Abstract
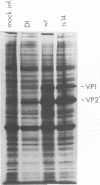
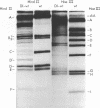
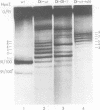
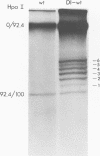
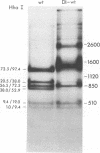
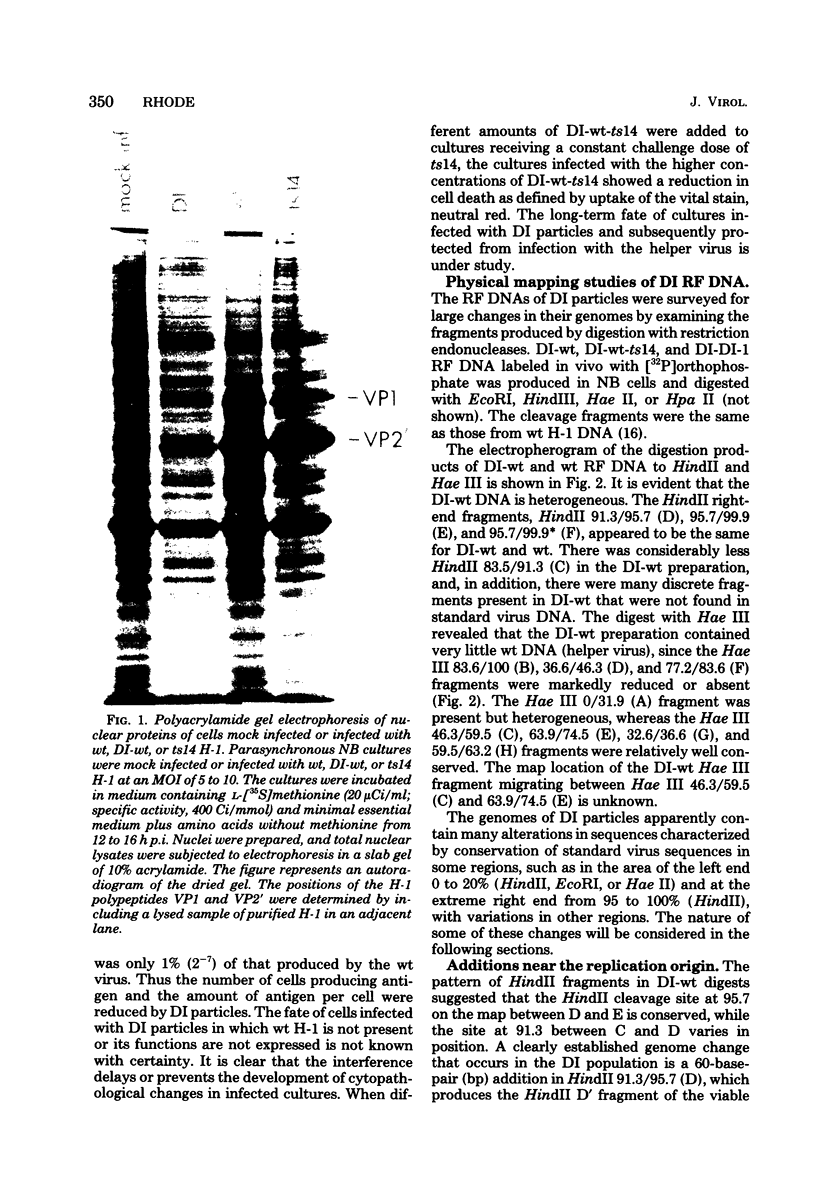
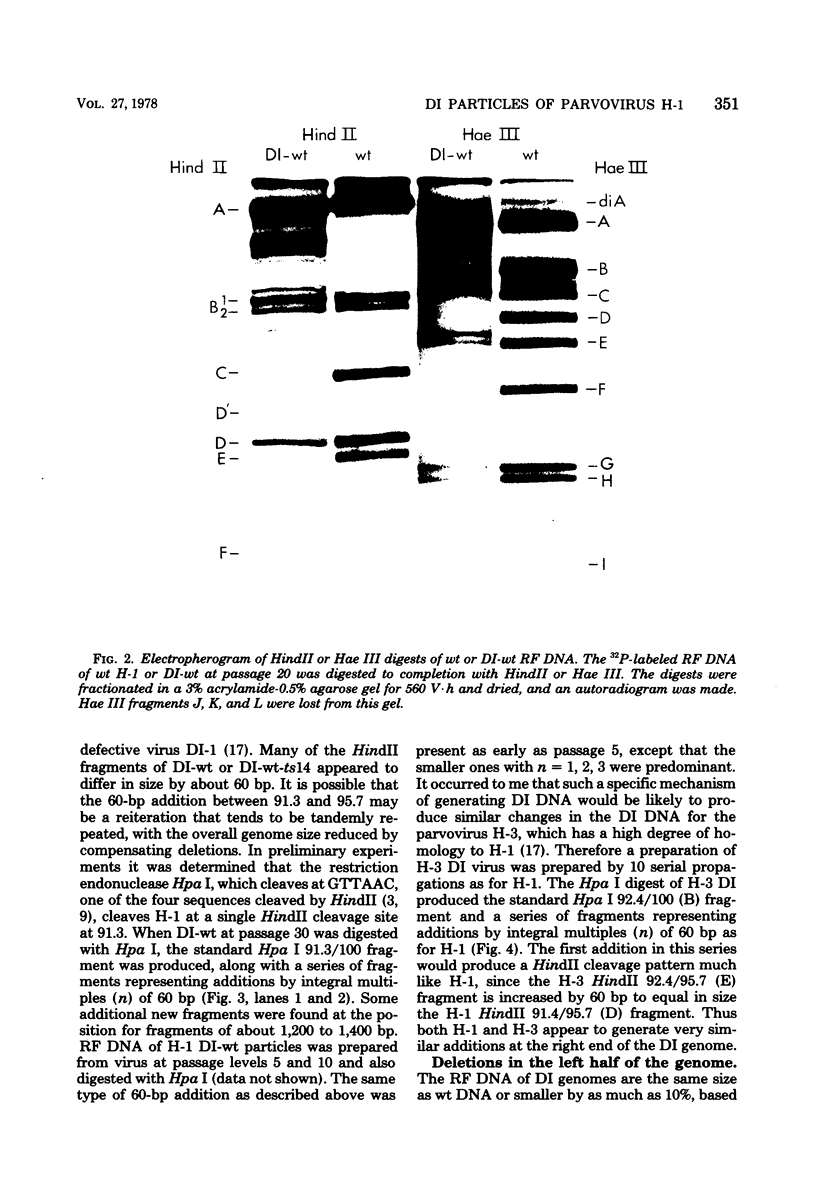
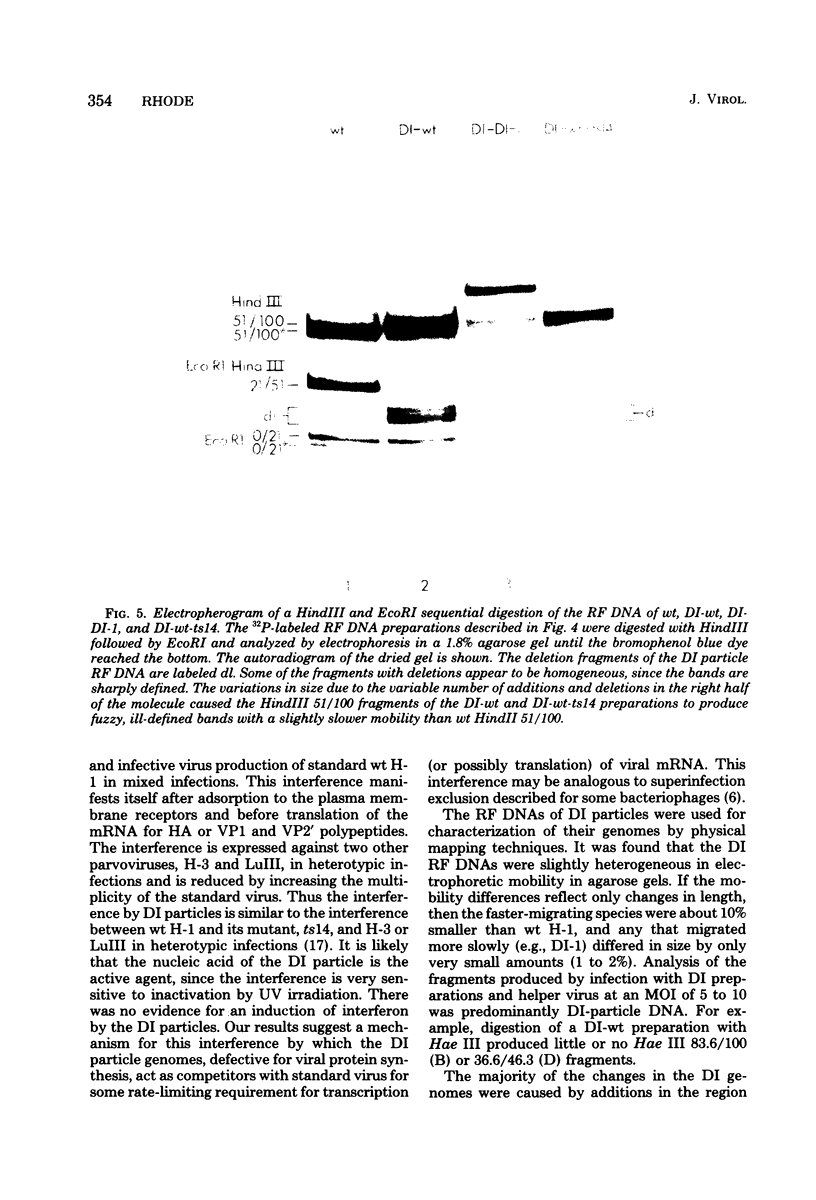
Defective interfering particles of the parvovirus H-1 were produced by serial propagation at high multiplicities of infection. Such particles interfere with the synthesis of capsid proteins and infectious virus of standard H-1. The interference is sensitive to UV irradiation, dependent on the multiplicity of the challenge virus, and is active in heterotypic infections against parvovirus H-3 or LuIII. Defective interfering particle genomes have alterations characterized by integral numbers (1 to 10 or more) of a 60-base-pair addition in the neighborhood of the origin of replicative-form DNA replication and deletions that are located primarily within two regions, 32 to 44 or 80 to 90 on the genome map. Some of the implications of these findings are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davoli D., Ganem D., Nussbaum A. L., Fareed G., Howley P. M., Khoury G., Martin M. A. Genome Structures of reiteration mutants of simian virus 40. Virology. 1977 Mar;77(1):110–124. doi: 10.1016/0042-6822(77)90411-1. [DOI] [PubMed] [Google Scholar]
- Enea V., Horiuchi K., Turgeon B. G., Zinder N. D. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977 Apr 25;111(4):395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- Garfin D. E., Goodman H. M. Nucleotide sequences at the cleavage sites of two restriction endonucleases from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jul 10;59(1):108–116. doi: 10.1016/s0006-291x(74)80181-6. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Ellem K. A., Singer I. I. Environmental pH and the recovery of H-1 parvovirus during single cycle infection. Virology. 1974 Aug;60(2):548–557. doi: 10.1016/0042-6822(74)90349-3. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Pfenning-Yeh M., Ponta H., Herrlich P., Schweiger M. A virus-specified mechanism for the prevention of multiple infection--T7- and T3-mutual and superinfection exclusion. Mol Gen Genet. 1976 Dec 22;149(3):243–249. doi: 10.1007/BF00268524. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kilham L., Buckler C. E., Ferm V. H., Baron S. Production of interferon during rat virus infection. Proc Soc Exp Biol Med. 1968 Oct;129(1):274–278. doi: 10.3181/00379727-129-33302. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Popescu M., Lehmann-Grube F. Defective interfering particles in mice infected with lymphocytic choriomeningitis virus. Virology. 1977 Mar;77(1):78–83. doi: 10.1016/0042-6822(77)90407-x. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: the maturation of recombination intermediates. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. IX. Physical mapping studies of the H-1 genome. J Virol. 1977 May;22(2):446–458. doi: 10.1128/jvi.22.2.446-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. X. Isolation of a mutant defective in replicative-form DNA replication. J Virol. 1978 Jan;25(1):215–223. doi: 10.1128/jvi.25.1.215-223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VIII. Partial denaturation mapping and localization of the replication origin of H-1 replicative-form DNA with electron microscopy. J Virol. 1977 Feb;21(2):724–731. doi: 10.1128/jvi.21.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W. The picodna viruses. H, RV, and AAV. Int Rev Exp Pathol. 1968;6:135–180. [PubMed] [Google Scholar]