Abstract
Serum samples from 489 free-ranging white-tailed deer (Odocoileus virginianus) were screened for antibodies against the Eastern equine encephalitis virus (EEEV) using plaque reduction neutralization tests (PRNTs). EEEV antibodies were detected in 10.2% of serum samples. This is the first evidence that EEEV is present in Vermont. Serum was collected from deer in all 14 counties in the state, and positive EEEV sera were found in 12 (85%) of 14 counties, suggesting statewide EEEV activity in Vermont. Analysis of the spatial distribution of PRNT-positive samples revealed a random distribution of EEEV throughout the state. The results indicate widespread EEEV activity in Vermont and suggest that EEEV is not a recent introduction to the state but that EEEV activity has not been detected until now.
Introduction
Eastern equine encephalitis (EEE) is an acute neurologic mosquito-borne disease caused by the EEE virus (EEEV), an alphavirus in the family Togavirdae.1 It was first established as a cause of human illness in 1938.1 Although it is an uncommon cause of illness, the severity of infection with EEEV makes it medically important. It is a highly virulent virus and results in death in approximately 31–75% of people who become ill.2–4 Up to 70% of survivors can have significant residual neurologic sequelae.1,3
EEEV is maintained in an enzootic cycle of bird-biting mosquitoes, primarily Culiseta (Climacura) melanura (Coquillett), and passerine birds in fresh water swamps.5,6 Humans, equids, camelids, and ratites are susceptible to illness from EEEV.1,7,8 The mosquito vectors responsible for transmitting the virus to mammals are thought to include those in the genera Coquillettidia, Culex, and Aedes.9,10 These vectors are known as bridge vectors, because they bite both birds and mammals.
In North America, EEEV has been found in foci along the Atlantic and Gulf Coasts up into southern Canada. However, before 2000, EEEV was rarely detected or suspected in northern New England. In 2005, New Hampshire experienced a resurgence in EEEV activity, with seven confirmed human cases of locally acquired disease. This was the first time that locally acquired human infection was detected in New Hampshire in 41 years of surveillance.2 In Maine, before 2009, EEEV activity had only rarely been reported from the southernmost part of the state. However, in 2009, EEEV infection was identified in 15 horses, 1 llama, and 3 flocks of pheasants, with much of the activity occurring in the central part of the state.11 In addition, Quebec reported the disease in 19 horses and a flock of emus in 2008. Before 2008, the disease had only been reported two times in Quebec.12 EEEV has also been found in humans, horses, and mosquitoes in New York, including two horses in Clinton County, which borders northern Vermont.13,14
Although EEEV had been reported in the surrounding areas, it had never been detected in Vermont. Passive surveillance for human and veterinary cases had not detected any illness attributed to this virus, and limited mosquito surveillance did not yield any EEEV-positive mosquitoes. Vermont has hardwood acidic swamps, which are thought to be the preferred habitat for Cs. melanura, and the use of resting box traps in recent years has yielded significant numbers of this species.15,16 In recent years, a lack of resources has meant that mosquito trapping was mostly limited to a small region of the state where nuisance mosquito species are particularly abundant. Cs. melanura have been collected from this region, which is in the western central part of Vermont, but no pools have tested positive for EEEV. Given that EEEV has caused human or animal illness in Vermont's bordering states, the virus is likely present in Vermont as well.
In the fall of 2010, the Centers for Disease Control and Prevention (CDC), the Vermont Department of Health, and the Vermont Agency of Agriculture, Food and Markets initiated a deer serosurvey project to try to document exposure to EEEV in wild white-tailed deer (Odocoileus virginianus) and determine the distribution of the virus within the state. The white-tailed deer has been shown to be a reasonable sentinel species that is frequently exposed to EEEV.17–20 White-tailed deer also represent a large proportion of the mammalian source for blood meals for the most common bridge vector species.21 In addition, white-tailed deer have a summer home range that is about 1 mile in radius, to which they return faithfully.22,23 This range makes it likely that the site of harvest is close to the site of exposure, and it can provide an accurate estimation of the distribution of EEEV. Finally, collecting blood samples at deer check stations during hunting season is a simple, efficient, and economical way to sample a large population of animals. In this article, we present the results of a preliminary serosurvey conducted to detect EEEV activity and determine the distribution of the virus in Vermont, a state in which EEEV activity had previously never been shown.
Methods
Serum samples.
Volunteers went to 19 deer check stations on youth weekend and opening weekend of rifle season to collect serum samples. Youth weekend heralds the opening of rifle season, and deer of any age can be harvested on that weekend as long as the shooter is 15 years old or younger. Youth weekend occurred on November 6 and 7 in 2010. During youth weekend, the busier check stations were manned by wildlife biologists from the Vermont Department of Fish and Wildlife, who were able to provide estimated ages as well as collect teeth for more precise cementum age analysis. Serum samples were also collected at deer check stations on November 13 and 14, which was the opening weekend of rifle season. During rifle season, hunting is limited to older bucks that have one antler with two or more points 1 in or longer.
Deer presented to the deer check stations are typically field-dressed, and therefore, all internal organs have been removed. Disposable plastic pipettes were used to collect blood that pooled in the body cavity of the deer. The blood was then place in a 7.5-mL tiger-top Vacutainer tube and placed in a cooler with ice packs. The deer tag number, town, and county of harvest were recorded on a log sheet. The place of harvest was provided by the hunter and marked on a DeLorme Vermont Atlas. Blood was stored for 24–72 hours on ice packs until it could be delivered to the Vermont Agency of Agriculture Laboratory, where it was centrifuged to separate out the serum. The serum samples were stored frozen at −20°C and shipped on dry ice to the CDC's laboratory in Fort Collins, CO for antibody screening.
Samples were collected from deer in all 14 Vermont counties. However, only three samples were obtained from Essex County in the northeastern part of the state, and only four samples were collected from Windham County in the southeastern part. In total, 489 deer blood samples were collected and tested for antibodies against EEEV.
Serologic tests.
Deer serum samples diluted 1:10 were screened for EEEV-neutralizing antibodies by plaque-reduction neutralization assay (PRNT).24 Any samples initially testing positive were retested and titrated in duplicate for confirmation. Serum samples were considered positive for EEEV antibodies if they neutralized 80% of a challenge dose of ∼100 plaque-forming units of EEE-Sindbis chimeric virus.25 Titers are presented as the reciprocal of the dilution showing ≥ 80% reduction in the number of plaque-forming units (PRNT80). To ensure that neutralization was specific to EEEV and not resulting from antibody cross-reactivity, samples with low neutralizing titers were also screened for antibodies to Highlands J virus. The Highlands J virus strain used for these PRNTs was MW8-5AD, which was isolated from a mosquito pool in Maryland in 1968.
Spatial analysis.
Of the 489 deer from which blood samples were collected, only 459 could be accurately geocoded to the location of collection. Each deer collection site identified by the hunter and marked on a DeLorme Vermont Atlas was geocoded and imported into a geographic information system (GIS; ArcGIS 10, ESRI, Redlands, CA). Results of serological testing were included as attributes of the deer collection data. Moran I statistic was used to determine whether samples were randomly distributed with regard to PRNT results. Results were considered to significantly deviate from a random spatial distribution when the P value was less than 0.05.
Results
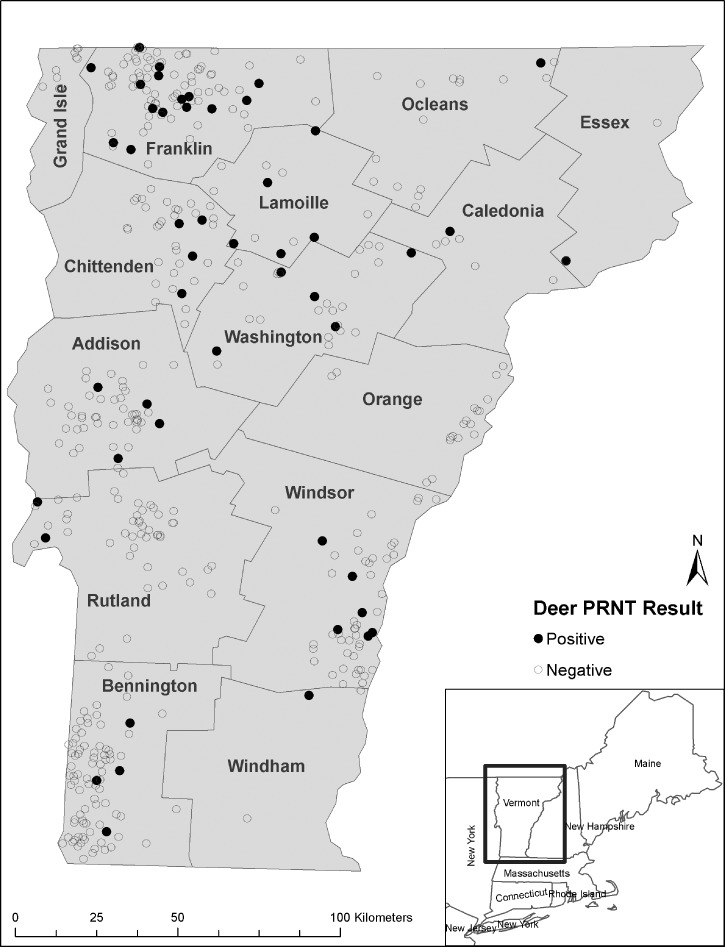
Deer serum samples (489) were tested for EEEV neutralizing antibodies by PRNT, and 50 (10.2%) samples tested positive for these antibodies; 12 of Vermont's 14 counties had samples that tested positive. Among the counties with at least 10 samples tested, Lamoille county had the highest proportion of positive samples (41.7%) followed by Washington (18.5%), Franklin (16.3%), Chittenden (11.1%), Windsor (9.2%), Addison (8.2%), Orleans (6.3%), Bennington (4.7%), and Rutland (3.8%) counties. Caledonia, Windham, and Essex counties each had 1 positive sample, but fewer than 10 samples were submitted from each of these counties. Orange and Grand Isle counties were the only two Vermont counties that did not have a positive sample (Figure 1 and Table 1). Because analysis of spatial patterns in point-based seroprevalence by administrative boundaries can bias the statistical significance of spatial analyses (know as the modifiable areal unit problem), we analyzed the spatial distribution of PRNT-positive or -negative sera obtained from deer based on their points of collection. Our analysis revealed a random distribution with respect to PRNT results (Moran I = 0.010, P = 0.092).
Figure 1.
Location of geocoded deer by county in Vermont. Deer serum samples were screened for EEE-neutralizing antibodies by PRNT. The area of interest is shown in the inset.
Table 1.
Number and percentage of EEEV antibody-positive deer sera by county in Vermont in 2010
| County | No. deer serum samples screened | No. EEEV antibody-positive sera (% [95% CI]) |
|---|---|---|
| Franklin | 92 | 15 (16.3 [9.8–24.9]) |
| Bennington | 86 | 4 (4.7 [1.5–10.8]) |
| Windsor | 65 | 6 (9.2 [3.8–18.2]) |
| Rutland | 53 | 2 (3.8 [0.6–11.9]) |
| Addison | 49 | 4 (8.2 [2.6–18.5]) |
| Chittenden | 36 | 4 (11.1 [3.6–24.7]) |
| Washington | 27 | 5 (18.5 [7.1–36.4]) |
| Orleans | 16 | 1 (6.3 [0.3–27.2]) |
| Lamoille | 12 | 5 (41.7 [17.2–68.0]) |
| Unknown | 11 | 1 (9.1 [0.5–37.3]) |
| Caledonia | 7 | 1 (14.3 [0.7–53.0]) |
| Windham | 4 | 1 (25.0 [1.3–75.8]) |
| Essex | 3 | 1 (33.3 [1.7–86.8]) |
| Orange | 18 | 0 (0–15.3) |
| Grand Isle | 10 | 0 (0–25.9) |
| Total | 489 | 50 (10.2 [7.8–13.2]) |
CI = confidence interval.
PRNT80 neutralizing antibody titers against EEEV ranged from ≥ 10 to ≥ 2,560; 34 (68%) samples had neutralizing titers greater than 160. Sixteen (32%) of the EEEV antibody-positive samples were collected from deer estimated to be 1.5 years or younger; 93 of the deer were aged more precisely by their teeth. Of these deer, 12 (13%) deer were EEEV antibody-positive, and 8 (67%) deer were determined to be yearlings. None of the samples had neutralizing antibodies against Highlands J virus.
Discussion
This study shows that free-ranging white-tailed deer in Vermont have evidence of exposure to EEEV and provides the first evidence that this virus is present in the state. The high prevalence of infection observed during this short-term surveillance effort, along with the spatial distribution of positive samples (e.g., random distribution of positive samples throughout the state as opposed to a focal cluster of positives), suggests that EEEV is well-established in Vermont. Furthermore, the random distribution of positive samples suggests contiguous distribution of EEEV activity in Vermont. The work by Morris9 argued that contiguous distribution was probably the original pattern of EEEV activity distribution in North America and that the commonly observed, geographically isolated foci of EEEV activity are a result of natural change and human development. The fact that human population density is low in Vermont suggests limited human development, which would favor contiguous distribution of EEEV activity. In addition, the finding of young animals with high titers of antibodies against EEEV suggests recent infections, although residual antibodies caused by passive immunity cannot be completely ruled out. Deer serosurveillance seems to be a sensitive and cost-effective method for the detection of EEEV activity in an area. The overall seropositivity rate of 10.2% in this study is similar to the 14% rate found in Georgia and the 7.1% rate found in Maine in 2010.17–19
Culiseta melanura, the primary enzootic vector, and Coquillettidia, Culex, and Aedes spp., which are likely bridge vectors of EEEV, have previously been documented in Vermont.9,16 These potential bridge vectors are widely distributed in Vermont, but the distribution of Cs. melanura is unknown.16 The limited trapping and testing that has been done in the state has not detected EEEV in mosquitoes. During the 2010 mosquito season, the use of resting boxes was emphasized to increase the number of Cs. melanura trapped for testing. In 2010, 409 mosquito pools consisting of 3,546 mosquitoes were tested for EEEV; 24% of the tested mosquitoes were Cs. melanura, which was three times as many as in 2009.16 However, this increase in testing still did not lead to the detection of EEEV. Mosquito surveillance has proven to be an insensitive method for detecting EEEV in Vermont. This insensitivity may be because EEEV transmission is typically focal, and therefore, finding infected mosquitoes has been difficult; also, it may be because the level of circulating EEEV is too low to detect with limited mosquito trapping.
In September of 2011, after the study described here, EEEV was found to be the cause of significant morbidity and mortality on an emu farm in Rutland County (Berl E., unpublished data). This case was the first time that EEEV has been documented as the cause of animal illness in Vermont. Considering these serosurvey results, it raises the possibility that other animal or human cases have been overlooked. Despite the large horse population in this rural state, Vermont has not recorded a single equine case of EEE. Although the equine EEEV vaccine is known to be very effective at preventing illness in horses, it is probable that not all horses in the state have been vaccinated.26 Furthermore, the availability of vaccine has not prevented outbreaks in horses in other regions.11,12 It is possible that equine cases have occurred and not been recognized or reported, despite annual efforts to educate veterinarians about diagnosing and reporting arboviral diseases. Our deer serosurvey results show that EEEV is widespread within the state of Vermont and may pose a greater threat to human and animal health than previously recognized. Furthermore, our findings suggest that deer can serve as effective sentinels for surveillance of EEEV.
ACKNOWLEDGMENTS
The authors would like to thank wildlife biologists from the Vermont Department of Fish and Wildlife for their help in collecting serum samples and aging deer on youth weekend. Many volunteers helped to collect serum samples. The authors would like to thank students from the University of Vermont, Green Mountain College, and Paul Smiths College and the staff from the Vermont Department of Health, the Vermont Agency of Agriculture, Food and Markets, the Vermont Department of Forest, Parks and Recreation, the Vermont Center for Ecostudies, and the United States Department of Agriculture, Animal, and Plant Health Inspection Service. We would also like to thank the hunters for allowing us to collect blood samples from their deer and their willingness to reveal their harvest sites.
Footnotes
Financial support: The work done in this study was supported by the Centers for Disease Control and Prevention, the Vermont Department of Health, and the Vermont Agency of Agriculture, Farm and Markets.
Authors' addresses: Erica Berl, Vermont Department of Health, Burlington, VT, E-mail: Erica.berl@state.vt.us. Rebecca J. Eisen, Katherine MacMillan, and John-Paul Mutebi, Centers for Disease Control and Prevention, Division of Vector-Borne Diseases, Fort Collins, CO, E-mails: dyn2@cdc.gov, iky4@cdc.gov, and jmutebi@cdc.gov. Bethany N. Swope, Inviragen, Inc., Fort Collins, CO, E-mail: bethany.swope+CDC@gmail.com. Kali D. Saxton-Shaw, Entomology Ecology Activity, Arboviral Diseases Branch, Centers for Disease Control and Prevention, Fort Collins, CO, E-mail: KSaxtonShaw@cdc.gov. Alan C. Graham and Jon P. Turmel, Vermont Agency of Agriculture, Food and Markets, Waterbury, VT, E-mails: Alan.graham@state.vt.us and Jon.turmel@state.vt.us.
References
- 1.Markott L. Alphaviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practices of Infectious Disease. 6th Ed. Philadelphia, PA: Churchill Livingstone; 2005. 1913. [Google Scholar]
- 2.Stull JW, Talbot EA, MacRae S, Montero JT, Matyas B, Cantor F, Konomi T, DeMaria A, Hayes EB, Smith TL, Nasci RS, Sejvar JJ, O'Leary DR, Campbell GL, Noga AJ, Lanciotti RS, Plotinsky RN, Schumacher S, Farnon EC. Eastern equine encephalitis – New Hampshire and Massachusetts, August–September 2005. MMWR Morb Mortal Wkly Rep. 2006;55:697–700. [PubMed] [Google Scholar]
- 3.Deresiewicz RL, Thaler SJ, Hsu L, Zamani A. Clinical and Neuroradiographic manifestations for eastern equine encephalitis. N Engl J Med. 1997;336:1867–1874. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 4.Przelomski MM, O'Rourke E, Grady GF, Berardi VP, Markley HG. Eastern equine encephalitis in Massachusetts: a report of 16 cases, 1970–1984. Neurology. 1988;38:736–739. doi: 10.1212/wnl.38.5.736. [DOI] [PubMed] [Google Scholar]
- 5.Howard JJ, Wallis RC. Infection and transmission of eastern equine encephalomyelitis virus with colonized Culiseta melanura (coquillett) Am J Trop Med Hyg. 1974;23:522–525. doi: 10.4269/ajtmh.1974.23.522. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong PM, Andreadis TG. Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerg Infect Dis. 2010;16:1869–1874. doi: 10.3201/eid1612.100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day JE, Start LM. Eastern equine encephalitis transmission to emus (Dromaius novaehollandiae) in Volusia County, Florida: 1992 through 1994. J Am Mosq Control Assoc. 1996;12:429–436. [PubMed] [Google Scholar]
- 8.Nolen-Walston R, Bedenice D, Rodriguez C, Rushton S, Bright A, Fecteau ME, Short D, Majdalany R, Tewari D, Pedersen D, Kiupel M, Maes R, Del Piero F. Eastern equine encephalitis in 9 South American camelids. Vet Intern Med. 2007;21:846–852. doi: 10.1892/0891-6640(2007)21[846:eeeisa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Morris CD. Eastern equine encephalomyelitis. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 1–20. [Google Scholar]
- 10.Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibney KB, Robinson S, Mutebi JP, Hoenig DE, Bernier BJ, Webber L, Lubelczyk C, Nett RJ, Fischer M. Eastern equine encephalitis: an emerging arboviral disease threat, Maine, 2009. Vector Borne Zoonotic Dis. 2011;11:637–639. doi: 10.1089/vbz.2010.0189. [DOI] [PubMed] [Google Scholar]
- 12.Chénier S, Côté G, Vanderstock J, Macieira S, Laperle A, Hélie P. An eastern equine encephalomyelitis (EEE) outbreak in Quebec in the fall of 2008. Can Vet J. 2010;51:1011–1015. [PMC free article] [PubMed] [Google Scholar]
- 13.Young DS, Kramer LD, Maffei JG, Dusek RJ, Backenson PB, Mores CH, Bernard KA, Ebel GD. Molecular epidemiology of eastern equine encephalitis virus, New York. Emerg Infect Dis. 2008;14:454–460. doi: 10.3201/eid1403.070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Department of Agriculture, Animal Plant and Health Inspection Service, Veterinary Services Summary of Eastern Equine Encephalitis Cases in the United States. 2009 March 13. 2008. http://www.aphis.usda.gov/vs/nahss/equine/ee/ Available at. Accessed February 29, 2012.
- 15.Sorenson E, Popp R, Lew-Smith M, Engstrom B, Lapin M, Ferguson M. Hardwood Swamps of Vermont: Distribution, Ecology, Classification, and Some Sites of Ecological Significance for Nongame and Natural Heritage Program. Vermont Fish and Wildlife Department, Agency of Natural Resources. Waterbury, (VT) 2004. http://www.vtfishandwildlife.com/library/reports_and_documents/nongame_and_Natural_Heritage/Natural_Communities/Hardwood%20Swamps%20of%20Vermont.pdf Available at. Accessed December 21, 2011.
- 16.Vermont Agency of Agriculture Food and Markets Annual Reports on Mosquito Testing and Trapping Waterbury (VT) 2011. http://www.vermontagriculture.com/ARMES/plantindustry/entomology/mosquito/MosquitoReports.html Available at. Accessed December 21, 2011.
- 17.Mutebi JP, Lubelczyk C, Eisen R, Panella N, MacMillan K, Godsey M, Swope B, Young G, Smith RP, Kantar L, Robinson S, Sears S. Using wild white-tailed deer to detect eastern equine encephalitis virus activity in Maine. Vector Borne Zoonotic Dis. 2011;11:1403–1409. doi: 10.1089/vbz.2011.0643. [DOI] [PubMed] [Google Scholar]
- 18.Tate CM, Howerth EW, Stallknecht DE, Allison AB, Fischer JR, Mead DG. Eastern equine encephalitis in a free-ranging white-tailed deer (Odocoileus virginianus) J Wildl Dis. 2005;41:241–245. doi: 10.7589/0090-3558-41.1.241. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt SM, Cooley TM, Fitzgerald SD, Bolin SR, Lim A, Schaefer SM, Kiupel M, Maes RK, Hogle SA, O'Brien DJ. An outbreak of eastern equine encephalitis virus in free-ranging white-tailed deer in Michigan. J Wildl Dis. 2007;43:635–644. doi: 10.7589/0090-3558-43.4.635. [DOI] [PubMed] [Google Scholar]
- 20.Hoff GL, Trainer DO, Richards SH. Arbovirus serology in North Dakota mule and white-tailed deer. J Wildl Dis. 1973;9:291–295. doi: 10.7589/0090-3558-9.4.291. [DOI] [PubMed] [Google Scholar]
- 21.Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals form 23 species of Aedes, Culex, Coquillettidia, Psorophora, and Uranotaeni. J Med Entomol. 2008;45:1143–1151. doi: 10.1603/0022-2585(2008)45[1143:hpopmv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.DeNicola AJ, VerCauteren KC, Curtis PD, Hygnstrom SE. Managing White-Tailed Deer in Suburban Environments. A Technical Guide. A Publication of the Cornell Cooperative Extension, the Wildlife Society-Wildlife Management Working Group, and Northeastern Wildlife Damage Research and Outreach Cooperative. New York. 2000. http://wildlifecontrol.info/pubs/Documents/Deer/Deer_management_mechs.pdf Available at. Accessed December 21, 2011. [Google Scholar]
- 23.Hohman EL. Ecology and Management of the White-Tailed Deer. Ottawa County Parks and Recreation Commission. West Olive (MI) 2006. http://grandhaven.org/uploads/pdf_documents/deer_management/management_information/Mgmt%20of%20White%20Tail.pdf Available at. Accessed January 25, 2012.
- 24.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennette EH, Lennette DA, Lennette ET, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 7th Ed. Washington, DC:: American Public Health Association; 1995. pp. 169–188. [Google Scholar]
- 25.Wang E, Petrakova O, Adams AP, Aguilar P, Kang W, Paessler S, Volk SM, Frolov I, Weaver SC. Chimeric sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine. 2007;25:7573–7581. doi: 10.1016/j.vaccine.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Association of Equine Practitioners Eastern/Western Equine Encephalomyelitis. Lexington, KY. 2008. http://www.aaep.org/eee_wee.htm Available at. Accessed February 17, 2012.