Abstract
Exclusion of malaria traditionally requires three negative serial thick and thin blood films. However, many clinical laboratories now routinely perform rapid diagnostic tests (RDTs) in addition to blood films when malaria is suspected. We sought to determine whether serial testing is necessary in this setting. We examined 388 cases of malaria diagnosed during 1999–2010 at three laboratories in Melbourne, Australia. For each case, we ascertained whether the diagnosis was made on initial or follow-up testing. Nine cases (3.5%) were diagnosed after a negative initial blood film and RDT: 7 Plasmodium vivax, 1 P. ovale, and 1 P. falciparum. Of four case-patients with P. vivax in which clinical data were available, all had recent exposure to antimalarial medication. Our data suggest that among patients who have not received recent anti-malarial therapy, and when RDTs are performed and blood films are prepared, most malaria diagnoses are made by using the first set of tests.
Introduction
Traditional teaching mandates that the diagnosis of malaria cannot be excluded by a single blood film. If the first blood film is negative, it is recommended that serial blood films be obtained 6–12 hours apart for 48 hours until three films have been found to be negative.1–3 The rationale is that parasitemia may be cyclical, at low levels in the early stages of illness, or sequestered in the case of Plasmodium falciparum and therefore missed if only one film is prepared. In addition, detection by light microscopy is operator and laboratory dependent,4–6 which may be overcome by serial examinations. However, the preparation and examination of blood films is time-consuming for laboratory staff, taking 30–60 minutes for experienced technicians. This limitation can have implications for laboratory workflow, particularly after-hours, as well as for emergency department or hospital flow when patients may be admitted for further testing before the diagnosis of malaria is excluded.
Rapid diagnostic tests (RDTs) are increasingly being used in clinical laboratories, in developed and developing settings.7 The performance of these tests varies according to manufacturer, type, and setting.8 The BinaxNOW malaria RDT has a reported sensitivity and specificity of 95% and 94% for P. falciparum and 68.9% and 99.8% for P. vivax (manufacturer's package insert: www.binaxnow.com). A recent report found that Binax NOW RDT had a negative predictive value of 100% for P. falciparum malaria and 98% for all malaria in a U.S. clinical laboratory setting.9 Despite the excellent capacity of RDTs to exclude malaria, serial testing is generally still recommended.2 However, fewer tests could dramatically improve patient waiting times, laboratory and hospital efficiency, and costs. Thus, in a setting in which blood films are routinely complemented by RDTs, we sought to evaluate the need for serial testing to exclude the diagnosis of malaria.
Materials and Methods
Setting.
Melbourne, Australia is a non-malaria–endemic setting, and all cases of malaria are imported. The hematology laboratories at The Royal Melbourne Hospital and the Alfred Hospital each provide diagnostic services to their own emergency departments and those of several smaller satellite hospitals; Melbourne Pathology provides diagnostic services to community practitioners as well as several hospitals. At all three laboratories, standard operating procedures currently stipulate that all clinical requests for thick and thin blood films for malaria should have a reflex RDT performed. In Melbourne, all suspected malaria diagnoses are sent to a central reference laboratory (the Victorian Infectious Diseases Reference Laboratory) for confirmation. Thus, records at this laboratory comprise the complete list of confirmed malaria cases diagnosed in the city.
Procedures.
Records at the reference laboratory identified all cases of malaria diagnosed at the three clinical laboratories. All confirmed malaria cases in adults (age > 16 years) during 1999–2010 at the Royal Melbourne hospital, during 2006–2010 at the Alfred hospital, and during 2005–2010 at Melbourne Pathology were identified. Names and birthdates of cases were cross-checked across the laboratories to ensure that cases were not included twice. Each case was reviewed and the number of thick and thin blood films and RDTs performed before the ultimate diagnosis made was determined. Cases where the first thick and thin blood film and the first RDT result were negative were determined, and these cases were reviewed in detail by accessing hospital records where available. The proportion of such cases was calculated. The analysis was categorized according to Plasmodia species and percentage of parasitemia. Wilcoxon rank sum tests were used to compare percentage of parasitemia between cases with positive and negative test results.
Rapid diagnostic tests in use.
The BinaxNOW® immunochromatographic test (Inverness Medical, Scarborough, ME) is a rapid antigen capture assay used to detect histidine-rich protein 2 (HRP-2) for the specific identification of P. falciparum, together with pan malarial Plasmodium aldolase antigen for identification of all species. This RDT was introduced in 2005 at the Royal Melbourne Hospital, in 2003 at Melbourne Pathology, and were in use from the time of study at the Alfred Hospital.
During 1999–2002 at The Royal Melbourne Hospital, RDT testing was routinely performed only for P. falciparum (ICT Malaria Pf; ICT Diagnostics, Sydney, New South Wales. Australia). Ethical approval for this study was obtained from the Human Research Ethics Committee at the Royal Melbourne Hospital.
Results
Cases included in the analysis.
A total of 388 patients with malaria were identified. Of these patients, 255 (64.7%) had RDTs performed and blood films prepared. All 388 patients had blood films prepared. However, 133 patients did not have RDT performed, most because the RDT had not been introduced in the laboratory at the time of testing. The types of malaria, modes of diagnosis, number of blood films prepared, and ages of the patients are shown in Table 1. Most (62.7%) cases were P. vivax.
Table 1.
Characteristics of 388 patients given a diagnosis of malaria, Melbourne, Australia*
| Variable | Median (95% CI) | Range | |
|---|---|---|---|
| Age (years) | 41.1(16.2–109.1) | 16–88 | |
| No. blood films | 2.1 (2.0–2.3) | 1–9 | |
| Variable | Categories | % (95% CI) | No. |
| Mode of diagnosis in patients in whom RDT and blood film were used (n = 255) | Diagnosis made on blood film only | 28.2 (22.7–33.8) | 72 |
| Diagnosis made on RDT only | 1.6 (0.03–3.1) | 4 | |
| Diagnosis made on blood film and RDT | 70.2 (64.5–75.8) | 179 | |
| Laboratory | Royal Melbourne | 69.7 (60.8–73.3) | 269 |
| The Alfred | 13.5 (10.1–16.9) | 52 | |
| Melbourne Pathology | 16.8 (13.1–20.6) | 67 | |
CI = confidence interval; RDT = rapid diagnostic test.
Cases not detected by initial blood film and RDT.
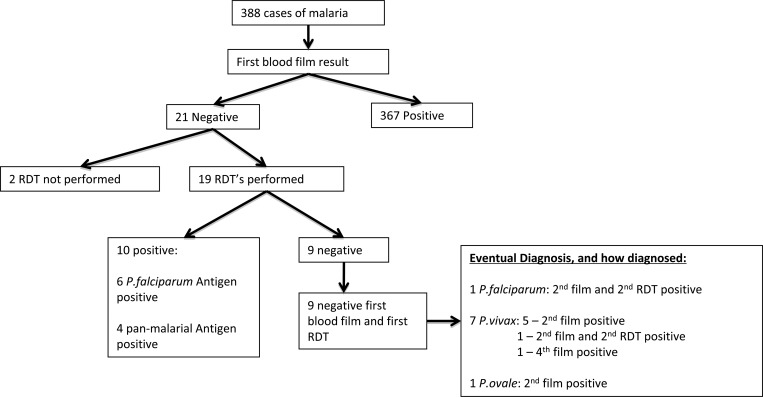
For 9 (3.5%) of 255 cases, the initial blood film and RDT result were negative. Of these cases, seven were P. vivax, one was P. ovale, and one was P. falciparum (Figure 1). The clinical details of these cases are shown in Table 2. All cases except one were diagnosed on the second set of tests. Of the seven cases of P. vivax, four were documented to have recently received antimalarial medication: two for treatment of suspected malaria and two for prophylaxis. Records were unavailable for the remaining three cases.
Figure 1.
Flow chart showing results of thick and thin films and rapid diagnostic tests (RDTs) for 388 cases of malaria, Melbourne, Australia.
Table 2.
Summarized clinical details of patients ultimately given a diagnosis of malaria, whose first TT and RDT results were negative, Melbourne, Australia*
| Diagnosis | Exposure | Presentation/clinical notes | Antimicrobial drugs |
|---|---|---|---|
| Plasmodium falciparum | Born in Tanzania and migrated to Australia 11 months before presentation. Travel to Tanzania for 3 months to visit friends and relatives | 21-year-old woman. Febrile illness while away treated locally. When arrived home, the patient was sick with vomiting and diarrhea. She was afebrile. Test results in the emergency department were negative for malaria. At a clinic review two weeks later, the patient had a fever for 24 hours before P. falciparum malaria was diagnosed. | Unknown antimicrobial drug received while overseas. No malaria prophylaxis taken. |
| P. vivax | Recently immigrated from Tanzania and Burundi | Fevers and aches. | No details available. |
| P. vivax | Australian born. Travel to Indonesia | 32-year-old man. Five days of vomiting and lethargy. | Doxycycline for malaria prophylaxis. |
| P. vivax | Australian born. Travel to Papua New Guinea | 48-year-old man. P. vivax malaria treated 5 days earlier. Fevers, lethargy, and cough. | Chloroquine and primaquine for treatment of P. vivax malaria finished two days before presentation. |
| P. vivax | Australian born. Working in Papua New Guinea for 18 months | 30-year-old man. 24 hours of fever and lethargy. | Self-treatment of malaria (malarone) completed one day before presentation. |
| P. vivax | Australian born. Travel to Vanuatu | 18-year-old woman. Three days of fever, sweats, and headache. | Doxycycline for malaria prophylaxis. |
| P. vivax | Travel to Vietnam and Laos | 19-year-old person. Two weeks of recurrent fevers and night sweats. Self limiting febrile illness while in Vietnam. | No details available. |
| P. vivax | Travel to Papua New Guinea four weeks before presentation | 49-year-old person. Febrile illness. | No details available. |
| P. ovale | Born in Africa and migrated to Australia. Travel to Africa for three months | 24-year-old man. Asymptomatic. Screening tests performed because of recent travel. | Doxycycline for malaria prophylaxis. |
TT = traditional test; RDT = rapid diagnostic test.
The single case of P. ovale malaria occurred in an asymptomatic African man who had thick and thin blood films examined as part of apparently unrelated screening tests performed by his local doctor. The single case of P. falciparum occurred in a young African woman who had migrated from Tanzania to Australia six months earlier, and had visited Tanzania one month before her presentation. She had a febrile illness during her trip for which she received local treatment (unspecified) and apparently made a full recovery. One month after her return to Australia, she came to the emergency department afebrile but had diarrhea. Results of a single RDT and blood film prepared at that time were negative. She was discharged, and at a prearranged outpatient follow-up one week later, she reported a fever over the previous 24 hours: results of repeat blood films and RDT performed at this time were positive for P. falciparum.
Cases not detected by initial RDT.
For 60 (13.6%) of 255 cases, the initial RDT result was negative. Laboratories would usually not repeat an RDT once a diagnosis of malaria had been made by blood film. However, for 17 cases, the RDT was repeated. Of these cases, 15 had parasites detected on the first blood film. In two cases, the first blood film was also negative, and a diagnosis of malaria was made on the second blood film and RDT. These cases are described in Table 3. In one case, three serial RDTs were performed despite P. vivax malaria at a percentage of 0.2% detected on the first blood film. In this case, the first two RDT results s were negative, but the third was positive.
Table 3.
Results of thick and thin blood films and rapid diagnostic tests in 388 patients who were ultimately given a diagnosis of malaria at three laboratories in Melbourne, Australia*
| Test type | Categories | Result | Proportion (95% CI) | No. |
|---|---|---|---|---|
| Result of first set of thick and thin blood films according to Plasmodia species (includes mixed infections) | All species | Positive | 94.4 (92.1–96.7) | 372 |
| Negative | 5.6 (3.3–7.8) | 22 | ||
| Plasmodium falciparum | Positive | 92.1 (86.7–97.4) | 93 | |
| Negative | 7.9 (2.6–13.3) | 8 | ||
| P. vivax | Positive | 94.7 (91.9–97.5) | 234 | |
| Negative | 5.3 (2.4–8.1) | 13 | ||
| All other species (other than P. falciparum and P. vivax) | Positive | 97.7 (92.9–102) | 42 | |
| Negative | 2.3 (–2.3 to 7.0) | 1 | ||
| Result of first RDT according to Plasmodia species (RDT co-reactive for HRP-2 and pan-malarial antigen are counted separately) | All species | Positive | 86.4 (82.7–90.1) | 211 |
| Negative | 13.6 (17.2–27.1) | 60 | ||
| P. falciparum | Positive | 96.3 (92.2–100) | 79 | |
| Negative | 3.7 (–0.5 to 7.8) | 3 | ||
| P. vivax | Positive | 72.0 (64.7–73.9) | 105 | |
| Negative | 33.5 (26.1–40.9) | 53 | ||
| All other species (other than P. falciparum and P. vivax) | Positive | 55.6 (35.5–75.6) | 15 | |
| Negative | 44.4 (24.4–64.5) | 12 | ||
| Median parasitemia of first blood film was positive, i.e., in cases in which initial blood films were negative, the parasitemia of the first blood was positive (%) | First RDT result and blood film were negative | 0.02 (0.003–0.12) | Test of difference, P = 0.3105† | |
| First RDT result or first blood film were positive | 0.06 (0.05–0.07) | |||
CI = confidence interval; RDT = rapid diagnostic test; HRP-2 = histidine-rich protein 2.
By Wilcoxon rank sum test.
Discussion
In this setting, we found that most patients are given a diagnosis of malaria on the initial blood film and RDT. Plasmodium falciparum malaria was diagnosed on the first diagnostic set in all but one case (99%). For non–P. falciparum malaria, initial tests detected > 97% of cases.
Each case not detected on the initial testing set was atypical. In most cases, the patients with these cases had been taking anti-malarial medications. Exposure to anti-malarial drugs lowers parasite density, modifies the appearance of parasites on blood films,10,11 and in the case of non–P. falciparum malaria, lowers concentrations of parasite antigen.12 These factors may lessen the sensitivity of microscopy4 and RDTs.13,14 Therefore, serial testing may be required. The single case of P. falciparum malaria that was not detected on initial testing appears to have occurred in a recently treated, possibly immune person, and the initial presentation when test results were negative may not have represented malarial illness. The case of P. ovale malaria occurred in an asymptomatic, likely immune person. In both of these cases, the clinical significance of parasitemia is unclear and may not have represented malarial illness.
We found a similar sensitivity (72%) of RDTs for non–P. falciparum malaria to the manufacturer's reported sensitivity (for P. vivax malaria, 68.9%)8 and other hospital-based reports of RDT performance (e.g., 61% and 86.7% in France and Canada, respectively).15–17 Because laboratories would usually not perform an RDT if a diagnosis of malaria had already been made by a blood film, RDTs were repeated in only a small number of cases. However, of those that did have a serial RDT, only two cases had positive subsequent test results for which the first RDT result and blood film had been negative, and only one case with a positive first blood film and a negative first RDT result had a subsequently positive RDT result on the third test.
The results of our study should be considered in the context of its limitations. First, data collection was retrospective with the attendant limitations of data collected in this manner. Second, laboratories in this study were well-resourced, tertiary centers with experienced microscopists. Microscopy is operator dependent, and thus it may be difficult to generalize the performance of microscopy to different laboratory settings. In particular, for non–P. falciparum malaria, for which most diagnoses were made by microscopy, laboratories with less experience may have lower sensitivity compared with our centers. For example, a study from Toronto, Canada, found that P. vivax infection was correctly diagnosed in only 26% of cases by laboratory personnel from a group of hospital laboratories and privately owned pathology companies.18 Third, most cases in this study were non-immune travelers and these data should not be extended to settings to which malaria is endemic.
Nevertheless, to our knowledge, this is the first study to formally evaluate the common practice of serial testing in the diagnosis of malaria in the era of routine RDTs. Our findings suggest that for patients with imported malaria who have not been exposed to anti-malarial drugs, the diagnosis is likely to be made on the first set of thick and thin blood films combined with RDTs. Larger, prospective studies are now required to assess the safety of this approach.
Footnotes
Authors' addresses: Janet M. Pasricha, Parkville, Melbourne, Victoria, Australia, E-mail: harper_janet@hotmail.com. Surender Juneja, Joseph Manitta, Sant-Rayn Pasricha, and Damon P. Eisen, Diagnostic Haematology Department, Royal Melbourne Hospital, Melbourne, Victoria, Australia, E-mails: juneja@mh.org.au, joseph.manitta@mh.org.au, santapasricha@hotmail.com, and damom.eisen@mhorg.au. Susan Whitehead, Diagnostic Haematology Department, The Alfred, Melbourne, Victoria, Australia, E-mail: s.whitehead@alfred.org.au. Ellen Maxwell and Wai-Keong Goh, Diagnostic Haematology Department, Melbourne Pathology, Melbourne, Victoria, Australia, E-mails: ellen.maxwell@mps.com.au and wk.goh@uqconnect.edu.au.
References
- 1.White NJ. The treatment of malaria. N Engl J Med. 1996;335:800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 2.Mandell GL, Bennett JE, Doli R. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 7th edition 2010. [Google Scholar]
- 3.The Malaria Working Party of the General Haematology Task Force of the British Committee for Standards Haematology The laboratory diagnosis of malaria. Clin Lab Haematol. 1997;19:165–170. [PubMed] [Google Scholar]
- 4.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RE, Sattabongkot J, Promstaporm S, Maneechai N, Tippayachai B, Kengluecha A, Rachapaew N, Zollner G, Miller RS, Vaughan JA, Thimasarn K, Khuntirat B. Comparison of PCR and microscopy for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Malar J. 2006;5:121. doi: 10.1186/1475-2875-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrheim DN, Becker PJ, Billinghurst K. Diagnostic disagreement: the lessons learnt from malaria diagnosis in Mpumalanga. S Afr Med J. 1997;87:1016. [PubMed] [Google Scholar]
- 7.World Health Organization WH World Malaria Report 2010. 2010. http://wwwwhoint/malaria/publications/atoz/9789241564106/en/indexhtml Available at.
- 8.Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauffer WM, Cartwright CP, Olson DA, Juni BA, Taylor CM, Bowers SH, Hanson KL, Rosenblatt JE, Boulware DR. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin Infect Dis. 2009;49:908–913. doi: 10.1086/605436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaudoin RL, Aikawa M. Primaquine-induced changes in morphology of exoerythrocytic stages of malaria. Science. 1968;160:1233–1234. doi: 10.1126/science.160.3833.1233. [DOI] [PubMed] [Google Scholar]
- 11.Jiang JB, Jacobs G, Liang DS, Aikawa M. Qinghaosu-induced changes in the morphology of Plasmodium inui. Am J Trop Med Hyg. 1985;34:424–428. doi: 10.4269/ajtmh.1985.34.424. [DOI] [PubMed] [Google Scholar]
- 12.Miller RS, McDaniel P, Wongsrichanalai C. Following the course of malaria treatment by detecting parasite lactate dehydrogenase enzyme. Br J Haematol. 2001;113:558–559. doi: 10.1046/j.1365-2141.2001.02782.x. [DOI] [PubMed] [Google Scholar]
- 13.Bell DR, Wilson DW, Martin LB. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 2005;73:199–203. [PubMed] [Google Scholar]
- 14.Gasser RA, Jr, Ruebush MA, Miller TK, Sirichaisinthop RS, Forney J, Jr, Bautista CT, Rhorer J, Wittes J, Wongsrichanalai C. Washington, DC: 2005. (Malaria diagnosis: performance of a NOW® ICT malaria in a large scale field trial. American Society of Tropical Medicine and Hygiene). 54th Annual Meeting. [Google Scholar]
- 15.Farcas GA, Zhong KJ, Lovegrove FE, Graham CM, Kain KC. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am J Trop Med Hyg. 2003;69:589–592. [PubMed] [Google Scholar]
- 16.De Monbrison F, Gerome P, Chaulet JF, Wallon M, Picot S, Peyron F. Comparative diagnostic performance of two commercial rapid tests for malaria in a non-endemic area. Eur J Clin Microbiol Infect Dis. 2004;23:784–786. doi: 10.1007/s10096-004-1202-9. [DOI] [PubMed] [Google Scholar]
- 17.Miller R. American Society of Tropical Medicine and Hygiene, 55th Annual Meeting; Atlanta, GA: 2006. (Comparison of performance characteristics of the Binax NOW Malaria test using venous and fingerstick samples). Abstract 553. [Google Scholar]
- 18.Kain KC, Tennyson S, Keystone JS. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27:142–149. doi: 10.1086/514616. [DOI] [PubMed] [Google Scholar]