Abstract
Beginning in 2005, the Centers for Disease Control and Prevention (CDC) expanded the overseas presumptive treatment of intestinal parasites with albendazole to include refugees from the Middle East. We surveyed the prevalence of helminths and protozoa in recent Middle Eastern refugees (2008–2010) in comparison with refugees from other geographical regions and from a previous survey (2001–2004) in Santa Clara County, California. Based on stool microscopy, helminth infections decreased, particularly in Middle Eastern refugees (0.1% versus 2.3% 2001–2004, P = 0.01). Among all refugees, Giardia intestinalis was the most common protozoan found. Protozoa infections also decreased somewhat in Middle Eastern refugees (7.2%, 2008–2010 versus 12.9%, 2001–2004, P = 0.08). Serology for Strongyloides stercoralis and Schistosoma spp. identified more infected individuals than stool exams. Helminth infections are increasingly rare in refugees to Northern California. Routine screening stool microscopy may be unnecessary in all refugees.
Introduction
Over the past decade, 30,000–80,000 refugees have entered the United States each year.1 California, the leading state of residence for these individuals, accepted between 8% and 24% of all refugees.2 This large population has unique health care needs. Among these, a high prevalence of intestinal parasites has been reported in various groups of refugees.3–6 Untreated intestinal parasites can have serious long-term health consequences. For example, anemia, growth retardation, and cognitive impairment can develop in children with chronic hookworm infection,7,8 and in adults, significant morbidity and mortality has been caused by hyperinfection with Strongyloides stercoralis.7–10 In 1999, after presumptive parasite treatment in immigrants was determined to be cost-effective, the Centers for Disease Control and Prevention (CDC) recommended single-dose albendazole to refugees > 2 years of age from sub-Saharan Africa and Southeast Asia.11,12
Since 2005, the number of refugees from the Middle East has increased 10-fold. Previously a mere 3–4% of all refugees, by 2008, Middle Eastern refugees comprised nearly one-third of all refugees entering the United States.1 During this period, the CDC also expanded presumptive pre-departure albendazole treatment to the Middle East, South Asia, and instead of only sub-Saharan Africa, the entire African continent. The epidemiology of intestinal parasites in refugees from the Middle East has not been well described. Furthermore, the extent to which albendazole has been used overseas is uncertain, as is the impact of the broader pre-departure treatment recommendations on the prevalence of intestinal parasites in recent Middle Eastern refugees. The objective of this study was to characterize the parasite infection profiles of Middle Eastern refugees in comparison with profiles of refugees from other geographical regions who arrived in Santa Clara County, California from 2008 to 2010. In addition, to estimate the extent to which albendazole has been effective for intestinal parasites, we compared the prevalence of helminths and protozoa in current refugees to that of refugees in 2001–2004.
Methods
Population.
The California Department of Public Health's Refugee Health Assessment Program (RHAP) serves as an initial health system contact for refugees who are referred within 90 days of arrival in the United States. Through the RHAP, incoming refugees receive a thorough health evaluation that includes screening for intestinal parasites. Santa Clara County is one of 10 counties in California to provide such screening evaluations, which take place at the Santa Clara County Tuberculosis Clinic and Refugee Health Assessment Program in San Jose, CA.13 Subjects consisted of all persons who were referred to the RHAP from June 1, 2008 until November 30, 2010. Informed consent was obtained from a convenience subset sample of 238 refugees who were examined in more detail. These individuals were healthy, 18–55 years of age, had been living in the United States for < 2 years, and spoke English, Arabic, Burmese, Chinese, Farsi, Spanish, or Vietnamese (languages for which we had translators).
To determine changes in immigration trends and parasite infections, we compared current refugees (2008–2010) with a historical cohort of previously described refugees from the Santa Clara county RHAP who were similarly evaluated from October 1, 2001 to January 30, 2004.14 The study was approved by the institutional review boards at Stanford University and Santa Clara Valley Medical Center.
Data collection.
Stool collection for ova and parasite (O&P) evaluation was carried out with the ParaPak ULTRA Stool Transport and Filtration System, which contained two 15-mL vials. One vial contained zinc sulfate and polyvinyl alcohol to preserve trophozoites and cysts, whereas the other contained 10% formalin for direct examination (Meridian Bioscience, Cincinnati, OH). Stool specimens were processed by the Santa Clara County Microbiology Laboratory, Parasite Division (San Jose, CA). Strongyloides and Schistosoma spp. serology assays (done for the subset of 238 individuals who gave informed consent) were conducted using commercial enzyme-linked immunosorbent assay kits according to manufacturer's instructions (Scimedx, Denville, NJ).
De-identified data for all refugees were obtained from the RHAP and included demographic characteristics, immigration status, country of birth, number of stool specimens submitted for examination, stool ova and parasite results, and species identification.
Analyses.
We compared the characteristics of refugees who completed the health assessment with those who did not complete evaluations offered through the RHAP. Country of birth was classified according to the United Nations categories of geographical regions as follows: Africa, East Asia (China, Korea, Mongolia), Middle East (Armenia, Azerbaijan, Georgia, Iran, Iraq, Jordan, Kuwait, Lebanon, Saudi Arabia, Syria, Turkey), South Asia (Afghanistan, Bhutan, India, Nepal, Kazakhstan, Pakistan), and Southeast Asia (Thailand, Vietnam, Burma, Cambodia, Indonesia, Malaysia, Philippines). Because of the low numbers of refugees, the following regions were grouped as “Other”: Eastern Europe (N = 10), South and Central America (N = 7), and South Europe (N = 1). Education information was available only for those who initiated the health assessment.
In the analyses of stool results, only individuals who turned in at least two stool specimens were included. Non-pathogenic parasites were excluded. We assessed the prevalence of protozoa and helminth infections by species count. As a result of the presence of multiple infections in samples from some subjects, species counts were greater than person counts. Regions with fewer than five infected individuals were excluded from analysis (East Asia and Other).
Differences between groups were assessed using the χ2, Fisher's exact test, or Wilcoxon rank-sum test as appropriate. Multivariate logistic regression was performed to estimate adjusted odds ratios and associated 95% confidence intervals for protozoa and helminth infections in separate models. In the comparisons of the 2001–2004 and the 2008–2010 cohorts, Southeast Asia was excluded as a result of low numbers of refugees from this region in the older cohort.
Analyses were carried out using SAS, version 9.3 (SAS Institute Inc., Cary, NC).
Results
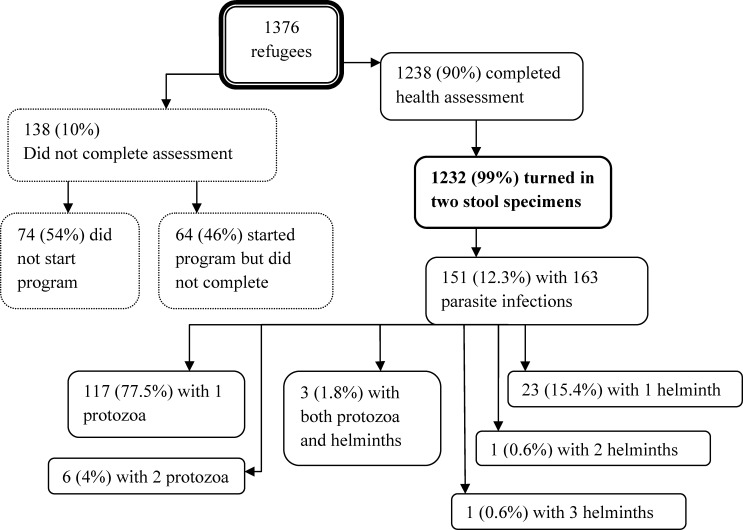
One thousand three hundred and seventy-six (1,376) refugees were referred to the RHAP from June 1, 2008 to November 30, 2010 (Figure 1). The great majority of them (N = 1238, 90%) completed the refugee health assessment. Of these, 1,232 (89.5%) turned in at least two stool specimens for ova and parasite testing and were included in our analysis. Of the 138 (10%) who did not complete the health evaluation, 74 (54%) did not start the program because: they could not be located (N = 34, 46%), moved out of the county (N = 34, 46%), chose another health care provider (N = 4, 5%), declined (N = 1, 1%), or died (N = 1, 1%). The 64 refugees (46%) who began but did not complete the health assessment consisted of 48 (75%) who moved out of the county, 13 (20%) who changed health care providers, and 3 (5%) who were lost to follow-up.
Figure 1.
Flow chart of 1,376 refugees referred to the Santa Clara Valley Refugee Health Assessment Program from June 1, 2008 until November 30, 2010. The 1,232 refugees turned in at least 2 stool specimens and were included in the analyses. There were 151 persons harboring 163 protozoa and/or helminth infections.
Characteristics of refugees completing the RHAP.
There were similar numbers of men and women refugees. The median age of all refugees was 29 years, and age ranged from 0 to 87 years. Children (< 18 years of age) were less likely to complete the evaluation than adults (P = 0.03). During 2008–2010, the region with the highest number of refugees referred to the RHAP was the Middle East (N = 777, 56%). A high proportion of Middle Eastern refugees, 93.7%, completed the RHAP. In contrast, East Asian refugees showed low participation, with only 48% of eligible refugees completing the health assessment. Among Africans, Congolese were the largest group (25.7%), followed by Ethiopians (19.4%), Eritreans (18.8%), and Somalis (15.3%). Among South Asians, refugees from Bhutan and Nepal made up 83.2% of the group. In Southeast Asia, Vietnam and Burma were the two most represented countries (92.7% cumulative). East Asia was almost entirely composed of refugees from China (80%). There were very few refugees from Europe and Latin America
Characteristics of Middle Eastern refugees.
Compared with refugees from other geographical regions, Middle Eastern refugees were somewhat older and had received more education (Table 1). The majority of Middle Eastern refugees had completed at least high-school level education (58.4% versus 27.7% in refugees from other regions, P = 0.001). Iranians and Iraqis comprised 96.4% of the refugees from the Middle East and 54.4% of all refugees. The great majority of Iranian refugees (N = 350, 87.5%) arrived in the United States through Austria, where almost all (N = 336, 96%) had lived for no more than 6 months before emigration. The second most common country of residence of Iranian refugees before emigration was Turkey (N = 41, 10.3%). For Iraqi refugees, the four most common countries of residence before emigration were Syria (N = 108, 31%), Jordan (N = 103, 29.5%), Iraq (N = 52, 14.9%), and Turkey (N = 47, 13.5%).
Table 1.
Characteristics of 777 Middle Eastern refugees and 599 refugees from other regions referred to the Santa Clara Valley Refugee Health Assessment Program (RHAP) from June 1, 2008 to November 30, 2010
| Middle East N = 777 | Other regions N = 599 | Total N = 1,376 | |||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 387 | 49.8% | 275 | 45.9% | 662 |
| Male | 390 | 50.2% | 324 | 54.1% | 714 |
| Age | |||||
| Median, Q1–Q3 | 31 | 22–47 | 25 | 17–40 | |
| 0–10 | 76 | 9.8% | 66 | 11.0% | 142 |
| 11–17 | 58 | 7.5% | 62 | 10.4% | 120 |
| 18–34 | 315 | 40.5% | 264 | 44.0% | 579 |
| ≥ 35 | 328 | 42.2% | 207 | 34.6% | 535 |
| Education* | |||||
| No formal education | 80 | 10.6% | 110 | 20.1% | 190 |
| 1–11 years | 234 | 31.0% | 285 | 52.2% | 519 |
| ≥ 12 years | 442 | 58.4% | 151 | 27.7% | 593 |
Data missing for 74 refugees who did not begin the RHAP.
Prevalence of intestinal parasites 2008–2010.
There were 368 stools that were positive for pathogens, representing 163 infections in 151 (12.3%) people (Table 2). Eleven refugees (7.3% of infected, 0.9% of all tested) had multiple parasites. Among the 140 infected with a single parasite, all three stools were positive in 61.1% and at least two stools were positive in 80.9%.
Table 2.
Geographical distribution of intestinal parasites identified through stool ova and parasite tests. Regions with fewer than five infected individuals were excluded
| Africa N = 120 | Middle East N = 721 | South Asia N = 111 | Southeast Asia N = 254 | Total N = 1,232* | |
|---|---|---|---|---|---|
| Protozoan infections† | |||||
| Blastocystis hominis | 18 (15.0%) | 52 (7.2%) | 33 (29.7%) | 25 (9.3%) | 132‡ (10.7%) |
| Dientamoeba fragilis | 0 | 4 (0.6%) | 4 (3.6%) | 6 (2.4%) | 14 (1.1%) |
| Entamoeba | 1 (0.8%) | 23 (3.2%) | 10 (9.0%) | 5 (2.0%) | 41 (3.3%) |
| histolytica/dispar | 5 (4.2%) | 15 (2.1%) | 5 (4.5%) | 1 (0.4%) | 26 (2.1%) |
| Giardia intestinalis | 12 (10.0%) | 10 (1.4%) | 14 (12.6%) | 13 (5.1%) | 51 (4.1%) |
| Helminth infections† | 5 (4.2%) | 1 (0.1%) | 3 (2.7%) | 21 (8.3%) | 31‡ (2.5%) |
| Ascaris lumbricoides | 0 | 0 | 0 | 0 | 0 |
| Clonorchis sinensis | 0 | 0 | 0 | 1 (0.4%) | 1 (0.1%) |
| Hookworm | 0 | 0 | 0 | 13 (5.1%) | 13 (1.1%) |
| Hymenolepsis nana | 1 (0.8%) | 1 (0.1%) | 3 (2.7%) | 0 | 5 (0.4%) |
| Schistosoma spp. | 1 (0.8%) | 0 | 0 | 0 | 1 (0.1%) |
| Strongyloides stercoralis | 3 (2.5%) | 0 | 0 | 5 (2.0%) | 8 (0.6%) |
| Trichuris trichuria | 0 | 0 | 0 | 2 (0.8%) | 3 (0.2%) |
| TOTAL persons infected | 23 (19.2%) | 53 (7.4%) | 31 (27.9%) | 39 (15.4%) | 151† (12.3%) |
The 26 refugees from East Asia, Eastern Europe, South & Central America, and South Europe are not represented separately because of small numbers.
Species counts exceed person counts because of mixed infections in 11 individuals.
Out of the geographical regions not represented separately because of small numbers of refugees, there were five infections with intestinal parasites: four protozoa and one helminth.
In total, 126 refugees (10.2%) had intestinal protozoa in their stool. Giardia intestinalis (syn. Giardia lamblia and Giardia duodenalis) was the most common protozoan identified (4.1% of all tested, and 41.1% of persons with protozoan infections), followed by Dientamoeba fragilis (3.4% of all tested, and 38.6% of protozoan infections) (Table 2, last column). In contrast to protozoa, helminth infections were rare overall. Only 28 refugees (2.3%) had positive stools for helminths, with hookworm being the most common (1.1% of all tested, 41.9% of helminth infections).
Based on stool O&P results, there was significant regional variability in the distribution of intestinal parasites. The Middle East had the lowest prevalence of both protozoa (7.2%) and helminths (0.1%). South Asia was the region with the highest prevalence of protozoan infections (29.7%). Although G. intestinalis was the most prevalent protozoa in refugees from Africa, South Asia, and Southeast Asia, in the Middle East, G. intestinalis was third to D. fragilis and E. histolytica/dispar. With regards to helminth infections, Southeast Asia was the region with the highest prevalence (8.3%, predominantly hookworm), whereas the Middle East had no hookworm infection and the only helminth infection was Hymenolepis nana.
Infection risk factors differed for protozoa and helminths (Table 3). Decreasing age was associated with protozoa but not helminth infection. Neither sex nor education level was associated with protozoan infection. However, men and those with less than a high school education were three and four times more likely to have helminths, respectively. Refugees from the Middle East had the lowest likelihood of having either protozoa or helminths in their stool. Compared with them, South Asians were three times more likely to harbor protozoa, and refugees from all geographic regions were much more likely to have helminths. Year of arrival in the United States was also a significant risk factor for both protozoa and helminth infections. The risk was highest in those arriving in the year 2008.
Table 3.
Multivariate logistic regression of risk factors for helminth and protozoa infections in 1,232 refugees. Adjusted odds ratios and 95% confidence intervals*
| Risk factor | Protozoa | P value | Helminth | P value |
|---|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | |||
| Age | ||||
| Per 10-year increase | 0.9 (0.8–1.0) | 0.04 | 1.1 (0.9–1.3) | 0.56 |
| Sex | ||||
| Male | 1.3 (0.9–2.0) | 0.15 | 3.3 (1.3–8.5) | 0.01 |
| Female | Reference | Reference | ||
| Region | ||||
| Africa | 2.4 (1.3–4.5) | 0.005 | 29.9 (3.3–269) | 0.002 |
| South Asia | 3.6 (2.1–6.1) | < 0.001 | 10.8 (1.1–108) | 0.04 |
| Southeast Asia | 1.1 (0.6–1.9) | 0.77 | 31.3 (4.1–240) | < 0.001 |
| Middle East | Reference | Reference | ||
| Education | ||||
| < 12 years | 0.9 (0.6–1.4) | 0.70 | 3.9 (1.1–13.6) | 0.04 |
| ≥ 12 years | Reference | Reference | ||
| Year of arrival | ||||
| 2008 | 2.3 (1.3–3.9) | 0.002 | 5.3 (1.6–18.3) | 0.008 |
| 2009 | 1.1 (0.7–1.9) | 0.69 | 2.7 (0.8–9.5) | 0.12 |
| 2010 | Reference | Reference | ||
AOR = adjusted odds ratio; CI = confidence interval.
Comparison with 2001–2004 historical cohort.
We compared the prevalence of intestinal protozoa and helminths in recent Middle Eastern refugees with the prevalence seen in similar refugees from 2001 to 2004 based on stool ova and parasite results (Table 4). Helminth infections decreased in all geographical regions, but most markedly in refugees from the Middle East, where a 95% reduction was observed from 2.3% to 0.1%. The prevalence of helminth infections also fell significantly in South Asia, by 75%. Overall, the proportion of refugees with protozoan infections remained unchanged, but depended on the region of birth of the refugees. Prevalence of protozoa decreased in the Middle East, and more than quadrupled in South Asia. African refugees continued to have the same rate of protozoa infections. In refugees from the Middle East, the prevalence of G. lamblia specifically decreased from 8.3% to 1.6%. The increase in protozoan infections in South Asian refugees was driven primarily by the high prevalence of Giardia infections in Bhutanese refugees (13.2% of those tested, 47.6% of the infected). There were no refugees from Bhutan in 2001 to 2004, but they comprised 61.8% of South Asians infected with protozoa in 2008 to 2010.
Table 4.
Prevalence of intestinal parasites in Middle Eastern and South Asian refugees, 2001–2004 versus 2008–2010, diagnosed by stool microscopy*
| 2001–2004 | % | 2008–2010 | % | P value | |
|---|---|---|---|---|---|
| Persons infected | Persons infected | ||||
| Middle East | N = 132 | N = 721 | |||
| Protozoa | 17 | 12.9% | 52 | 7.2% | 0.08 |
| Helminths | 3 | 2.3% | 1 | 0.1% | 0.01 |
| South Asia | N = 167 | N = 111 | |||
| Protozoa | 11 | 6.6% | 33 | 29.7% | < 0.001 |
| Helminths | 18 | 10.8% | 3 | 2.7% | 0.02 |
| Africa | N = 142 | N = 120 | |||
| Protozoa | 16 | 11.3% | 18 | 15.0% | 0.37 |
| Helminths | 12 | 8.5% | 5 | 4.2% | 0.21 |
| TOTAL | N = 533 | N = 1232 | |||
| Protozoa | 51 | 9.6% | 126 | 10.2% | 0.67 |
| Helminths | 33 | 6.6% | 28 | 2.3% | < 0.001 |
Eastern Europe, East Asia, and Southeast Asia comparisons not done because of insufficient participants and/or infected persons in one or both cohorts.
Results of Strongyloides and Schistosoma serology tests.
To examine the extent of possible missed diagnoses of Strongyloides and Schistosoma spp., we tested serologies for these parasites in a subset of 238 adult refugees. Although stool O&P identified only 2 individuals with Strongyloides and none with Schistosoma, serology identified 56 individuals with Strongyloides (23.5% of 238 tested, Table 5), and 18 individuals with Schistosoma (7.6% of 238 tested). Strongyloides serology was positive in both persons who had positive stool O&P. The 56 refugees who had positive Strongyloides serology were from Iran (N = 20, 21% of Iranians tested), Bhutan (N = 10, 67%), Iraq (N = 8, 21%), Ethiopia (N = 7, 50%), Burma (N = 6, 30%), Eritrea (N = 2, 18%), Cuba (N = 1, 50%), Thailand (N = 1, 100%), and Vietnam (N = 1, 5%). The 18 refugees with positive Schistosoma serology were from Iran (N = 4, 4%), Eritrea (N = 3, 27%), Vietnam (N = 3, 16%), Bhutan (N = 2, 13%), Burma (N = 2, 10%), Ethiopia (N = 1, 7%), India (N = 1, 50%), Iraq (N = 1, 3%), and Somalia (N = 1, 50%). Fourteen of the African refugees tested for Schistosoma immigrated to the United States after overseas presumptive therapy with praziquantel was initiated in sub-Saharan Africa. Only four of them were positive. Three (5.4%) persons with positive Strongyloides antibodies (including 1 person with Strongyloides in the stool) had eosinophilia. Only 1 (5.6%) person with positive Schistosoma antibodies had eosinophilia.
Table 5.
Characteristics of refugees with positive serology for Strongyloides or Schistosoma spp. among 238 refugees who were tested*
| Africa | South Asia | Southeast Asia | Middle East | South & Central America | Total | |
|---|---|---|---|---|---|---|
| Number tested | 29 | 27 | 40 | 136 | 2 | 238 |
| Strongyloides+ | 9 (31%) | 10 (37%) | 8 (20%) | 28 (21%) | 1 (50%) | 56 (24%) |
| Male | 8 (33%) | 6 (32%) | 6 (23%) | 12 (15%) | 0 | 32 (21%) |
| Female | 1 (20%) | 4 (50%) | 2 (14%) | 16 (30%) | 1 (100%) | 24 (29%) |
| Age | ||||||
| 18–25 | 1 (14%) | 6 (40%) | 2 (13%) | 9 (23%) | 0 | 18 (23%) |
| 26–35 | 4 (31%) | 3 (30%) | 2 (13%) | 13 (19%) | 1 (50%) | 23 (21%) |
| 36–45 | 4 (44%) | 1 (50%) | 4 (50%) | 5 (19%) | 0 | 14 (30%) |
| 46–55 | 0 | 0 | 0 | 1 (33%) | 0 | 1 (25%) |
| Stool O&P+ | 0 | 0 | 2 | 0 | 0 | |
| Schistosoma+ | 5 (17%) | 3 (11%) | 5 (13%) | 5 (4%) | 0 | 18 (8%) |
| Male | 4 (16%) | 3 (16%) | 2 (8%) | 0 | 0 | 9 (6%) |
| Female | 1 (20%) | 0 | 3 (21%) | 5 (9%) | 0 | 9 (11%) |
| Age | ||||||
| 18–25 | 0 | 1 (7%) | 3 (20%) | 2 (5%) | 0 | 6 (8%) |
| 26–35 | 5 (38%) | 2 (20%) | 1 (6%) | 2 (3%) | 0 | 10 (9%) |
| 36–45 | 0 | 0 | 1 (13%) | 1 (4%) | 0 | 2 (4%) |
| 46–55 | 0 | 0 | 0 | 0 | 0 | 0 |
% = positive persons/persons tested × 100. Four East Asians tested and with all negative results are not represented.
Discussion
A recent large study by the CDC showed that the rate of helminth infections in refugees from Africa and Southeast Asia decreased from 20.4% to 4.7% after the first recommendation for overseas treatment with single-dose albendazole.15 In our study of refugees relocating to Northern California, helminth infections appear to be rapidly disappearing from the Middle East and South Asia as well. Although we have no data for the extent of overseas treatment, helminths were less likely to be found in refugees arriving in 2010 versus 2008, after adjusting for all other recorded variables including region of birth. These findings are consistent with a possible effect from the implementation of overseas albendazole treatment of refugees from the Middle East and South Asia.
Our results support the use of serology rather than stool O&P to diagnose Strongyloides and Schistosoma spp. infections—the two parasites that cause the greatest morbidity and mortality among refugees. Even though stool examination is the gold standard for screening refugees for intestinal parasites, our data are consistent with the literature reporting increased sensitivity of serology for the diagnosis of these two infections specifically.6,16 In 2005, in an effort to improve the overseas treatment of Strongyloides and Schistosoma spp., the CDC expanded the recommended drug regimen with ivermectin and praziquantel. However, because of funding constraints, these changes were not implemented until more recently. Ivermectin has only been used since July 2011 in Burmese refugees emigrating from Thailand. Praziquantel was not started until January 2010. Therefore, the positive serologies in our study are likely true infections despite the negative results from stool microscopy. Four refugees from sub-Saharan Africa had positive Schistosoma serology and immigrated to the United States after the initiation of praziquantel overseas treatment in January 2010. We do not know if these positive results are in fact false positives (residual positive antibodies after treated infection) because we do not know if the refugees actually received praziquantel. The disadvantage of only using serology in refugees with no documentation of overseas treatment is possible overdiagnosis because antibodies can persist transiently before waning for Strongyloides and persist indefinitely for schistosomiasis after treatment.17,18 However, repeated serology testing after some time has elapsed could help clarify the diagnosis, perhaps even for Schistosoma infection as scientists develop newer assays for antibodies that wane with time after treatment.19–22 The presence of eosinophilia may help confirm infection with positive serology, though because it is often transient and rarely found, its absence cannot exclude infection.
The two protozoa in our study that cause the most morbidity were G. lamblia and Entamoeba histolytica. Although we found a high prevalence of Giardia infections among the refugees, particularly those from South Asia and Africa where the prevalence was 12.6% and 10%, respectively, our findings suggest that routine stool O&P is also probably unnecessary. More than three fourths of Giardia infections are asymptomatic, and it is not clear whether asymptomatic infection is detrimental to the host and by extension, whether treatment is beneficial.23,24 In children, perhaps some of the most vulnerable to infection, the indications for screening and treatment are the most controversial. For example, although some studies found that subclinical or asymptomatic Giardia in children was associated with growth delay, failure to thrive, or carbohydrate malabsorption, various other groups found no ill effects.25–27 Furthermore, other investigators report a possible protective effect with decreased gastrointestinal and respiratory illnesses among daycare children with asymptomatic Giardia infection.28,29 From a public health perspective, there have been no reported incidents of Giardia outbreaks originating from refugees. For those with symptomatic Giardia infection, diagnostic testing—rather than screening tests—can be done, although even in this group, disease is usually self-limited.30 Perhaps a conservative approach could be to only test adults with symptoms and children regardless of symptoms. E. histolytica/dispar, the second protozoan to cause the most morbidity, had a low overall prevalence in our study (2.1%). This number also overestimates the true prevalence of E. histolytica because microscopically, the organism is indistinguishable from the non-pathogenic E. dispar. Furthermore, E. dispar is far more common than E. histolytica, making up to 90% of E. histolytica/dispar reported.6 For both Giardia and E. histolytica, stool antigen tests or PCR-based tests are more sensitive and accurate than stool microscopy.31–33 Whether to replace stool O&P examinations with antigen testing for all refugees, only in those with symptoms, or only in children, needs to be evaluated.
Our finding of exceedingly rare helminth infections identified through stool O&P exams is consistent with the current CDC recommendation to omit domestic screening with stool O&P in refugees with documentation of pre-departure anti-helminthic treatment. Furthermore, our findings suggest that even in the absence of this documentation, routine screening stool O&P may no longer be necessary for most refugees. Instead, symptom-based testing, or targeted screening according to region of birth may be more appropriate. For example, Southeast Asians had the highest rate of hookworm infections, and selected stool O&P in these refugees might lead to higher diagnostic yield than routine screening O&P for all refugees without documentation of overseas treatment.
Our study was limited by the absence of data for overseas treatment. We also lacked data on symptoms that could have informed the clinical significance of directed stool O&P exams. We had limited serology data in adults and none in children, who have the highest risk for long-term sequelae of chronic intestinal parasites from missed diagnoses. Furthermore, we know very little about intestinal parasites in recent East Asian refugees as a result of their very low participation rate in the RHAP. Finally, we describe patterns of infections based on broad geographic regions, but recognize that refugees from specific countries can deviate significantly from the group, and their influx depends on not wholly predictable global political climates.
In conclusion, helminth infections are rapidly decreasing in refugees settling in Northern California. Although Giardia infections are still prevalent in some groups of refugees, routine screening stool microscopy, especially in adult refugees, is probably unnecessary. Evaluation of the feasibility and cost-effectiveness of new domestic screening protocols, such as symptom-based testing, serology, and/or stool antigen testing for intestinal parasites is warranted.
ACKNOWLEDGMENTS
We thank Gulshan Bhatia and the staff at the Santa Clara County Tuberculosis Clinic and Refugee Health Program, who collected the refugee data in our county; Marisa Ramos and the California Department of Public Health, Public Health Policy and Research Branch, Refugee Health Program, for their assistance in obtaining the data; IVD Research, Carlsbad, CA, who developed the ELISA kits used for serology tests for Strongyloides and Schistosoma and provided the kits at no charge; and Scimedx, Denville, NJ, who eventually purchased the ELISA kits from IVD Research and continued to provide the kits to us at no charge.
Footnotes
Financial support: This research was funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, grants R01AI042801 and R01AI042801-10SI (J.P.), K23AI091688 (A.H.C.), and K23AI054443 (S.P.).
Authors' addresses: Alicia H. Chang, Division of Infectious Diseases and Geographic Medicine, Stanford University, Stanford, CA, E-mail: achang2@stanford.edu. Sharon Perry, Medecins sans Frontieres, France Malawi/Chiradzulu, E-mail: shnperry@stanford.edu. Jenny N. T. Du, Touro University Nevada, Henderson, NV, E-mail: jenntdu@gmail.com. Abdulkareem Agunbiade, University of Chicago, Pritzker School of Medicine, Biological Sciences Learning Center, Chicago, IL, E-mail: kareema@uchicago.edu. Andrea Polesky, Tuberculosis Clinic and Refugee Health Assessment Program, San Jose, CA, E-mail: andrea.polesky@hhs.sccgov.org. Julie Parsonnet, Division of Infectious Diseases and Geographic Medicine, Stanford University, Stanford, CA, E-mail: parsonnt@stanford.edu.
References
- 1.Homeland Security Refugees and Asylees: 2010. 2011. http://www.dhs.gov/files/statistics/publications/gc_1304623329455.shtm Available at: Accessed May 8, 2012.
- 2.CDPH California Arrival Report. 2011. http://www.cdph.ca.gov/programs/Pages/RHP-ArrivalDemographics.aspx Available at. Accessed May 8, 2012.
- 3.Lurio J, Verson H, Karp S. Intestinal parasites in Cambodians: comparison of diagnostic methods used in screening refugees with implications for treatment of populations with high rates of infestation. J Am Board Fam Pract. 1991;4:71–78. [PubMed] [Google Scholar]
- 4.Varkey P, Jerath AU, Bagniewski S, Lesnick T. Intestinal parasitic infection among new refugees to Minnesota, 1996–2001. Travel Med Infect Dis. 2007;5:223–229. doi: 10.1016/j.tmaid.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Shah JJ, Maloney SA, Liu Y, Flagg EW, Johnston SP, Young SA, Weston R, Merritt S, Wilkins PP, Keane V, Calderon J, Sharp DJ, Causer L, Maguire JH, Cetron MS. Evaluation of the impact of overseas pre-departure treatment for infection with intestinal parasites among Montagnard refugees migrating from Cambodia to North Carolina. Am J Trop Med Hyg. 2008;78:754–759. [PubMed] [Google Scholar]
- 6.CDC Guidelines for the U.S. Domestic Medical Examination for Newly Arriving Refugees. 2012. http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/domestic-guidelines.html Available at. Accessed May 9, 2012.
- 7.Sakti H, Nokes C, Hertanto WS, Hendratno S, Hall A, Bundy DA, Satoto Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop Med Int Health. 1999;4:322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121((Suppl)):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 9.Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102:314–318. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Roxby AC, Gottlieb GS, Limaye AP. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;49:1411–1423. doi: 10.1086/630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC Overseas Refugee Health Guidelines: Intestinal Parasites. 2010. http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html Available at. Accessed May 8, 2012.
- 12.Muennig P, Pallin D, Sell RL, Chan MS. The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med. 1999;340:773–779. doi: 10.1056/NEJM199903113401006. [DOI] [PubMed] [Google Scholar]
- 13.CDPH Refugee Health Program. 2011. http://www.cdph.ca.gov/programs/Pages/RefugeeHealthProgram.aspx Available at. Accessed May 9, 2012.
- 14.Garg PK, Perry S, Dorn M, Hardcastle L, Parsonnet J. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73:386–391. [PubMed] [Google Scholar]
- 15.Swanson S, Phares C, Mamo B, Smith K, Cetron M, Stauffer W. Albendazole therapy and enteric parasites in United States-bound refugees. N Engl J Med. 2012;366:1498–1507. doi: 10.1056/NEJMoa1103360. [DOI] [PubMed] [Google Scholar]
- 16.Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha JL, Seet RC, Summer AP, Nield LS, Supparatpinyo K, Chaiwarith R, Walker PF. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med. 2007;120:e1–8. doi: 10.1016/j.amjmed.2006.05.072. 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page WA, Dempsey K, McCarthy JS. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg. 2006;100:1056–1062. doi: 10.1016/j.trstmh.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Biggs BA, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80:788–791. [PubMed] [Google Scholar]
- 19.Hamadto HH, Rashed SM, el Said A, Elhayawan IA. Humoral and cellular immune response in schistosomiasis pre and post praziquantel therapy. J Egypt Soc Parasitol. 1990;20:667–672. [PubMed] [Google Scholar]
- 20.Kanamura HY, Hoshino-Shimizu S, Kimura RT, Matsumoto TK, da Silva LC, Lima DM, Abrantes-Lemos CP. Decay of antibody isotypes against early developmental stages of Schistosoma mansoni after treatment of schistosomiasis patients. Rev Inst Med Trop Sao Paulo. 1997;39:271–277. doi: 10.1590/s0036-46651997000500005. [DOI] [PubMed] [Google Scholar]
- 21.Duus LM, Christensen AV, Navntoft D, Tarp B, Nielsen HV, Petersen E. The Schistosoma-specific antibody response after treatment in non-immune travellers. Scand J Infect Dis. 2009;41:285–290. doi: 10.1080/00365540902756505. [DOI] [PubMed] [Google Scholar]
- 22.Bligh J, Schramm G, Chiodini PL, Doenhoff MJ. Serological analysis of the outcome of treatment of Schistosoma mansoni infections with praziquantel. Ann Trop Med Parasitol. 2010;104:511–520. doi: 10.1179/136485910X12786389891245. [DOI] [PubMed] [Google Scholar]
- 23.Lopez CE, Dykes AC, Juranek DD, Sinclair SP, Conn JM, Christie RW, Lippy EC, Schultz MG, Mires MH. Waterborne giardiasis: a communitywide outbreak of disease and a high rate of asymptomatic infection. Am J Epidemiol. 1980;112:495–507. doi: 10.1093/oxfordjournals.aje.a113019. [DOI] [PubMed] [Google Scholar]
- 24.Addiss DG, Juranek DD, Spencer HC. Treatment of children with asymptomatic and nondiarrheal Giardia infection. Pediatr Infect Dis J. 1991;10:843–846. doi: 10.1097/00006454-199111000-00010. discussion 846–848. [DOI] [PubMed] [Google Scholar]
- 25.Hollm-Delgado MG, Gilman RH, Bern C, Cabrera L, Sterling CR, Black RE, Checkley W. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol. 2008;168:647–655. doi: 10.1093/aje/kwn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Morais MB, Suzuki HU. Weight gain in children with asymptomatic giardiasis and iron-deficiency anaemia during oral iron therapy. J Trop Pediatr. 1997;43:121–122. doi: 10.1093/tropej/43.2.121. [DOI] [PubMed] [Google Scholar]
- 27.Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology. 2005;131:51–56. doi: 10.1017/s0031182005007353. [DOI] [PubMed] [Google Scholar]
- 28.Ish-Horowicz M, Korman SH, Shapiro M, Har-Even U, Tamir I, Strauss N, Deckelbaum RJ. Asymptomatic giardiasis in children. Pediatr Infect Dis J. 1989;8:773–779. doi: 10.1097/00006454-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Bilenko N, Levy A, Dagan R, Deckelbaum RJ, El-On Y, Fraser D. Does co-infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol. 2004;19:877–883. doi: 10.1023/b:ejep.0000040533.75646.9c. [DOI] [PubMed] [Google Scholar]
- 30.Solaymani-Mohammadi S, Singer SM. Giardia duodenalis: the double-edged sword of immune responses in giardiasis. Exp Parasitol. 2010;126:292–297. doi: 10.1016/j.exppara.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque R, Faruque AS, Hahn P, Lyerly DM, Petri WA., Jr Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J Infect Dis. 1997;175:734–736. doi: 10.1093/infdis/175.3.734. [DOI] [PubMed] [Google Scholar]
- 32.Leo M, Haque R, Kabir M, Roy S, Lahlou RM, Mondal D, Tannich E, Petri WA., Jr Evaluation of Entamoeba histolytica antigen and antibody point-of-care tests for the rapid diagnosis of amebiasis. J Clin Microbiol. 2006;44:4569–4571. doi: 10.1128/JCM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boia MN, Carvalho-Costa FA, Sodre FC, Eyer-Silva WA, Lamas CC, Lyra MR, Pinto VL, Jr, Cantalice Filho JP, Oliveira AL, Carvalho LM, Gross JB, Sousa AL, Moraes TI, Bermudez-Aza EH, Martins EB, Coura JR. Mass treatment for intestinal helminthiasis control in an Amazonian endemic area in Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:189–195. doi: 10.1590/s0036-46652006000400003. [DOI] [PubMed] [Google Scholar]