TriFast according to the manufacturer's protocol (Peqlab, Erlangen, Germany). Total RNA (2 µg) were reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed on a Roche LightCycler 480 (Roche Diagnostics, Palo Alto, CA) using the QuantiFastTM SYBR® Green PCR Kit (Qiagen, Valencia, CA). Samples were analyzed in duplicate and normalized to the expression of cyclophilin A. Expression profiles and associated statistical parameters were calculated using the public domain program Relative Expression Software Tool – REST 2008 (http://www.gene-quantification.com/download.html). Primer sequences are available upon request.
TG hydrolase activity assay
TG hydrolase activity was assayed as previously described (12). Briefly, intestinal protein was isolated from HFD-fed mice. One hundred micrograms of protein in 100 µl of 100 mM potassium phosphate lysis buffer was incubated with 100 µl TG substrate (25 nmol triolein/assay and 40,000 cpm/nmol [9,10-3H]triolein; PerkinElmer, Boston, MA) and 35.5 µg mixed micelles of phosphatidylcholine and phosphatidylinositol (3:1, w/w), respectively. After incubation at 37°C for 1 h, the reaction was terminated by adding 3.25 ml methanol-chloroform-heptane (10:9:7, v/v/v) and 1 ml 100 mM potassium carbonate (pH 10.5 with boric acid). After centrifugation (800 g, 15 min, 4°C), the radioactivity in 1 ml of the upper phase was determined by liquid scintillation counting.
Tissue lipid analysis
ATGLiKO and control littermates were fed chow or HFD for 6 weeks. After a fasting period of 4 h, three parts of the small intestine (duodenum, jejunum, ileum) and livers were collected. Lipids were extracted by the Folch extraction method. The lipid extract was dried under a stream of nitrogen. One hundred microliters 1% Triton-X100 in chloroform was added, and lipids were dried again under a stream of nitrogen gas. Thereafter, the samples were dissolved in 100 μl ddH2O, and TG and TC concentrations were measured using the above-mentioned kits. The readings were normalized to protein concentrations.
Intestinal TG absorption
Overnight-fasted mice were intraperitoneally injected with the lipase inhibitor tyloxapol (500 mg/kg in PBS; Sigma-Aldrich, St. Louis, MO) to prevent peripheral lipolysis. Thirty minutes after injection, mice were gavaged with 200 μl corn oil containing 2 μCi [3H]trioleate to assess dietary fat absorption. Plasma was collected 3 and 6 h, and livers and intestines were collected 6 h after gavage. Radioactivity was determined by liquid scintillation counting. For determination of the distribution of lipid classes, duodena were lyophilized overnight, and lipids from 40 mg tissue were extracted in chloroform:methanol 2:1 and separated by thin-layer chromatography using n-hexane-diethylether-acetic acid (80:20:2, v/v/v). TG-, DG-, FFA-, and PL-corresponding bands were cut, and radioactivity was measured by liquid scintillation counting. TG uptake was studied in overnight-fasted mice gavaged with 2 µCi [3H]trioleate provided in 200 µl corn oil. Mice were euthanized 30 min after gavage. Intestines were collected, and radioactivity was determined by liquid scintillation counting. For fecal fat measurements, feces of HFD-fed mice were collected and weighed, and lipids were extracted as described above. To determine FFA uptake over a period of 3 days, mice were gavaged with 200 µl corn oil containing 5 µCi [3H]trioleate. Feces were collected daily, and radioactivity was measured in lipid extracts.
Gut transit
Overnight-fasted mice were gavaged with 200 µl Evans blue suspension (5% Evans blue, 5% gum Arabic in PBS). Afterward mice had free access to food and water, and the time until the detection of Evans blue in the feces was recorded.
TG uptake from the blood
To investigate if ATGLiKO mice accumulate TG taken up from the basolateral side of enterocytes, mice were injected with 500 µl Intralipid (Fresenius Kabi Austria GmbH, Graz, Austria) containing 7 µCi [3H]oleate. Mice were euthanized 6 h after injection, and small intestines were collected. To reduce gallbladder emptying, mice were fasted during this experiment. Lipids were extracted from 30 mg of lyophilized duodenum, jejunum, and ileum. Lipid extracts of duodenum were separated by thin-layer chromatography. TG-, DG-, FFA-, and PL-corresponding bands were cut, and radioactivity was measured by liquid scintillation counting.
Intestinal cholesterol uptake and absorption
Cholesterol uptake and absorption was performed as previously described (21) with minor modifications. Briefly, mice fed a chow diet were fasted for 4 h before gavage with 200 µl corn oil containing 2 µCi [3H]cholesterol (ARC Inc., St. Louis, MO) and 200 µg cholesterol. Plasma, livers, and intestines were collected 4 h after gavage, and radioactivity was determined by liquid scintillation counting.
Fractional cholesterol absorption was measured by the fecal dual-isotope ratio method as described (21). Briefly, mice were fasted for 4 h before they were given a single intragastric dose of [3H]sitostanol (0.2 µCi; ARC Inc.) and 0.1 µCi [14C]cholesterol (ARC Inc.) in 100 µl corn oil. Feces were collected for 48 h. Fecal lipids were extracted using the Folch extraction method, and radioactivity was determined by liquid scintillation counting. Fractional cholesterol absorption was calculated by the following formula: % absorption = dose [14C]:[3H]fecal [14C]:[3H])/dose [14C]:[3H] × 100.
Oil Red O staining
Jejunum was isolated and fixed in 4% neutral-buffered formalin (Carl Roth GmbH, Vienna, Austria). Serial sections (8 μm) of the jejunum were cut (HM 560 Cryo-Star; Microm International GmbH, Walldorf, Germany) and stained with oil Red O and Mayer's hematoxylin. Microscopic images were taken using a Nikon Eclipse E600 equipped with a Nikon Digital Sight DS-U1 unit (Spach Optics Inc., New York, NY).
Statistical analysis
Statistical differences between groups were analyzed using unpaired Student's t-test (GraphPad Prism 5.0, San Diego, CA). Data are represented as means ± SEM for the specified number of animals. P values ≤ 0.05 were considered statistically significant.
RESULTS
ATGL is efficiently knocked out in the small intestine of ATGLiKO mice
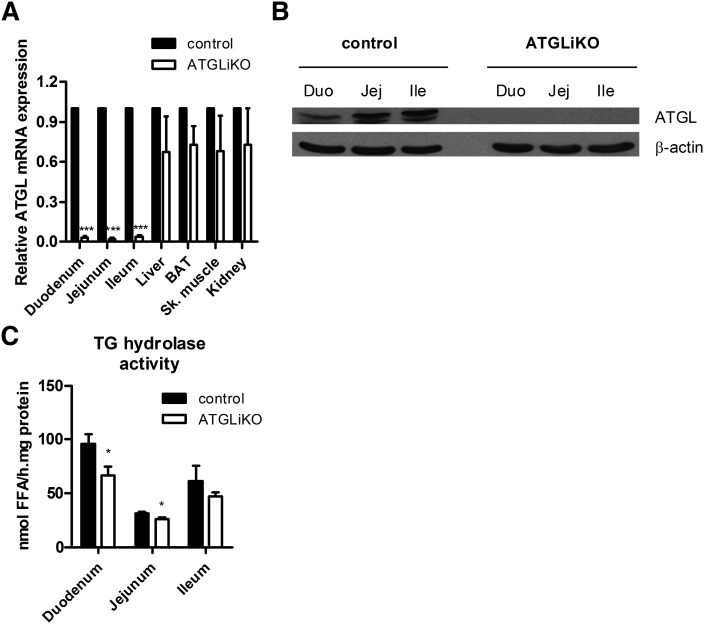
To elucidate the physiological function of intestinal ATGL on whole-body lipid homeostasis and to exclude systemic effects of whole-body ATGL deficiency on intestinal lipid metabolism, we eliminated ATGL from the intestinal epithelium. To confirm ATGL deletion in adult ATGLiKO intestine, we isolated three parts of the small intestine (duodenum, jejunum, and ileum) and examined ATGL expression. ATGL mRNA was markedly down-regulated in duodenum, jejunum, and ileum of ATGLiKO mice compared with control mice, whereas no differences were observed in liver, brown adipose tissue, skeletal muscle, and kidney (Fig. 1A). ATGL protein expression was undetectable in ATGLiKO duodenum, jejunum, and ileum (Fig. 1B). TG hydrolase activity was reduced in the duodenum and jejunum of ATGLiKO mice (31% and 15%, respectively) (Fig. 1C).
Fig. 1.
ATGL is knocked out specifically in the small intestine. A: ATGL mRNA was drastically down-regulated in all three parts of the small intestine (duodenum, jejunum, ileum) but unchanged in control tissues (liver, brown adipose tissue, skeletal muscle, kidney). Data represent mean values ± SEM (n = 3). ***P ≤ 0.001. :) Protein lysates of pools from three mice of each genotype were separated by SDS-PAGE. ATGL protein expression was analyzed by Western blotting. The expression of β-actin served as loading control. C: TG hydrolase activity was determined in duodenum, jejunum, and ileum of HFD-fed mice. Data represent mean values ± SEM (n = 4). *P ≤ 0.05.
Intestinal ATGL deficiency has no impact on plasma lipid parameters and body weights
Next, we determined plasma TG and cholesterol concentrations in chow-fed and HFD-fed ATGLiKO mice. Plasma TG, TC, and NEFA concentrations were comparable between ATGLiKO and control mice, as were food intake and body weight before and after feeding a HFD (Table 1).
TABLE 1.
Plasma lipid parameters of 4 h fasted mice fed chow (aged 8 weeks) or HFD (aged 14 weeks) for 6 weeks and body weights before and after feeding. TG, TC, and NEFA concentrations were determined enzymatically. Data are expressed as mean values ± SEM
| Chow |
HF |
||||||||
| TC | TG | NEFA | Body weight | TC | TG | NEFA | Body weight | Food intake | |
| mg/dl | mg/dl | mM | g | mg/dl | mg/dl | mM | g | g/mouse/day | |
| Control | 85 ± 5.9 | 76 ± 5.1 | 0.70 ± 0.1 | 16.15 ± 1 | 182 ± 12.6 | 48 ± 3.7 | 0.54 ± 0.02 | 27.1 ± 0.9 | 3.13 ± 0.1 |
| ATGLiKO | 92 ± 8.8 | 87 ± 11.4 | 0.57 ± 0.1 | 17.18 ± 1 | 167 ± 8.0 | 58 ± 3.1 | 0.58 ± 0.02 | 27.0 ± 0.8 | 3.36 ± 0.2 |
TGs accumulate in small intestines of ATGLiKO mice
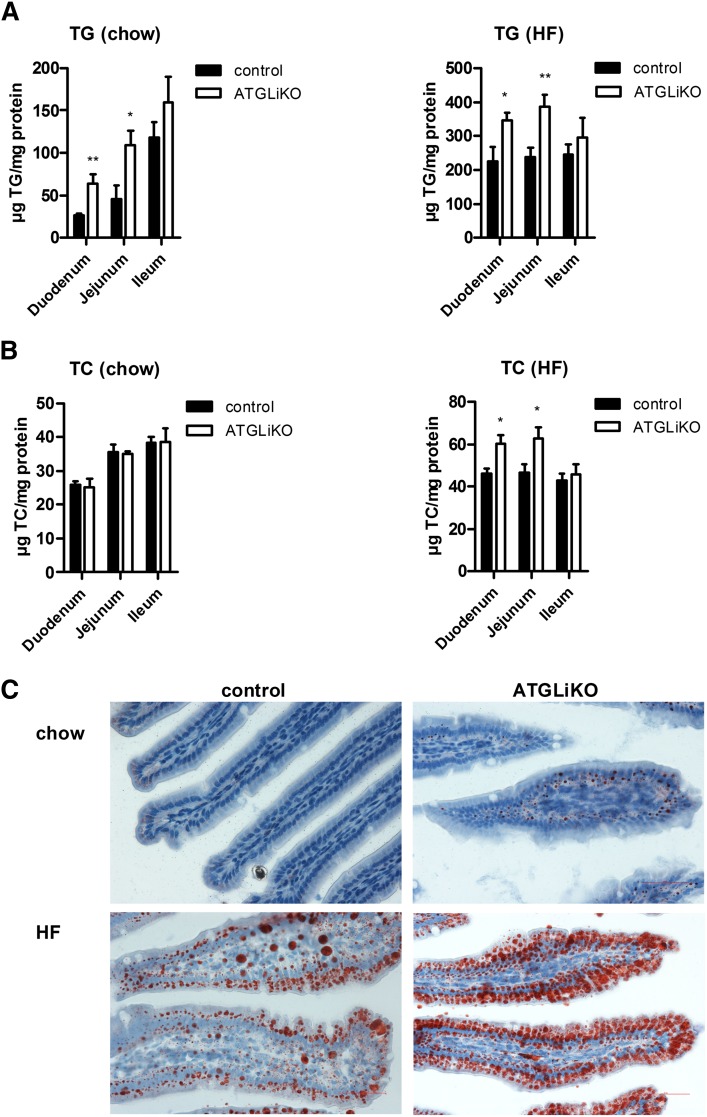
Analyses of intestinal TG and cholesterol concentrations in chow and HFD-fed ATGLiKO mice revealed a markedly elevated TG content in duodenum (2.4- and 1.5-fold, respectively) and jejunum (2.4- and 1.6-fold, respectively) (Fig. 2A). Intestinal cholesterol content was unchanged in chow diet-fed mice but increased in duodenum (1.3-fold) and jejunum (1.4-fold) after HFD feeding (Fig. 2B). Oil Red O staining of jejuna confirmed an increased number of lipid droplets in chow-fed (Fig. 2C, upper panel) and HFD-fed ATGLiKO mice compared with control mice (Fig. 2C, lower panel). Hepatic TG concentrations were comparable in ATGLiKO and control mice (supplementary Fig. I).
Fig. 2.
Intestinal TG and TC accumulation in ATGLiKO mice. TG (A) and TC (B) concentrations in duodenum, jejunum, and ileum from mice fed chow or HFD for 6 weeks. Data represent mean values ± SEM (n = 5–6). *P ≤ 0.05; **P ≤ 0.01. C: Oil Red O staining of jejunum in mice fed chow (upper panel) or HFD (lower panel) for 6 weeks. Images were taken using a Nikon Eclipse E600 microscope equipped with a Nikon Digital Sight DS-U1 unit. Magnification, ×40. Scale bars: 50 µm.
ATGLiKO mice accumulate TGs in the small intestine from dietary sources
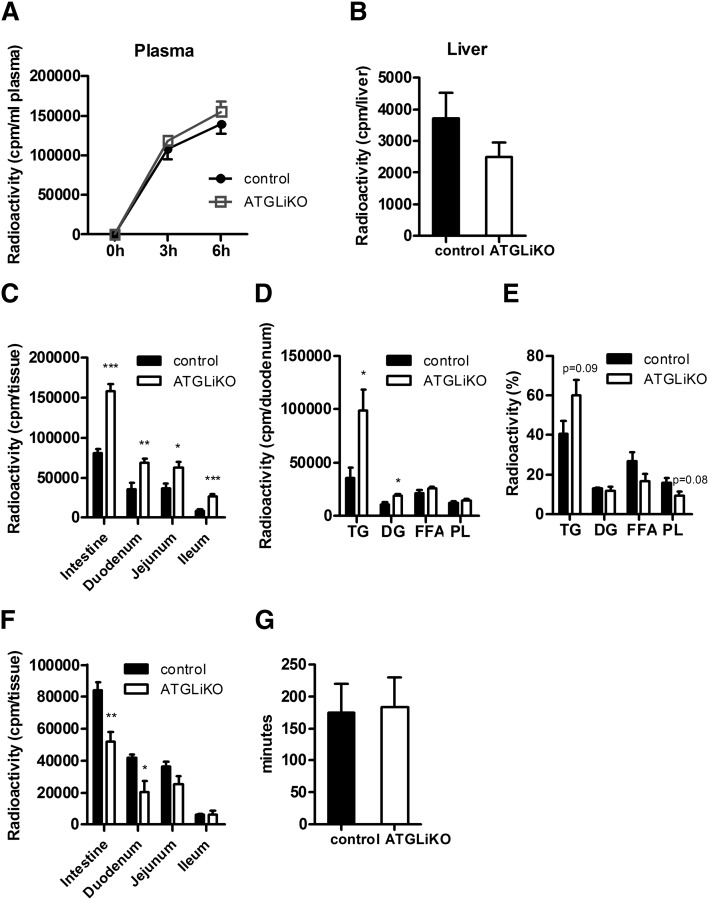
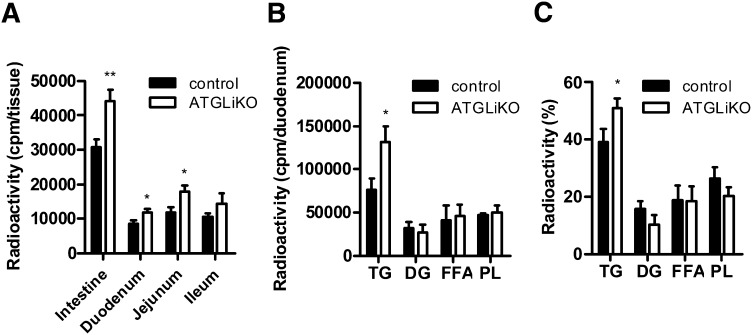
To determine whether ATGL is directly involved in absorption of dietary TGs, we blocked peripheral lipolysis by tyloxapol injection before gavaging mice with 200 µl corn oil containing 2 µCi [3H]trioleate. Absorption into plasma (Fig. 3A) and liver (Fig. 3B) was comparable between genotypes. However, 6 h after gavage, ATGLiKO mice had significantly increased radioactivity in all three parts of the small intestine (Fig. 3C), indicating that dietary lipids accumulate in ATGLiKO enterocytes. Further analysis of the disposal of this radioactivity from dietary lipids into specific lipid classes within duodenal enterocytes revealed increased radioactivity in TGs (2.8-fold) and DGs (1.7-fold) but not in FFAs or PLs of ATGLiKO compared with control mice (Fig. 3D). Radioactivity accumulation within specific subclasses expressed as a percentage of total lipids further revealed a trend to a relative increase in accumulation into TGs and decreased accumulation into PLs (Fig. 3E). These data suggest that ATGL action may be important for transfer of diet-derived lipids from TGs into PLs.
Fig. 3.
Accumulation of dietary TG in small intestines of ATGLiKO mice. A–E: Mice fed chow diet were injected with tyloxapol to inhibit peripheral lipolysis. Thereafter they were gavaged with 200 µl corn oil containing 2 µCi [3H]trioleate. A: Radioactivity in the plasma was determined 3 and 6 h after gavage. Mice were euthanized 6 h after gavage, and radioactivity in liver (B) and whole small intestine, duodenum, jejunum, and ileum (C) was measured by scintillation counting. Distribution (D) and relative distribution (E) of radioactivity in lipid species of duodenum 6 h after gavage. Data represent mean values ± SEM (n = 5). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. F: Radioactivity in the whole small intestine, duodenum, jejunum, and ileum was determined 30 min after gavage of 200 µl corn oil containing 2 µCi [3H]trioleate. Data represent mean values ± SEM (n = 4). *P ≤ 0.05; **P ≤ 0.01. G: Mice were gavaged with 200 µl Evans blue, and gut transit was determined by recording the time until appearance in the feces. Data represent mean values ± SEM (n = 3).
To determine whether the intestinal uptake in the early phase of absorption is disturbed in ATGLiKO mice, we isolated the small intestines 30 min after gavage of [3H]trioleate. Radioactivity was decreased by 38% in ATGLiKO small intestines, consistent with a delayed uptake of FFAs into enterocytes (Fig. 3F). This effect was most pronounced in the duodenum (49% reduction in ATGLiKO compared with control mice). Differences in enterocyte TG accumulation were independent on gut transit, which was identical in both genotypes (Fig. 3G).
Fecal fat is increased but FFA uptake is unchanged in ATGLiKO mice
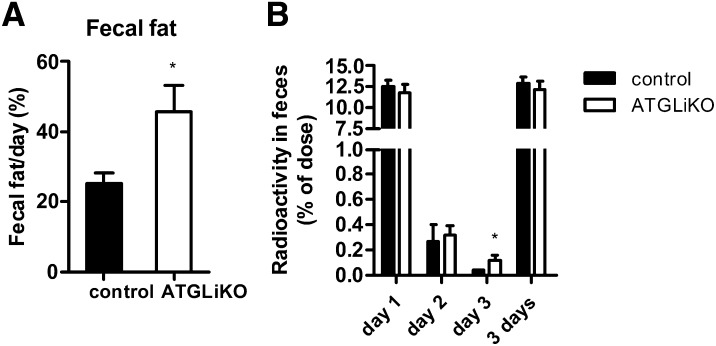
We then investigated whether intestinal ATGL deficiency affects FFA uptake from the intestinal lumen. Fecal fat weight was increased by 1.8-fold in HFD-fed ATGLiKO mice (Fig. 4A). In addition, we collected the feces of [3H]trioleate-gavaged mice over a period of 3 days. We found comparable amounts of radioactivity at day 1 and 2 but increased counts at day 3 (Fig. 4B). These data indicate that the observed increase in fecal fat weight is likely due to sloughed lipid-filled enterocytes, whereas the quantity of FFA uptake is unaffected.
Fig. 4.
Increased fecal fat loss but unchanged fat absorption in ATGLiKO mice. A: Feces of HFD-fed mice were collected over a period of 3 days, and weights of lipid extracts were determined. Data represent mean values ± SEM (n = 5–8). *P < 0.05. B: Mice were gavaged with 5 µCi [3H]trioleate, and feces were collected over a period of 3 days. Radioactivity was measured in lipid extracts from each day. Data represent mean values ± SEM (n = 5–6). *P ≤ 0.05.
ATGLiKO mice accumulate TGs in the small intestine from the systemic circulation
To elucidate whether ATGLiKO enterocytes accumulate TGs from the systemic circulation via the basolateral membrane of enterocytes, we intraperitoneally injected [3H]oleate into fasted ATGLiKO and control mice and determined the radioactivity in various parts of the small intestine and different lipid classes 6 h after injection. ATGLiKO mice showed a 1.4-fold increase in radioactivity in the small intestine mainly due to elevated counts in duodenum and jejunum (1.4- and 1.5-fold, respectively) (Fig. 5A). ATGLiKO duodena showed a marked increase of radioactivity (1.7-fold) in the TG fraction (Fig. 5B). Calculation of the relative distribution revealed a trend switch from DGs and PLs to TGs in ATGLiKO duodena (Fig. 5C). These results demonstrate that enterocytes lacking ATGL accumulate TGs derived from FFAs taken up from the systemic circulation by the basolateral side of enterocytes.
Fig. 5.
Accumulation of basolaterally absorbed TG in the small intestine of ATGLiKO mice. A–C: Mice were injected with 500 µl Intralipid containing 7 µCi [3H]oleate. Six hours after injection, mice were euthanized, and small intestines were isolated. A: Radioactivity in the whole small intestine, duodenum, jejunum, and ileum. Absolute (B) and relative lipid distribution (C) in the duodenum. Data represent mean values ± SEM (n = 6–9). *P ≤ 0.05; **P ≤ 0.01.
ATGL deficiency results in down-regulation of intestinal PPARα target genes
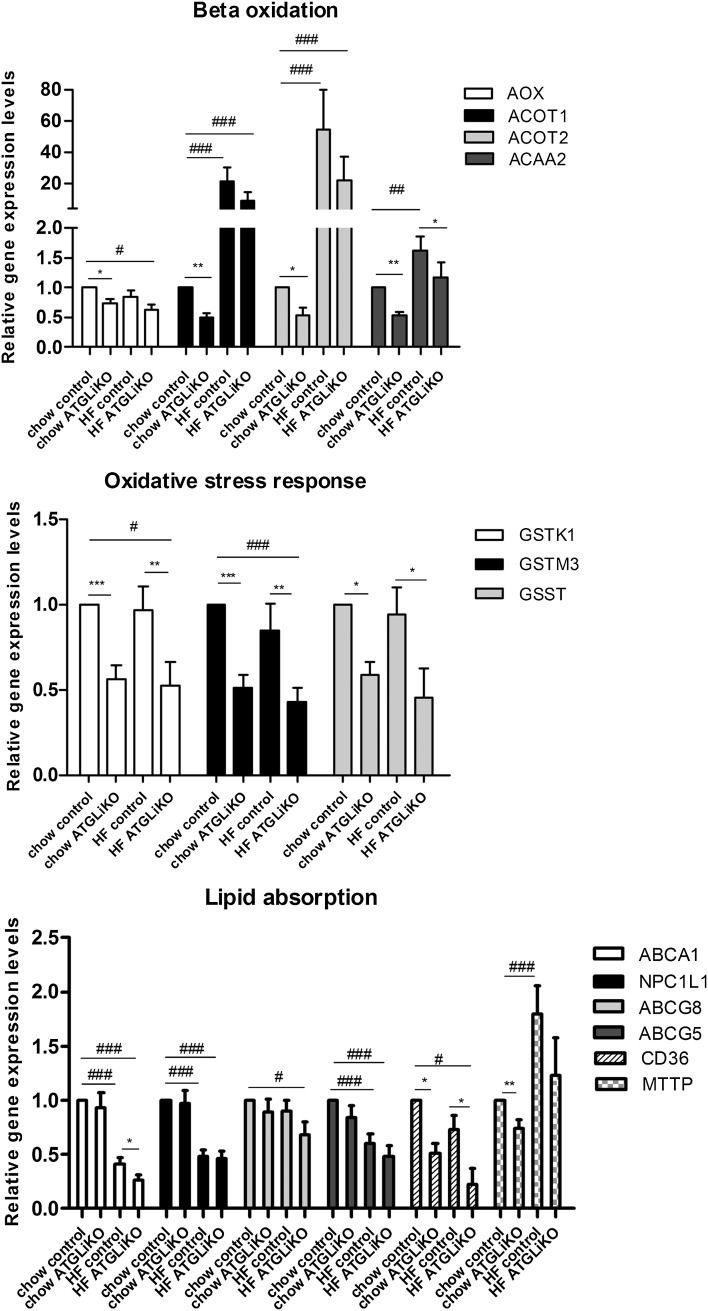
ATGL deficiency has been shown to modulate PPARα target gene expression in several tissues (11, 14, 15). We therefore determined the expression of intestinal PPARα target genes, which regulate β-oxidation, oxidative stress response, and cholesterol absorption. In mice fed a chow diet, we observed decreased mRNA expression of genes involved in β-oxidation (acyl-CoA oxidase, acyl-CoA thioesterase 1, acyl-CoA thioesterase 2, acetyl-CoA acyltransferase 2) and oxidative stress (glutathion-S-transferase kappa 1, glutathion-S-transferase mu 3, glutathion-S-transferase teta) (Fig. 6A). mRNA expression of genes involved in lipid absorption were unchanged except for CD36 antigen (CD36) and microsomal triglyceride transfer protein, which were down-regulated in jejunum of ATGLiKO mice (Fig. 6). In HFD-fed mice, relative transcript levels of genes modulating β-oxidation tended to be lower but did not reach statistical significance, except for acetyl-CoA acyltransferase 2. Genes involved in oxidative stress response were down-regulated, suggesting reduced adaptation of ATGLiKO intestines to oxidative stress. Decreased mRNA expression levels of Abca1 and CD36 further implicate ATGL in FFA uptake and cholesterol absorption in the setting of HFD feeding (Fig. 6). mRNA expression in jejuna isolated from fed mice revealed unchanged PPARα target gene expression in ATGLiKO mice (supplementary Fig. II). These results indicate that in the small intestine ATGL is mainly important for PPARα activation during negative energy balance.
Fig. 6.
mRNA expression of intestinal PPARα target genes are down-regulated in the jejunum of ATGLiKO mice. Jejunal mRNA expression after 4 h fasting of mice fed chow or HFD for 3 weeks. Data represent mean values ± SEM (n = 3–4). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (control compared with ATGLiKO mice). #P ≤ 0.05; ##P ≤ 0.01;l ###P ≤ 0.001 (chow-fed controls compared with HFD-fed control and ATGLiKO mice).
ATGLiKO mice have delayed cholesterol absorption
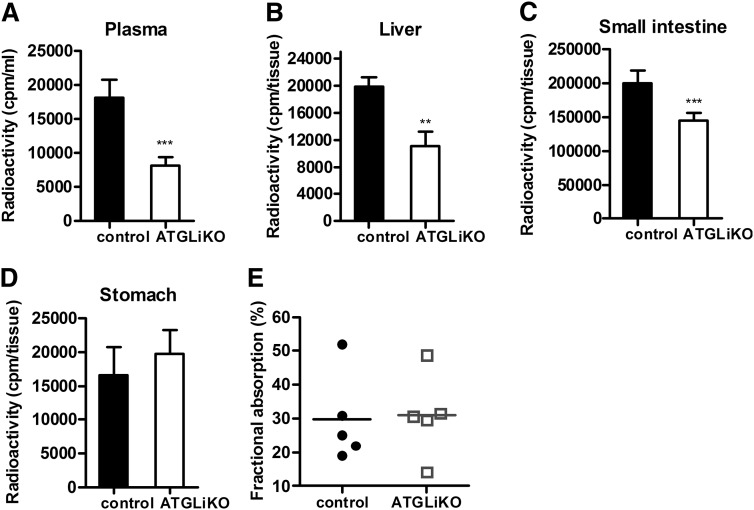
Because CD36 was down-regulated in the jejunum of ATGLiKO mice, we tested whether cholesterol absorption is affected by intestinal ATGL deficiency. We found markedly reduced radioactivity in plasma (Fig. 7A), liver (Fig. 7B), and small intestine (Fig. 7C) of ATGLiKO compared with control mice 4 h after gavage of [3H]cholesterol. Comparable amounts of radioactivity in the stomach (Fig. 7D) suggest unaltered gastric retention. Fractional cholesterol absorption, however, was unchanged in ATGLiKO mice (Fig. 7E). These data indicate that equivalent amounts of cholesterol are absorbed into the body of ATGLiKO and control mice with delayed absorption in ATGLiKO mice.
Fig. 7.
Delayed cholesterol absorption in ATGLiKO mice. A–D: Mice were gavaged with 200 µl corn oil containing 2 µCi [3H]cholesterol and 200 µg cholesterol. Radioactivity in plasma (A), liver (B), small intestine (C), and stomach (D) was measured by liquid scintillation counting 4 h after gavage. Data represent mean values ± SEM (n = 6–7). **P ≤ 0.01; ***P ≤ 0.001. E: Fractional cholesterol absorption determined by the fecal dual-isotope ratio method. Data represent mean values ± SEM (n = 5).
Because bile acids are able to influence cholesterol absorption, we determined mRNA expression of apical sodium-dependent bile acid transporter and farnesoid X receptor. Unchanged gene expression (supplementary Fig. III), however, suggests that intestinal ATGL deficiency does not modulate bile acid metabolism.
DISCUSSION
During the postprandial period, dietary TGs are transiently stored in CLDs of enterocytes (22), thereby providing substrates for the formation of chylomicrons during the intraprandial period. Because CLDs become smaller during absorption (22), this pathway likely requires efficient TG hydrolysis by lipase(s) (5). ATGL is known to catalyze the initial step in lipolysis by hydrolyzing TGs into DGs and FFAs (23–25). Consequently, ATGL deficiency results in TG accumulation in essentially all tissues and cells (12, 13, 26). We therefore hypothesized that ATGL is a possible candidate for TG catabolism in the small intestine.
Although TG hydrolase activity was reduced in ATGLiKO mice, these results revealed that, besides ATGL, additional lipases are involved in intestinal TG degradation, leading to the observed relatively high residual TG hydrolase activity. We have previously shown that HSL contributes to intestinal TG hydrolase activity in vitro (8). Mahan and colleagues (7) reported the intestinal expression of iPTL, which also exhibits TG hydrolase activity. Moreover, several carboxylesterases, such as Ces1, Ces3, and AADA, were shown to be expressed in the small intestine and might also contribute to intestinal lipid metabolism (as reviewed in Ref. 27). High residual TG hydrolase activity in the absence of ATGL was also reported in other organs, such as the brain [26]) or the liver (15), suggesting that additional (maybe so far unknown) lipases contribute to neutral TG breakdown in these tissues.
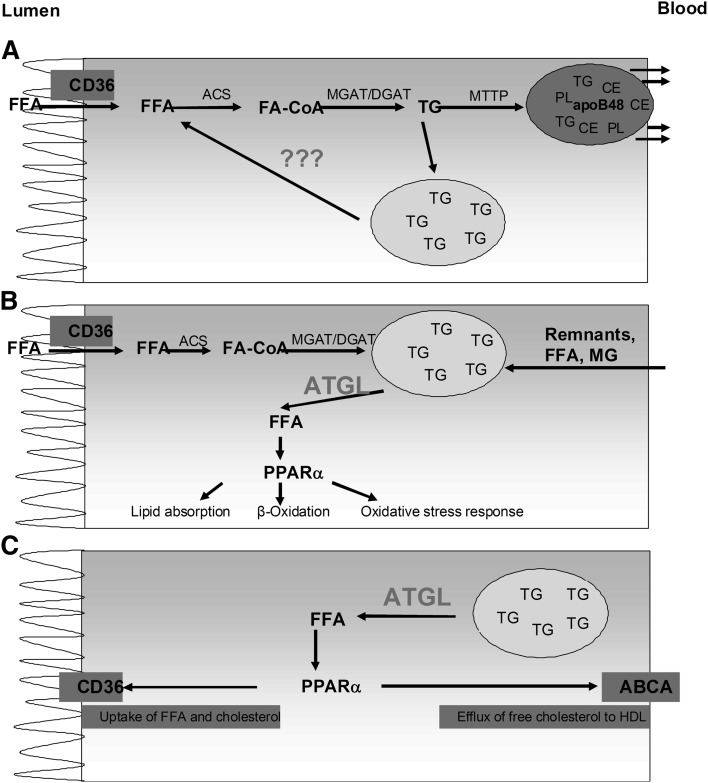
Here we show that the disruption of intestinal ATGL results in modulation of intestinal lipid metabolism. Although we found that radiolabeled TGs accumulated in the small intestine in response to a dietary TG challenge, we observed comparable amounts of radioactivity in plasma and liver. A direct involvement of ATGL in dietary TG absorption would have led to an accumulation of radioactive TGs in the small intestine as observed but also to reduced amounts of radioactivity in plasma and liver. These findings indicate that ATGL hydrolyzes CLD TGs but is not directly involved in TG absorption or TG release from enterocytes into the periphery (Fig. 8A). Our observations are in agreement with data obtained in livers from hepatocyte-specific ATGL-deficient mice, in which very low-density lipoprotein (VLDL) release was comparable to control mice (28). Unchanged TG absorption in the small intestine demonstrated in this study and unchanged VLDL release shown by others (15, 28) suggest that ATGL is not required for providing lipids for lipoprotein assembly in the small intestine or liver.
Fig. 8.
Role of ATGL in enterocytes. A FFAs taken up from the intestinal lumen are esterified and packed into chylomicrons or stored within cytosolic lipid droplets. The responsible enzyme providing FFA as TG hydrolysis products for re-esterification into TGs, which are further released via chylomicrons, is unknown. B: Lipids taken up from the apical (intestinal lumen) and the basolateral side (blood) are stored in CLDs and hydrolyzed by ATGL. FFAs released by ATGL activate PPARα, thereby modulating mRNA expression of genes involved in lipid absorption, β-oxidation, and oxidative stress response. C: ATGL regulates the expression of CD36 and ABCA1 via PPARα, thereby mediating FFA uptake from the apical surface and cholesterol absorption. ACS, acetyl-CoA synthase.
Treatment of mice with tyloxapol, which impedes plasma lipolytic activity, thereby blocking the formation of FFAs and chylomicron remnants, leads to the inhibition of the basolateral uptake of lipids into enterocytes. The fact that during this experimental approach TGs accumulate in ATGLiKO intestines demonstrates that ATGL hydrolyzes TGs after lipid uptake from the intestinal lumen. In addition, intraperitoneal administration of [3H]oleate resulted in increased radioactivity in the TG fraction of ATGLiKO small intestines. Because mice were fasted during this experiment, thereby avoiding contraction of the gallbladder, we reduced the contribution of tracer derived from the bile. We cannot completely exclude, however, a possible uptake of biliary lipids from the lumen. We therefore conclude that ATGL within enterocytes additionally mobilizes FFAs absorbed from the bloodstream (Fig. 8B). The process by which enterocytes take up lipids from the basolateral side has not been elucidated. It is known that enterocytes express essentially all important transporters necessary to take up lipoproteins (29). Moreover, it was reported that the small intestine is able to take up chylomicron remnants (30, 31) and FFAs (4). FFAs taken up from the bloodstream are mainly used for oxidative purposes and for PL synthesis. TGs taken up from the intestinal lumen, however, are predominantly transported in chylomicrons via the lymphatic system into the blood (4). These observations, together with data from the present study, provide evidence that different TG pools exist in enterocytes, whereby ATGL preferentially hydrolyzes TGs from the CLD pool used for oxidative purposes and PL synthesis.
When ATGLiKO mice were fed a HFD, we observed increased fecal fat weight. Reduced lipid uptake (32) might have been one possible explanation. Gavage of [3H]trioleate, however, resulted in comparable TG absorption and unchanged amounts of radioactivity in lipid extracts from feces, suggesting that the amount of FFAs taken up is unaltered in ATGLiKO mice. Notably, the lifespan of enterocytes is only about 2 to 3 days (33). Dead enterocytes are expelled into the intestinal lumen (34), and the lipid content of sloughed enterocytes also adds to fecal fat weight (35). Because ATGLiKO enterocytes accumulate TGs, it is likely that sloughing of fat-filled enterocytes is the cause for increased fecal fat weight in ATGLiKO mice. Plasma lipid parameters, body weights, and liver TG concentrations are unaffected in ATGLiKO mice, demonstrating that intestinal ATGL deficiency has negligible effects on whole-body lipid metabolism within the time frame of this study. This finding might be explained by unchanged absolute TG and cholesterol absorption in ATGLiKO mice.
Recent studies revealed that ATGL is an important player in the regulation of PPARα target genes (11, 14, 15). Intestinal PPARα regulates genes involved in lipid absorption (19, 36), β-oxidation (37), and defense against oxidative stress (18). In liver and heart (14, 15), ATGL deficiency resulted in decreased mRNA expression of genes regulating β-oxidation. We observed a similar down-regulation of these genes in the small intestine of 4 h fasted ATGLiKO mice. In addition, mRNA expression of the transport protein CD36 was reduced in ATGLiKO small intestine. In the small intestine, CD36 is regulated by PPARα (38) and is involved in the uptake of FFAs and cholesterol from the intestinal lumen (39). We therefore conclude that the down-regulation of CD36 was the reason for the delayed uptake of FFAs when small intestines were isolated 30 min after gavage of radioactive [3H]trioleate. Unchanged mRNA abundances of PPARα target genes in small intestines of fed mice suggest that ATGL is crucial to regulate PPARα-dependent processes exclusively in the fasted state.
In addition to CD36, mRNA abundance of Abca1 (mediating the efflux of unesterified cholesterol to HDL [40]) is down-regulated in ATGLiKO HFD-fed mice. Reduced abundance of CD36 and Abca1 mRNA suggested that ATGL might be involved in cholesterol homeostasis. Intestinal ABCA1 deficiency is associated with decreased HDL cholesterol, delayed cholesterol absorption into the blood, and increased intestinal cholesterol concentrations (40). Moreover, activation of PPARα was reported to increase Abca1 expression and consequently intestinal HDL production (41). An acute cholesterol uptake experiment revealed delayed uptake of cholesterol into the small intestine and diminished release of cholesterol into the plasma of ATGLiKO mice, which might be seen as a consequence of reduced CD36 expression. mRNA levels of the main cholesterol importer NPC1L1 and the cholesterol exporters ABCG5/G8 were unaltered. In accordance, fractional cholesterol absorption determined by the fecal dual-isotope method showed unchanged cholesterol absorption. This result is not surprising because CD36 was shown to be crucial only for cholesterol uptake of the proximal small intestine (39). We speculate that elevated cholesterol levels in intestines from HFD-fed ATGLiKO mice are the consequence of ABCA1 down-regulation because intestinal Abca1-deficient mice exhibit unchanged fractional cholesterol absorption but increased intestinal cholesterol concentrations (40). The modulation of FFA uptake and cholesterol absorption by ATGL is summarized in Fig. 8C.
In summary, this study identifies ATGL as an important TG hydrolase of the small intestine. Accumulation of TGs and modulation of PPARα signaling, thereby influencing the rate of apical FFA uptake and basolateral cholesterol efflux, highlight the role of ATGL in intestinal lipid homeostasis.
Supplementary Material
Acknowledgments
The authors thank A. Ibovnik for excellent technical assistance and I. Hindler for mice care.
Footnotes
Abbreviations:
- AADA
- arylacetamide deacetylase
- ATGL
- adipose triglyceride lipase
- ATGLiKO
- intestine-specific ATGL-deficient
- CD36
- CD36 antigen
- CLD
- cytosolic lipid droplet
- DG
- diglyceride
- HFD
- high-fat diet
- HSL
- hormone sensitive lipase
- iPTL
- intestinal pancreatic triglyceride lipase
- NEFA
- non-esterified fatty acid
- PL
- phospholipid
- PPARα
- peroxisome proliferator-activated receptor alpha
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by the Austrian Science Fund (P22832, SFB-LIPOTOX F30, DK-MCD W1226, and P19186) and the Austrian National Bank (12929). S.O. is a fellow of and P.G.C. and J.V.P. were supported by the PhD Program Molecular Medicine of the Medical University of Graz. Additional funding was provided by NIH grant R01DK090166 and HHMI Early Career Award (E.E.K.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Whitcomb D. C., Lowe M. E. 2007. Human pancreatic digestive enzymes. Dig. Dis. Sci. 52: 1–17 [DOI] [PubMed] [Google Scholar]
- 2.Iqbal J., Hussain M. M. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee B., Zhu J., Wolins N. E., Cheng J. X., Buhman K. K. 2009. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim. Biophys. Acta. 1791: 1173–1180 [DOI] [PubMed] [Google Scholar]
- 4.Storch J., Zhou Y. X., Lagakos W. S. 2008. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J. Lipid Res. 49: 1762–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niot I., Poirier H., Tran T. T., Besnard P. 2009. Intestinal absorption of long-chain fatty acids: evidence and uncertainties. Prog. Lipid Res. 48: 101–115 [DOI] [PubMed] [Google Scholar]
- 6.Grober J., Lucas S., Sorhede-Winzell M., Zaghini I., Mairal A., Contreras J. A., Besnard P., Holm C., Langin D. 2003. Hormone-sensitive lipase is a cholesterol esterase of the intestinal mucosa. J. Biol. Chem. 278: 6510–6515 [DOI] [PubMed] [Google Scholar]
- 7.Mahan J. T., Heda G. D., Rao R. H., Mansbach C. M., 2nd 2001. The intestine expresses pancreatic triacylglycerol lipase: regulation by dietary lipid. Am. J. Physiol. Gastrointest. Liver Physiol. 280: G1187–G1196 [DOI] [PubMed] [Google Scholar]
- 8.Obrowsky S., Chandak P. G., Patankar J. V., Pfeifer T., Povoden S., Schreiber R., Haemmerle G., Levak-Frank S., Kratky D. 2012. Cholesteryl ester accumulation and accelerated cholesterol absorption in intestine-specific hormone sensitive lipase-null mice. Biochim. Biophys. Acta. 1821: 1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trickett J. I., Patel D. D., Knight B. L., Saggerson E. D., Gibbons G. F., Pease R. J. 2001. Characterization of the rodent genes for arylacetamide deacetylase, a putative microsomal lipase, and evidence for transcriptional regulation. J. Biol. Chem. 276: 39522–39532 [DOI] [PubMed] [Google Scholar]
- 10.Lass A., Zimmermann R., Oberer M., Zechner R. 2011. Lipolysis: a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50: 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadian M., Abbott M. J., Tang T., Hudak C. S., Kim Y., Bruss M., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., et al. 2011. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., et al. 2010. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285: 20192–20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737 [DOI] [PubMed] [Google Scholar]
- 14.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong K. T., Mashek M. T., Bu S. Y., Greenberg A. S., Mashek D. G. 2011. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 53: 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J. W., Wang S. P., Casavant S., Moreau A., Yang G. S., Mitchell G. A. 2012. Fasting energy homeostasis in mice with adipose deficiency of desnutrin/adipose triglyceride lipase. Endocrinology. 153: 2198–2207 [DOI] [PubMed] [Google Scholar]
- 17.Bunger M., van den Bosch H. M., van der Meijde J., Kersten S., Hooiveld G. J., Muller M. 2007. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiol. Genomics. 30: 192–204 [DOI] [PubMed] [Google Scholar]
- 18.de Vogel-van den Bosch H. M., Bunger M., de Groot P. J., Bosch-Vermeulen H., Hooiveld G. J., Muller M. 2008. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genomics. 9: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Bosch H. M., Bunger M., de Groot P. J., van der Meijde J., Hooiveld G. J., Muller M. 2007. Gene expression of transporters and phase I/II metabolic enzymes in murine small intestine during fasting. BMC Genomics. 8: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madison B. B., Dunbar L., Qiao X. T., Braunstein K., Braunstein E., Gumucio D. L. 2002. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277: 33275–33283 [DOI] [PubMed] [Google Scholar]
- 21.Chandak P. G., Obrowsky S., Radovic B., Doddapattar P., Aflaki E., Kratzer A., Doshi L. S., Povoden S., Ahammer H., Hoefler G., et al. 2011. Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim. Biophys. Acta. 1811: 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J., Lee B., Buhman K. K., Cheng J. X. 2009. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J. Lipid Res. 50: 1080–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. 2004. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975 [DOI] [PubMed] [Google Scholar]
- 24.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. 2004. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279: 47066–47075 [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386 [DOI] [PubMed] [Google Scholar]
- 26.Etschmaier K., Becker T., Eichmann T. O., Schweinzer C., Scholler M., Tam-Amersdorfer C., Poeckl M., Schuligoi R., Kober A., Chirackal Manavalan A. P., et al. 2011. Adipose triglyceride lipase affects triacylglycerol metabolism at brain barriers. J. Neurochem. 119: 1016–1028 [DOI] [PubMed] [Google Scholar]
- 27.Quiroga A. D., Lehner R. 2012. Liver triacylglycerol lipases. Biochim. Biophys. Acta. 1821: 762–769 [DOI] [PubMed] [Google Scholar]
- 28.Wu J. W., Wang S. P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K. G., Yang G., Mitchell G. A. 2011. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 54: 122–132 [DOI] [PubMed] [Google Scholar]
- 29.Temel R. E., Brown J. M. 2012. Biliary and nonbiliary contributions to reverse cholesterol transport. Curr. Opin. Lipidol. 23: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansbach C. M., 2nd, Dowell R. F. 1995. Role of the intestine in chylomicron remnant clearance. Am. J. Physiol. 269: G144–G152 [DOI] [PubMed] [Google Scholar]
- 31.Soued M., Mansbach C. M., 2nd 1996. Chylomicron remnant uptake by enterocytes is receptor dependent. Am. J. Physiol. 270: G203–G212 [DOI] [PubMed] [Google Scholar]
- 32.Franck P., Sallerin J. L., Schroeder H., Gelot M. A., Nabet P. 1996. Rapid determination of fecal fat by Fourier transform infrared analysis (FTIR) with partial least-squares regression and an attenuated total reflectance accessory. Clin. Chem. 42: 2015–2020 [PubMed] [Google Scholar]
- 33.Tsuboi K. K., Kwong L. K., Sunshine P., Castillo R. O. 1992. Mechanism of maturational decline of rat intestinal lactase-phlorizin hydrolase. Biochem. J. 282: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminsky L. S., Zhang Q. Y. 2003. The small intestine as a xenobiotic-metabolizing organ. Drug Metab. Dispos. 31: 1520–1525 [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086 [DOI] [PubMed] [Google Scholar]
- 36.Knight B. L., Patel D. D., Humphreys S. M., Wiggins D., Gibbons G. F. 2003. Inhibition of cholesterol absorption associated with a PPAR alpha-dependent increase in ABC binding cassette transporter A1 in mice. J. Lipid Res. 44: 2049–2058 [DOI] [PubMed] [Google Scholar]
- 37.Kimura R., Takahashi N., Murota K., Yamada Y., Niiya S., Kanzaki N., Murakami Y., Moriyama T., Goto T., Kawada T. 2011. Activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) suppresses postprandial lipidemia through fatty acid oxidation in enterocytes. Biochem. Biophys. Res. Commun. 410: 1–6 [DOI] [PubMed] [Google Scholar]
- 38.Uchida A., Slipchenko M. N., Cheng J. X., Buhman K. K. 2011. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, alters triglyceride metabolism in enterocytes of mice. Biochim. Biophys. Acta. 1811: 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauli A. M., Nassir F., Zheng S., Yang Q., Lo C. M., Vonlehmden S. B., Lee D., Jandacek R. J., Abumrad N. A., Tso P. 2006. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 131: 1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colin S., Briand O., Touche V., Wouters K., Baron M., Pattou F., Hanf R., Tailleux A., Chinetti G., Staels B., et al. 2012. Activation of intestinal peroxisome proliferator-activated receptor-alpha increases high-density lipoprotein production. Eur. Heart J.In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.