Abstract
Leishmania sp. is an intracellular parasite that causes a variable degree of clinical manifestations, especially in the skin. We present the case of a 38-year-old male with a chronic history of mucocutaneous disease present since childhood that generated deformity, loss of cartilage in the ears and nose, and scarring that limited his range of motion. The parasite was identified as L. mexicana mexicana. The patient was treated with a 3-month course of oral miltefosine with overwhelming results.
Introduction
Leishmanisis is a vector-borne disease caused by obligate intracellular protozoa of the genus Leishmania found in the tropics that is transmitted by the Phlebotomine sandflies.1
There are different clinical presentations of the disease that include visceral, mucosal, and cutaneous, but the latter is the most common.2 Approximately 21 species of Leishmania can infect humans. These species include the L. donovani complex, L. mexicana complex, L. tropica, L. major, L. aethiopica, and the subgenus Viannia. Species of Leishmania are morphologically indistinguishable by conventional methods, but they can be differentiated by isoenzyme analysis, molecular methods, or monoclonal antibodies. The anergic or diffuse disease in Mexico is very rare, especially in immunocompetent patients without a travel history to endemic areas. Standard treatment of leishmaniasis is based on antimonials, but a variety of drugs are being used in patients with mucocutaneous disease with promising results.3,4 Miltefosine is a phosphocholine analog that is administered orally; although originally used in visceral disease, it has been used for cutaneous disease with variable results.
We present a case of mucocutaneous disease caused by L. mexicana in a non-immunocompromised Mexican patient without travel history that was refractory to combined treatment and responded to miltefosine.
Case presentation
A 38-year-old man, a native and resident of Mendez County, Tamaulipas (93.7 miles south of the Mexico–Texas border), with no travel history outside the region presented to the emergency room with stridor.
He had a history of anergic leishmaniasis since his childhood and had been treated during that time with inconsistent results. The patient had been lost to follow-up during the previous 15 years and was in his usual state of health until the previous 6 months, when he noticed vocal changes with reduced tone and volume. He developed progressive dysphagia and malaise and subsequently, lost 10 kg body weight. Three months before arrival, he noticed progressive dyspnea and stridor. The latter became more intense, and he finally sought medical attention.
He was transferred to our hospital from his local medical clinic for definitive treatment. His blood pressure was 110/70 mmHg, pulse was 80 bpm, respiratory rate was 16 breaths per minute, axilary temperature was 36°C, and pulseoximetry was 98% while on room air. The patient had a disseminated asymmetrical polymorphic dermatosis characterized by multiple non-tender nodules of 1–7 cm in diameter. The nodules were polypoid and pink/flesh-colored with an elastic consistency; some were superficial, and some were fixed to deep tissue (Figure 1A). Cartilage destruction was present in both ears and nose. During direct laryngoscopy, the oropharingeal mucosa had a pavement aspect, and granulomatous lesions were observed in the soft palate and extended to epiglottis and glottis. Severe laryngeal stenosis was documented, and an emergency tracheostomy was performed. The rest of the physical exam was unremarkable.
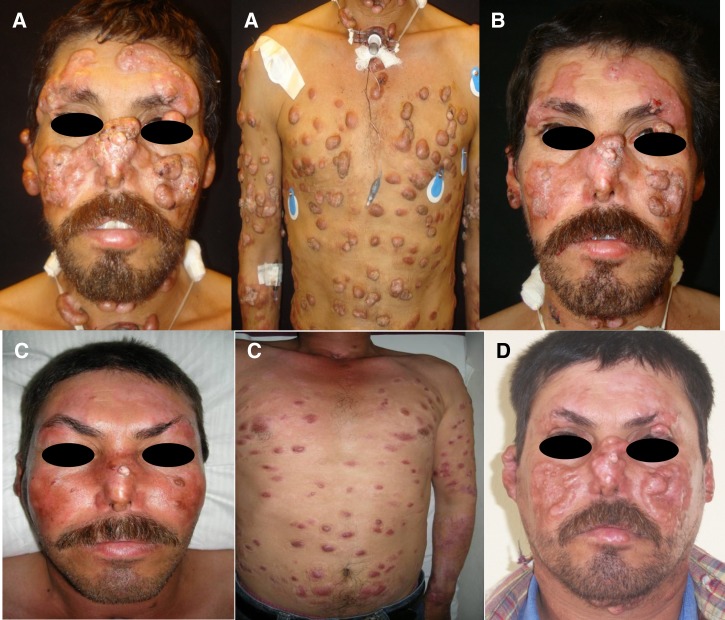
Figure 1.
(A) The patient's face and thorax show a disseminated dermatosis characterized by polypoid nodules eucromic with an elastic consistency (some superficial and some fixed to deep tissue). Cartilage destruction was present in both ears and nose. (B) After combined treatment with meglumine amphothericine B, there was a moderate reduction of nodules. (C) After treatment with miltefosine for 3 months, a marked reduction in nodules from his face and thorax was observed. (D) Six months after the last dose of miltefosine, the nodules reappeared.
Supraglottic biopsies showed amastigotes compatible with leishmaniasis associated with papillomatous changes. Various skin biopsies also showed amastigotes from Leishmania spp. The species was subsequently identified as Leishmania mexicana by polymerase chain reaction (PCR).
Blood count, serum electrolytes, and liver and kidney function tests were normal. A chest X-ray, electrocardiogram (EKG), and abdominal ultrasound were all normal. The patient is human immunodeficiency virus (HIV) -negative on repeated test. Lymphocytes CD3 count was 1,103 cells/mL (68.6%), lymphocytes CD3/CD4 count was 567 cells/mL (35.2%), lymphocytes CD3/CD8 count was 593 cells/mL (36.8%), natural killer cells count was 51.3 cells/mL (3.1%), lymphocytes CD19 count was 129 (8.0%), immunoglobulin A (IgA) count was 14.7 mg%, IgE count was 8.3 mg%, IgG count was 727.4 mg%, IgM count was 22.9 mg%, complement C3 count was 57.1 mg%, complement C4 count was 17.6 mg%, and CH50 count was 13.8 mg% (Table 1).
Table 1.
Results from tests performed to assess the patient's immunologic status
| Test | Result | Normal range |
|---|---|---|
| Lymphocytes CD3 | 1,103 cells/mL (68.6%) | 60–85% |
| Lymphocytes CD3/CD4 | 567 cells/mL (35.2%) | 25–60% |
| Lymphocytes CD3/CD8 | 593 cells/mL (36.8%) | 20–50% |
| Natural killer cells | 51.3 cells/mL (3.1%) | 8–19% |
| Lymphocytes CD19 | 129 (8.0%) | 7–23% |
| IgA | 14.7 mg% | 0–11 mg% |
| IgE | 8.3 mg% | 1.3–14.9 mg% |
| IgG | 727.4 mg% | 645–1,244 mg% |
| IgM | 22.9 mg% | 5–30 mg% |
| Complement C3 | 57.1 mg% | 50–120 mg% |
| Complement C4 | 17.6 mg% | 20–50 mg% |
| CH50 | 13.8 mg% | 20–50 UCH 50/mL |
CH50 = complement hemolyzing 50; UCH = units of complement hemolizing 50.
Treatment was initiated with intravenous (IV) meglumine (20 mg/kg per day), omeprazole per Ora (PO) (20 mg/day), and IV amphotericin B (0.5–0.75 mg/kg per day). Gradual improvement was observed on day 28 of treatment with no toxicity, but the lesions had not disappeared (Figure 1B). We extended the treatment to 38 days, with a total of 38 doses of meglumine and a cumulative dose of amphotericin B of 1,145 mg. Repeated EKGs and liver and renal screens were normal.
The patient requested voluntary discharge, and by 60 days follow-up, although no new lesions were noticed, the previous lesions had not disappeared completely. He was decanulated after glottis plasty without complications.
The patient returned after 5 months with new lesions on his extremities and eyelids (Figure 1B). We started treatment with 100 mg miltefosine (50 mg bis in die [BID] PO). An early and marked improvement was observed with miltefosine. Treatment was continued for 3 months. No toxicity was documented (Figure 1C). Unfortunately, 6 months after the patient stopped miltefosine treatment, the lesions gradually reappeared with their usual distribution and characteristics (Figure 1D).
Discussion
The clinical spectrum of cutaneous leishmaniasis in humans includes localized cutaneous leishmaniasis, mucocutaneous leishmaniasis, disseminated cutaneous leishmaniasis, and diffuse cutaneous leishmaniasis. The clinical manifestations are determined by the characteristics and the immune response of the infected patient.5
Localized cutaneous leishmaniasis is characterized by a single or multiple erythematous papules, usually located in the exposed region, that develop into ulcers with raised borders. The involvement of the nasal mucosa, palate, pharynx, larynx, and vocal cords may occur in up to 5% of the patients.
Diffuse cutaneous leishmaniasis (DCL) is a rare variant that usually begins in infancy and is characterized by the appearance of erythematous or skin-colored papules, plaques, or nodules with a continuous and uncontrolled dissemination; in advanced stages, there may be a parasitic invasion to nasopharyngeal and oral mucosa in more than 30% of the patients.6
It is necessary to establish the clinical difference with the disseminated cutaneous leishmaniasis, which is characterized by the presence of multiple polymorphic lesions that may present as multiple papules and acneiform-type lesions with few ulcers and mucosal involvement in 38% of cases. It is accompanied by symptoms such as fever and malaise. The immunological aspects are also distinct, because there is an abnormality in cellular immunity that is restored after treatment.7
For the pathogenesis of anergic DCL, there is a deficiency in cellular immune response that leads to poor parasite clearance and chronicity of the disease. It is found that there is an alteration in the cellular response T-helper (Th)-2 type, which is associated with a specific cell anergy to leishmania antigens and manifested by a negative Montenegro reaction.8 Also, there is a low level of CD-8 T lymphocytes, which plays an important role against infection by being a potential target for a vaccine development.9
Important for development of immunotherapy agents, they can exert their effect by directly or indirectly augmenting the host natural defenses, thereby restoring the impaired effector functions or decreasing host excessive and deleterious response to the parasite. Initially, dead promastigotes were used for the development of prophylactic vaccines in Brazilian patients.10 The work by Pereira and others11 reported the use of a vaccine containing killed promastigotes of Leishmania associated with the Bacillus Calmette–Guerin (BCG) alone or combined with chemotherapy. This vaccine may an alternative for patients who are intolerant or resistant to conventional treatment. The new vaccines are based on highly purified biological compounds and cytokines, interleukin-2 (IL-2), IL-4, IL-12, and interferon (INF-γ) which have the specific function of regulating the cellular response of Th-1and Th-2.12
DCL is associated with a poorer prognosis; the most challenging issue is the refractory nature of the condition to treatment, making it a demanding and often frustrating task.
This case illustrates the difficulty in obtaining sustained response to treatment in diffuse cutaneous leishmaniasis. This presentation of the disease is very rare in Mexico.
To our knowledge, no other cases similar to this case have been reported in the northern region of the country during the last 40 years.13 L. mexicana has been well-documented in Texas, and it causes human and animal infections, with the majority being single cutaneous lesions.14–16
This case was first treated by combining meglumine and amphotericin B (both medications are commonly used as monotherapy) in addition to using omeprazole, which induces changes in the acid pH that favors the proliferation of amastigotes in macrophages17; combination with antiparasitic drugs has been described mainly in visceral leishmaniasis (VL), and some studies are showing encouraging results.18–20 In this patient, the combination was successful as initial treatment without evidence of potentially toxic effects after 5 weeks of treatment. Although cardiovascular, liver, and kidney toxicity have been described with both medications,2,20 in our patient, strict cardiovascular monitoring did not show any abnormalities during and after combination therapy.
Miltefosine has shown a cure rate in immunocompetent patients with VL of 94% in India and about 90% in Ethiopia, where L. donovani is the cause of VL; it is listed as an option for VL in geographic areas where L. donovani is the predominant cause of VL.2 For geographical areas where L infantum is the cause of VL (examples: Mediterranean Basin, Middle East, Central Asia, and South America), miltefosine is not recommended.2
In an non-randomized trial using miltefosine (2.5 mg/kg per day for 28 days) for L. braziliensis, the cure rate for the 36 patients who had extensive disease (involving the palate, pharynx, and larynx) was 58%. For the contemporary group that was receiving amphotericin B (45 mg/kg over 90 days), the cure rate was 7 (50%) of 14 patients.21 We decided to start treatment with miltefosine and extend it for 3 months based on the encouraging results.
Relapse after miltefosine treatment has been described previously22–23 in a patient with diffuse cutaneous leishmaniasis caused by L. mexicana. Our patient followed a similar relapse pattern, although the time free of new lesions in our patient was longer (6 versus 2 months). The treatment of DCL can be very frustrating, because there is no sustained response to treatment. In our case, the response to a combined therapy with amphothericin B and meglumine was transitory like the response to miltefosine. In our case, the advantage with miltefosine is the outpatient setting and the possibility of extended treatment.
Conclusion
Mucocutaneous leishmaniasis caused by L. mexicana clinically responds to treatment with miltefosine with a high probability of relapse. Duration of treatment beyond 28 days seems to be well-tolerated with no toxicity. This finding suggests that miltefosine could be a useful maintenance therapy in DCL in an outpatient setting. Because of the immune deficit and physiopathology of DCL, a curative treatment is still not available.
Footnotes
Authors' addresses: Alejandro Ordaz-Farias, Ana Arana-Guajardo, and Adrian Camacho-Ortiz, Hospital Universitario–Internal Medicine, Monterrey, Nuevo Leon, Mexico, E-mails: ordaz7@hotmail.com, ana.aranag@gmail.com, and acamacho_md@yahoo.com. Fania Z. Muñoz-Garza, Farah K. Sevilla-Gonzalez, and Jorge Ocampo-Candiani, Hospital Universitario–Dermatology, Monterrey, Nuevo Leon, Mexico, E-mails: faniazamantta@hotmail.com, farahkaty82@hotmail.com, and jocampo2000@yahoo.com.mx. Nancy Treviño-Garza, Secretary of Health, National Center for Epidemiologic Surveillance and Infection Control, Mexico, Mexico, E-mail: nantrevino@hotmail.com. Ingeborg Becker, National Autonomous University of Mexico, Experimental Medicine, Mexico DF, Mexico, E-mail: becker@servidor.unam.mx.
References
- 1.Maroli M, Khoury C. Prevention and control of leishmaniasis vectors: current approaches. Parassitologia. 2004;46:211–215. [PubMed] [Google Scholar]
- 2.World Health Organization Control of Leishmaniases. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf Available at. Accessed August 2, 2012.
- 3.Mondal S, Bhattacharya P, Ali N. Current diagnosis and treatment of visceral leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:919–944. doi: 10.1586/eri.10.78. [DOI] [PubMed] [Google Scholar]
- 4.Richard JV, Werbovetz KA. New antileishmanial candidates and lead compounds. Curr Opin Chem Biol. 14. 2010:447–455. doi: 10.1016/j.cbpa.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Almeida OL, Santos JB. Advances in the treatment of cutaneous leishmaniasis in the new world in the last ten years: asystematic literature review. An Bras Dermatol. 2011;86:497–506. doi: 10.1590/s0365-05962011000300012. [DOI] [PubMed] [Google Scholar]
- 6.Zerpa O, Ulrich M, Blanco B, Polegre M, Avila A, Matos N, Mendoza I, Pratlong F, Ravel C, Convit J. Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses. Br J Dermatol. 2007;156:1328–1335. doi: 10.1111/j.1365-2133.2007.07872.x. [DOI] [PubMed] [Google Scholar]
- 7.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Jr, Carvalho EM. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–1834. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz JH, Becker I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol. 2007;29:671–678. doi: 10.1111/j.1365-3024.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Ruiz J, Salaiza-Suazo N, Carrada G, Escoto S. RuizRemigioA, RosensteinY, ZentellaA, BeckerI. CD8 cells of patients with diffuse cutaneous leishmaniasis display funconal exhaustion: the latteris reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayrink W, Botelho AC, Magalhães PA, Batista SM, Lima Ade O, Genaro O, Costa CA, Melo MN, Michalick MS, Williams P, Dias M, Caiaffa WT, Nascimento E, Machado-Coelho GL. Immunotherapy, immunochemotherapy and chemotherapy for American cutaneous leishmaniasis treatment. Rev Soc Bras Med Trop. 2006;39:14–21. doi: 10.1590/s0037-86822006000100003. [DOI] [PubMed] [Google Scholar]
- 11.Pereira LI, Dorta ML, Pereira AJ, Bastos RP, Oliveira MA, Pinto SA, Galdino H, Jr, Mayrink W, Barcelos W, Toledo VP, Lima GM, Ribeiro-Dias F. Increase of NK cells and proinflammatory monocytes are associated with the clinical improvementof diffuse cutaneous leishmaniasis after immunochemotherapy with BCG/Leishmania antigens. Am J Trop Med Hyg. 2009;81:378–383. [PubMed] [Google Scholar]
- 12.Okwor I, Uzonna JE. Immunotherapy as a strategy for treatment of leishmaniasis: a review of the literature. Immunotherapy. 2009;1:765–776. doi: 10.2217/imt.09.40. [DOI] [PubMed] [Google Scholar]
- 13.Simpson MH, Mullins JF, Stone OJ. An Autochthonous Case in Texas and the Mexican States of Tamaulipas and Nuevo Leon. Arch Dermatol. 1968;97:301–303. doi: 10.1001/archderm.97.3.301. [DOI] [PubMed] [Google Scholar]
- 14.Trainor KE, Porter BF, Logan KS, Hoffman RJ, Snowden KF. Eight cases of feline cutaneous leishmaniasis in Texas. Vet Pathol. 2010;47:1076–1081. doi: 10.1177/0300985810382094. [DOI] [PubMed] [Google Scholar]
- 15.McHugh CP. Cutaneous leishmaniasis in Texas. J Am Acad Dermatol. 2010;62:508–510. doi: 10.1016/j.jaad.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Wright NA, Davis LE, Aftergut KS, Parrish CA, Cockerell CJ. Cutaneous leishmaniasis in Texas: a northern spread of endemic areas. J Am Acad Dermatol. 2008;58:650–652. doi: 10.1016/j.jaad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Nilforoushzadeh MA, Jaffary F, Ansari N, Siadat AH, Nilforoushan Z, Firouz A. A comparative study between the efficacy of systemic meglumineantimoniate therapy with standard or low dose plus omeprazole in the treatment of cutaneous leishmaniasis. J Vector Borne Dis. 2008;45:287–291. [PubMed] [Google Scholar]
- 18.Van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect Dis. 2010;10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 19.Shakya N, Sane SA, Vishwakarma P, Bajpai P, Gupta S. Improved treatment of visceral leishmaniasis (kala-azar) by using combination of ketoconazole, miltefosine with an immunomodulator-Picroliv. Acta Trop. 2011;119:188–193. doi: 10.1016/j.actatropica.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Shamsi Meymandi S, Javadi A, Dabiri S, Shamsi Meymandi M, Nadji M. Comparative histological and immunohistochemical changes of dry type cutaneous leishmaniasis after administration of meglumineantimoniate, imiquimod or combination therapy. Arch Iran Med. 2011;14:238–243. [PubMed] [Google Scholar]
- 21.Kashani MN. Evaluation of meglumineantimoniate effects on liver, kidney and pancreas function tests in patients with cutaneous leishmaniasis. Eur J Dermatol. 2007;17:513–515. doi: 10.1684/ejd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 22.Soto J, Toledo J, Valda L, Balderrama M, Rea I, Parra R, Ardiles J, Soto P, Gomez A, Molleda F, Fuentelsaz C, Anders G, Sindermann H, Engel J, Berman J. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin Infect Dis. 2007;44:350–356. doi: 10.1086/510588. [DOI] [PubMed] [Google Scholar]
- 23.Calvopina M, Gomez E, Sindermann H, Cooper P, Hashiguchi Y. Relapse of new world diffuse cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana after miltefosine treatment. Am J Trop Med Hyg. 2006;75:1074–1077. [PubMed] [Google Scholar]