Abstract
Results of studies on the associations of maternal helminth infection and malaria-helminth co-infection on birth outcomes have been mixed. A group of 696 pregnant women from the Kwale district in Kenya were recruited and tested for malaria and helminth infection at delivery. Birthweight was documented for 664 infants. A total of 42.7% of the mothers were infected with Plasmodium falciparum, 30.6% with Schistosoma haematobium, 36.2% with filariasis, 31.5% with hookworm, and 5.9% with Trichuris trichiura; co-infection was present in 46.7%. Low birthweight (LBW) (weight < 2,500 grams) was present in 15.4% of the offspring, and 8.3% had a weight z-score ≤ 2 SD below the World Health Organization mean. Only gravida, age, and locale had a significant association with LBW. The high prevalence of maternal infection coupled with a higher than expected percentage of LBW highlight a need for further investigation of the association of maternal co-infection with LBW.
Introduction
The impact on morbidity and mortality of helminth infections has been underestimated, and they continue to represent significant individual and public health problems in much of the developing world.1 Furthermore, there is growing evidence that co-infection with multiple helminth species as well as with helminths and malaria parasites are the rule in many places with co-infection, resulting in synergistic adverse consequences.2–4 Helminth infections with schistosomes, filarial worms and soil-transmitted helminths such as hookworm and Trichuris trichiura, are the cause of common and important neglected tropical diseases.
Certain sub-populations, namely pregnant women and preschool age children, are at a higher risk for neglected tropical diseases. In sub-Saharan Africa and other disease-endemic areas, pregnant women carry a high burden of single helminth infection and of co-infection, or polyparasitism, with multiple helminths or with helminths and malaria.5–7 Historically, pregnant women have been left out of many mass drug administration programs in endemic areas because of concern about potential effects of anti-helminthic agents on the developing fetus.8,9 Consequently, a woman in these disease-endemic areas may not receive therapy for years while she is either pregnant or breastfeeding.8 These chronic helminth infections can thus persist and affect each subsequent pregnancy of an individual woman.10
The effects of maternal malaria infection during pregnancy on the infant are well defined, and malaria contributes to as much as 20% of low birthweight (LBW) deliveries, 36% of preterm deliveries, and 70% of intrauterine growth retardation in malaria-endemic areas.11,12 Furthermore, co-infection with malaria and helminths has been found to be an important factor in adverse birth outcomes.13,14
However, studies of helminth infections during pregnancy and of the benefits of treating pregnant women are still few in number and have shown mixed results.6,15,16 The consensus is now shifting to giving pregnant women anti-helminth treatment because of apparent safety after years of inadvertent doses given to women unknowingly pregnant. Lack of adverse outcomes in animal studies, reports from mass campaigns, and a randomized trial in Uganda of ivermectin and albendazole also support treating pregnant women.9,10 A study in Nepal, where rates of hookworm infection in pregnant women were high, suggested an improvement in maternal anemia, birthweight, and infant mortality in the group of women receiving albendazole during pregnancy.15 However, a subsequent randomized controlled trial in Uganda did not find a difference in birth outcomes in pregnant women treated with albendazole or praziquantel.6 Helminth infection intensities were low, however, and the data suggested a benefit on maternal anemia with albendazole in those women with heavy hookworm infections.6 A study with mebendazole in Peru showed a reduction in very low birthweight in those receiving anti-helminthic drugs, but not an overall improvement in mean birthweights.16 Based on these data and others, a Cochrane review demonstrated no definitive benefit of anti-helminth treatment in pregnant women and did not recommend routine use.17
Given these mixed results and the potential harm of these infections, more data on the effects of helminth infections and particularly, co-infection with malaria on perinatal outcomes are clearly needed. Through a cross-sectional analysis, we aimed to determine if maternal infections and co-infections with malaria, schistosomiasis, filariasis, hookworm, and trichuriasis are associated with low birthweights of term infants in a population in a disease-endemic area on the coast of Kenya.
Materials and Methods
Study participants and study design.
We applied a cross-sectional analysis to a subset of data collected from a cohort of pregnant women and their offspring from the Kwale District in Kenya. The pregnant women were recruited from the antenatal clinic during 2000–2005 at Msambweni District Hospital, Kwale District in Coast Province, Kenya. As described, mothers provided written informed consent for participation in the study and assent for their infants.18 The antenatal clinic participated in a malaria intermittent preventative treatment protocol with sulfadoxine-pyrimethamine, which was given to women at the beginning of the second and third trimesters. A total of 464 women were tested for human immunodeficiency virus (HIV). Women answered a questionnaire on their education level, household income, and spouse's occupation. Maternal venous blood, cord blood, and placental intervillous blood were collected at the time of delivery.18 Only infants born at term, defined as a gestational age of 38–42 weeks, were included in this study.
The infection status for malaria, schistosomiasis, filariasis, hookworm, and Trichuris spp. were derived either from the maternal venous blood, stool, or urine samples as described below. Birthweight was documented by the nurse present at delivery to a precision of at least 100 grams.
Laboratory methods.
Maternal venous blood was tested for Plasmodium falciparum infection by either light microscopy or real-time quantitative polymerase chain reaction, as described.18 Stool and urine were collected from the mothers at the time of delivery and were tested for intestinal helminths and Schistosoma ova, respectively.18 To test for infection with the filarial parasite, Wuchereria bancrofti, night blood samples were tested for circulating microfilariae just before delivery. Alternatively, a circulating antigen assay (Og4C3 antigen detection assay) of maternal venous blood was used to diagnose W. bancrofti infection. Recent exposure to schistosomiasis was also tested by an IgG4 enzyme-linked immunosorbent assay specific for soluble worm antigen preparation. Mothers infected with schistosomiasis or intestinal helminths were treated after delivery, but treatment for filarial infection was deferred.18
Statistical analysis.
To determine the association of individual infections and co-infection on birthweight, we first converted the birthweights into z-scores using Anthro software version 3.0.1, (World Health Organization [WHO], Geneva, Switzerland) and defined low weight-z- score (WZS) as a z-score ≤ 2 SD below the WHO mean. We also used the generally accepted term of LBW, which is a weight less than 2,500 grams, as an additional outcome. After initial univariate analysis with a 2 × 2 contingency table, we then applied multivariate logistic regression to determine the effect of infection and other variables on the likelihood of LBW or low WZS. The association of infection (single or multiple) with the other variables was also investigated by using multivariate logistic regression. The regression applied to the relationship between infections and birthweight was adjusted for the following variables: maternal age, socioeconomic status, education level, marital status, gravida, and area of residence. The number of HIV-positive women was low (n = 20), and as a variable, HIV infection was only evaluated with a 2 × 2 contingency table and not included in the multivariate analysis. The odds ratios with 95% confidence intervals (CI) were obtained from the models. A linear model was also performed and mean birthweights were compared based on infection status. Data analysis was performed using the statistical software R 2.13.1 (Vienna, Austria).
Ethical considerations.
Approval for this study was obtained from the Kenya Medical Research Institute National Ethical Review Committee and the Institutional Review Board for Human Studies at Case Western Reserve University.
Results
Demographics.
Demographic data for the mothers are shown in Table 1. There were 696 live births, with birthweights documented for 664 offspring. Of these mothers and newborns, 593 mother-baby pairs were included in the statistical analysis (data on age, socioeconomic status, and educational level were missing for 103 mothers). Most women had no more than a primary school education and most had a low-to-medium income (as measured in monthly income in shillings). Most women were married or cohabitating.
Table 1.
Demographic variables and gravida of women in the study, coastal Kenya*
| Variable | No. (%), n = 593 | Subgroup of infected women (%)* |
|---|---|---|
| Age (years) | (57 missing value) | |
| < 20 | 16.8 | 18.7 |
| 20–24 | 30.4 | 31.9 |
| 25–30 | 30.2 | 22.8 |
| > 30 | 22.6 | 26.6 |
| Socioeconomic status: monthly income (shillings) | (27 missing value) | |
| Very low (< 1,000) | 1.8 | 2.1 |
| Low (1,000–2,999) | 23.8 | 24.8 |
| Medium (3,000–4,999) | 57.2 | 56.9 |
| High (≥ 5,000) | 17.2 | 16.2 |
| Education | (20 missing value) | |
| None | 20.4 | 21.0 |
| Lower primary | 5.8 | 5.7 |
| Upper primary | 61.2 | 62.2 |
| Secondary | 12.6 | 11.1 |
| Marital status | (40 missing value) | |
| Single | 13.9 | 15.2 |
| Not single† | 76.1 | 84.8 |
| Gravida | (0 missing value) | |
| First pregnancy | 27.3 | 28.5 |
| 2–4 | 49.3 | 49.7 |
| > 4 | 23.4 | 21.8 |
| Geographic locale | (0 missing value) | |
| Mswambweni District | 58.7 | 57.4 |
| Vanga | 41.3 | 42.6 |
Women with ≥ 1 of the infections tested.
Married, cohabitating, separated, or divorced.
Maternal infection.
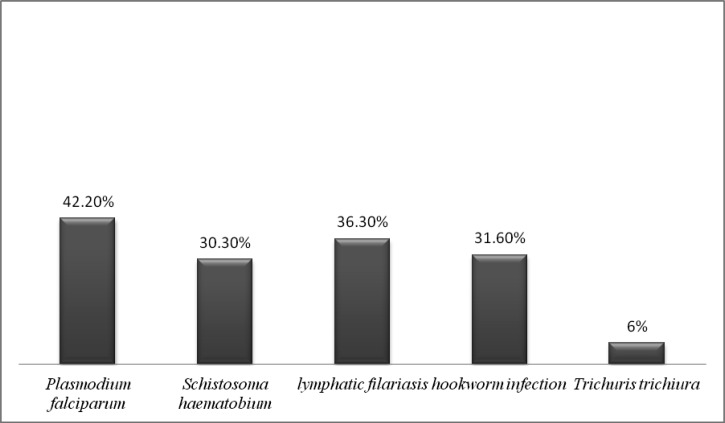
Of the 696 women, 42.7% of the mothers were infected with P. falciparum, 30.6% with S. haematobium, 36.2% with filariasis, 31.5% with hookworm, and 5.9% with T. trichiura (Figure 1). When grouped, 13.2% of the women had P. falciparum alone and 41.1% were infected with one or more helminth without malaria. Helminth-malaria co-infection was present in 29.5% of women and 16.2% had none of the tested infections. The total rate of co-infection with more than one parasitic infection was 46.7%. The HIV prevalence rate in the mothers was 8.4%.
Figure 1.
Maternal infection rates without taking into account co-infection, coastal Kenya.
Offspring data.
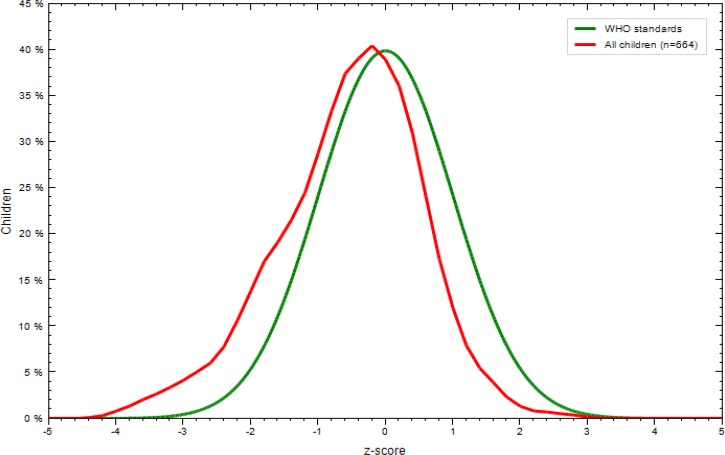
The mean (SD) birthweight for the 664 offspring was 3.06 (0.48) kg, and 8.3% had a low WZS. The distribution of z-scores in the tested newborns was below that considered standard by WHO (Figure 2). The number of children having LBWs was 15.4% when LBW (weight < 2.5 kg) was used as a criterion.
Figure 2.
Comparison of World Health Organization (WHO) mean z-scores (green) to mean z-scores of study infants (red) at day 0 of life at term (≥ 38 weeks gestation), coastal Kenya. Z-scores are not adjusted for gestational age.
Multivariate analysis.
Uninfected women showed a higher risk of giving birth to babies with low WZS and LBW (Table 2) based on univariate analysis. The multivariate logistic regression models showed that the individual infections or co-infection with malaria did not increase the risk of LWB or a low WZS (Table 3). Conversely, infection with helminths was associated with a significantly lower risk for low WZS when grouped as one or more helminths, and for LBW when grouped as single helminth infection. (Table 3). When Schistosoma infections were separated according to whether mothers were given a diagnosis on the basis of egg count versus serologic analysis alone, there was no significant association with adverse birthweight measured by WZS or LBW. The numbers were too low to analyze the data according to infection intensity of either Schistosoma infection or filarial infection.
Table 2.
Univariate analysis with 2 × 2 tables of infections associated with low WZS and LBW, coastal, Kenya*
| Infection | Low WZS | LBW | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| No infection versus | ||||
| Any type of infection | 0.54† | 0.33–0.92† | 0.24† | 0.13–0.42† |
| HIV | 0.81 | 0.75–1.12 | 0.76 | 0.62–1.22 |
| Malaria | 0.56 | 0.2–1.12‡ | 0.46† | 0.23–0.94† |
| Filariasis | 0.43† | 0.23–0.78† | 0.28† | 0.12–0.62† |
| Schistosomiasis | 0.67 | 0.37–1.23 | 0.47† | 0.22–0.99† |
| Trichuriasis | 0.61 | 0.25–1.55 | 0.79 | 0.79–2.21 |
| Hookworm | 0.44† | 0.23–0.81† | 0.33† | 0.14–0.72† |
| Single or multiple helminths | 0.55† | 0.30–0.93† | 0.34† | 0.16–0.70† |
| Coinfection with malaria and helminths | 0.53† | 0.29–0.99† | 0.45† | 0.21–0.98† |
WZS = weight-z-score; LBW = low birthweight; OR = odds ratio; CI = confidence interval; HIV = human immunodeficiency virus.
P < 0.05.
P < 0.1.
Table 3.
Infectious risk associated with low WZS and LBW outcomes, coastal Kenya*
| Infection | Low WZS | LBW | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Referent: no infection | ||||
| Malaria | 0.44 | 0.12–1.63 | 0.52 | 0.18–1.49 |
| Filariasis | 0.84 | 0.28–2.52 | 1.05 | 0.48–2.31 |
| Schistosomiasis | 2.40 | 0.78–7.32 | 1.29 | 0.59–2.82 |
| Trichuriasis | 2.36 | 0.42–13.36 | 0.95 | 0.23–3.91 |
| Hookworm | 1.19 | 0.40–3.51 | 0.87 | 0.40–1.91 |
| Single or multiple helminths | 0.21† | 0.05–0.97† | 0.57 | 0.19–1.75 |
| One helminth | 1.07 | 0.47–2.45 | 0.57† | 0.32–0.93† |
| Helminth co-infection | 1.32 | 0.351–1.58 | 1.32 | 0.72–2.54 |
| Coinfection with malaria and helminths | 0.45 | 0.07–2.79 | 0.77 | 0.20–2.99 |
WZS = weight-z-score; LBW = low birthweight; OR = odds ratio; CI = confidence interval. OR was adjusted for age, primigravid, education, social status, marital status, and residence locale.
P < 0.05.
Variables, apart from the infections, that were significantly associated with either a low WZS or LBW were first pregnancy and older age (Table 4). With regard to locale, residence in Vanga was a protective factor associated with less LBW (Table 4). The results of the models used to evaluate the effect of social factors on the prevalence of infection are shown in Table 5. Mothers who lived in Vanga had a higher risk of filariasis and helminth infections (grouped together and not shown in table) but a lower risk of schistosomiasis. Some of the women more than 20 years of age had a lower risk of hookworm infection and malaria-helminth co-infection (Table 5). Education was a significant protective factor against hookworm infection and malaria-helminth co-infection (Table 5). Women who were not single had a higher risk of hookworm infection.
Table 4.
Non-infectious risk factors associated with low WZS and LBW adjusted for infection status of mother, coastal Kenya*
| Factor | Low WZS | LBW | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Low socioeconomic status | 1.11 | 0.55–2.34 | 0.92 | 0.42–1.99 |
| Single | 1.43 | 0.47–4.40 | 0.43 | 0.15–2.49 |
| Education | 0.50 | 0.21–1.12† | 0.63 | 0.31–1.26 |
| First pregnancy | 3.71‡ | 1.52–9.07‡ | 5.22‡ | 2.53–10.77‡ |
| Age (referent < 20 years) | ||||
| 20–24 | 2.78 | 0.53–14.47 | 2.01 | 0.83–4.86 |
| 25–30 | 4.22‡ | 1.10–16.24‡ | 3.33‡ | 1.22–9.13‡ |
| > 30 | 6.12‡ | 1.40–26.84‡ | 2.38 | 0.80–7.05 |
| Vanga residence | 0.81 | 0.38–1.74 | 0.51‡ | 0.27–0.97‡ |
WZS = weight-z-score; LBW = low birthweight; OR = odds ratio; CI = confidence interval.
P < 0.05.
P < 0.1.
Table 5.
Variables associated with infection, coastal Kenya*
| Variable | Filariasis | Schistosomiasis | Hookworm infection | Trichuriasis | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Not single | 0.99 | 0.58–1.69 | 0.70 | 0.40–1.20 | 1.84† | 1.01–3.34† | 1.55 | 0.42–5.60 |
| Education | 1.20 | 0.71–2.02 | 0.92 | 0.54–1.57 | 0.56† | 0.33–0.92† | 0.74 | 0.25–2.13 |
| Age (referent < 20 years) | ||||||||
| 20–24 | 0.93 | 0.53–1.64‡ | 1.06 | 0.59–1.90 | 0.57 | 0.32–1.02‡ | 0.17† | 0.04–0.71† |
| 25–30 | 0.57 | 0.31–1.06 | 0.55 | 0.29–1.04‡ | 0.36† | 0.19–0.67† | 0.75 | 0.25–2.22 |
| > 30 | 0.70 | 0.38–1.26 | 0.61 | 0.32–1.13 | 0.42† | 0.22–0.77† | 0.30 | 0.08–1.06‡ |
| Vanga residence | 2.47† | 1.63–3.75† | 0.62† | 0.39–0.96† | 1.18 | 0.77–1.81 | 0.44 | 0.16–1.21 |
| Variable | Malaria | Single helminth | Helminth co-infection | Malaria-helminth co-infection | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Not single | 0.76 | 0.46–1.28 | 0.99 | 0.56–1.77 | 0.93 | 0.52–1.67 | 0.83 | 0.48–1.44 |
| Education | 0.71 | 0.44–1.17 | 1.29 | 0.74–2.23 | 0.96 | 0.55–1.74 | 0.52† | 0.31–0.86† |
| Age (referent < 20 years) | ||||||||
| 20–24 | 0.70 | 0.4–1.22 | 1.11 | 0.52–1.89 | 0.86 | 0.47–1.52 | 0.60 | 0.32–1.07‡ |
| 25–30 | 0.67 | 0.37–1.21 | 0.75 | 0.25–2.22 | 0.85 | 0.44–1.64 | 0.47† | 0.25–0.89† |
| > 30 | 0.73 | 0.41–1.31 | 0.85 | 0.42–1.61 | 0.74 | 0.39–1.41 | 0.69 | 0.37–1.26 |
| Vanga residence | 0.71 | 0.45–1.04‡ | 1.25 | 0.80–1.95 | 0.83 | 0.53–1.32 | 0.76 | 0.48–1.17 |
OR = odds ratio; CI = confidence interval.
P < 0.05.
P < 0.1.
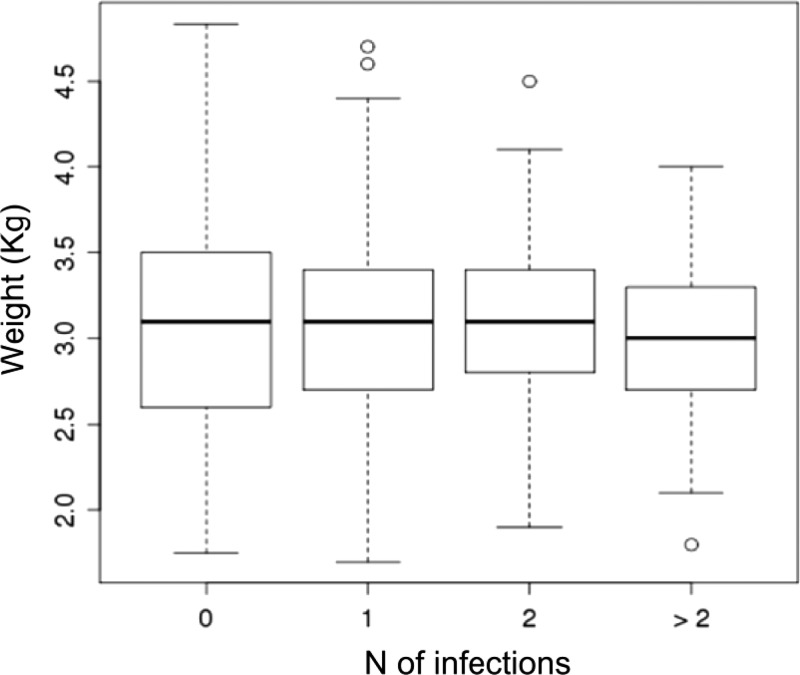
When using the linear model, no statistically significant differences were found in mean birthweights across the different infection groups. However, there was a trend towards lower birthweights in the mothers who were co-infected with more than two parasites (Figure 3).
Figure 3.
Boxplot of birthweights in offspring of mothers with 0, single, or multiple infections (x-axis), coastal Kenya. Solid black lines indicate the medias, white boxes indicate interquartile ranges, and dotted bars indicate extreme values of data (1.5 times interquartile ranges).
Discussion
Prevalence of either or both P. falciparum and helminth infection in our study population was high; only 16% of women were uninfected at the time of delivery. Interestingly, these uninfected women, as a group, had children with lower average birthweights than infected women. When we controlled for income and education level, as well as gravida and maternal age, neither the individual infections nor combination of helminth-malaria infection were associated with LBWs or low WZS in the offspring. Conversely, it appeared that the mothers infected with helminths alone were associated with a higher WZS. As a group, the women without any infection were associated with a lower mean z-score when compared with the other groups and therefore may have impacted the above association of the helminth alone group with higher WZS. Because Vanga residence appeared to be protective for LBW, it was worth looking in greater detail at the attributes of those mothers. Although they had more filarial infection, they had less schistosomiasis, and trended towards less malaria infection. It is possible that either maternal filarial infection has a protective effect on birthweight or the absence of malaria infection positively impacted the birthweight and thus these mothers were represented more in the helminth alone group. Furthermore, this village tended to have more food security and the women could have potentially been better nourished. Otherwise, there were no known factors during recruitment of the women that would explain the trend of lower birthweights in the uninfected group.
The fact that first pregnancy was associated with LBW and low WZS is not surprising because primigravid women are more likely to be affected by malaria infection.11 However, increased age was associated with lower birthweight, which is harder to explain because younger women are more likely to be primigravid. Although the linear model also did not show significant associations of the infections with birthweight, there was an interesting trend of lower mean birthweights in mothers harboring more than two parasites. A larger study may have uncovered a significant association.
Although the outcome of our analysis did not prove as expected based on past reports, one of the striking results is the high prevalence of infection with polyparasitism in the mothers.13–15 These data are consistent with those of recent studies in sub-Saharan Africa.5,6 Another important outcome to draw from this data is the high percentage of infants born with LBW and a low WZS. This population of infants had a percentage of LBW of 15.4%, which was well above the average of 11% for Kenya and 8% for the United States per WHO statistics in 1998.19 Furthermore, these national statistics included premature infants, whereas our study contains only term infants, making these findings even more significant. In terms of study limitations, we did not include other measures of adverse birth outcomes such as preterm deliveries or still births. Assessing these outcomes may have uncovered more effects of helminth infection or co-infection on birthweight. Also, infection was tested only at the time of delivery, and given the high prevalence of infection, it is probable that some of the uninfected women were, in fact, infected at some point during their pregnancy. Other maternal factors not included in this study that may have been confounders include maternal height and smoking status. Furthermore, the numbers were too low to separate high versus low intensity infections for schistosomiasis and filariasis. It is possible that an effect on birthweight may have been found at high intensity infection.
Although there are many factors involved, including unmeasured ones such as the nutritional status of the mother and maternal anemia, it is hard to ignore the high prevalence of malaria and helminth infection in these mothers and the potential effects on the health of the offspring. Maternal anemia in pregnancy is known to have negative effects on mother and fetus, including LBW.20 With the high prevalence of malaria and helminths that are known to cause anemia, it follows that there would be adverse effects on the pregnancy and perinatal outcomes.21,22 Although not consistently found across studies, both hookworm infection and schistosomiasis have been found to be independent risk factors for anemia in pregnancy.23–25 A recent study has also shown an adverse effect of T. trichiura infection as well as co-infection with hookworm and T. trichiura on maternal anemia.26 Maternal hemoglobin may therefore be an appropriate surrogate marker of adverse birth outcomes. In studies one can control for iron supplementation, which may make it easier to detect an impact of the parasitic infections.
In terms of co-infection with malaria and helminths, a study in Ghana demonstrated that co-infection with malaria and intestinal helminths substantially increased the risk of maternal anemia. And in those women with anemia, co-infection was associated with an increased risk of LBW, preterm delivery, and small size for gestational age, which was not completely explained by the presence of malaria infection.13 Another study in Nigeria showed a high prevalence of malaria infected pregnant women who were co-infected with helminths (45%) and suggested a trend towards lower maternal hemoglobin levels in those co-infected.14
Apart from maternal anemia and other measures of perinatal health such as birthweight, prematurity, and stillbirths, there are other potential morbidities from helminth infection and polyparasitism on fetuses that warrant attention. These morbidities include maternal and fetal inflammation, potential placental and congenital infection with schistosomiasis, early childhood growth, and early childhood response to vaccine8,27–29 Kurtis and others reported significantly more inflammation in maternal, placental, and cord blood in mothers infected with Schistosoma japonicum as measured by proinflammatory cytokines. This inflammation was associated with a statistically significant lower birthweight.29 Early childhood susceptibility to infection could also be impacted by maternal infection. This susceptibility has been demonstrated in the cohort reported in this study. Infants sensitized to malaria antigens in utero had higher susceptibility to malaria infection and lower hemoglobin levels.18 There is also evidence that maternal filarial infection can influence infant susceptibility to filarial infection.30 This finding may hold true for other helminth infections or co-infections.
In summary, our study with a small group of uninfected women and a high prevalence of infections did not show an effect of the parasitic infections studies on birthweight of term infants. However, our data, along with the other studies discussed, highlight the complexities of investigating this subject and the need for further studies on the impact of helminth infection, and in particular, polyparasitism, on birth outcomes. More studies looking at some of these other markers of morbidity such as maternal anemia, inflammatory cytokines, and response to vaccines along with traditional measures of perinatal outcomes are needed.
ACKNOWLEDGMENTS
We thank Victoria Saidi, Hashora Mwanguku, Zaituni Mwakileo, Fatuma Ngare, Ruth Notina, and Florence Wambua for helping to recruit women to participate in the study, collecting blood samples, and caring for women and infants; Elton Mzungu, Kefar Wambua, Charles N'gan'ga, and Alex Osore for performing many of the parasitologic examinations and immunologic assays; Grace Mathenge and Christine Lucas for data entry and management; and the women living in the Msambweni District and Vanga for participating in the study.
Footnotes
Financial support: This study was supported by the National Institute of Child Health and Human Development and National Institute of Health (grant no. A1064687).
Authors' addresses: Jessica K. Fairley, Division of Infectious Diseases, Emory University School of Medicine, Medical Office Tower, Atlanta, GA, E-mail: jessica.fairley@emory.edu. Donal Bisanzio and Uriel Kitron, Department of Environmental Studies, Emory University, Math and Science Center, Atlanta, GA, E-mails: dbisanz@emory.edu and ukitron@emory.edu. Charles H. King, Peter Mungai, Christopher L. King, and Indu Malhotra, Center for Global Health and Diseases, Case Western Reserve University, Biomedical Research Building, Cleveland, OH, E-mails: chk@case.edu, plmungai@yahoo.com, christopher.king@emory.edu, and ijm@case.edu. Eric Muchiri, Division of Vector Borne Diseases, Nairobi, Kenya, E-mail: ericmmuchiri@gmail.com.
References
- 1.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 2.Ashford RW, Craig PS, Oppenheimer SJ. Polyparasitism on the Kenya coast. 1. Prevalence, and association between parasitic infections. Ann Trop Med Parasitol. 1992;86:671–679. doi: 10.1080/00034983.1992.11812724. [DOI] [PubMed] [Google Scholar]
- 3.Ezeamama AE, McGarvey ST, Acosta LP, Zierler S, Manalo DL, Wu HW, Kurtis JD, Mor V, Olveda RM, Friedman JF. The synergistic effect of concomitant schistosomiasis, hookworm, and Trichuris infections on children's anemia burden. PLoS Negl Trop Dis. 2008;2:e245. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez PJ. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Adegnika AA, Ramharter M, Agnandji ST, Ateba Ngoa U, Issifou S, Yazdanbahksh M, Kremsner PG. Epidemiology of parasitic co-infections during pregnancy in Lambarene, Gabon. Trop Med Int Health. 2010;15:1204–1209. doi: 10.1111/j.1365-3156.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 6.Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, Oweka J, Kizindo R, Duong T, Kleinschmidt I, Muwanga M, Elliott AM. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clin Infect Dis. 2010;50:531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navitsky RC, Dreyfuss ML, Shrestha J, Khatry SK, Stoltzfus RJ, Albonico M. Ancylostoma duodenale is responsible for hookworm infections among pregnant women in the rural plains of Nepal. J Parasitol. 1998;84:647–651. [PubMed] [Google Scholar]
- 8.Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23:159–164. doi: 10.1016/j.pt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi District, western Uganda. Am J Trop Med Hyg. 2008;79:856–863. [PubMed] [Google Scholar]
- 10.Allen HE, Crompton DW, de Silva N, LoVerde PT, Olds GR. New policies for using anthelmintics in high risk groups. Trends Parasitol. 2002;18:381–382. doi: 10.1016/s1471-4922(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 11.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 12.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 13.Yatich NJ, Jolly PE, Funkhouser E, Agbenyega T, Rayner JC, Ehiri JE, Turpin A, Stiles JK, Ellis WO, Jiang Y, Williams JH. The effect of malaria and intestinal helminth coinfection on birth outcomes in Kumasi, Ghana. Am J Trop Med Hyg. 2010;82:28–34. doi: 10.4269/ajtmh.2010.09-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egwunyenga AO, Ajayi JA, Nmorsi OP, Duhlinska-Popova DD. Plasmodium/intestinal helminth co-infections among pregnant Nigerian women. Mem Inst Oswaldo Cruz. 2001;96:1055–1059. doi: 10.1590/s0074-02762001000800005. [DOI] [PubMed] [Google Scholar]
- 15.Christian P, Khatry SK, West KP., Jr Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet. 2004;364:981–983. doi: 10.1016/S0140-6736(04)17023-2. [DOI] [PubMed] [Google Scholar]
- 16.Larocque R, Casapia M, Gotuzzo E, MacLean JD, Soto JC, Rahme E, Gyorkos TW. A double-blind randomized controlled trial of antenatal mebendazole to reduce low birthweight in a hookworm-endemic area of Peru. Trop Med Int Health. 2006;11:1485–1495. doi: 10.1111/j.1365-3156.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 17.Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD005547.pub3. CD005547. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, Muchiri E, Tisch DJ, King CL. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization, UNICEF . Low Birthweight: Country, Regional and Global Estimates. Geneva: World Health Organization; 2004. [Google Scholar]
- 20.ACOG Committee on Practice Guidelines ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol. 2008;112:201–207. doi: 10.1097/AOG.0b013e3181809c0d. [DOI] [PubMed] [Google Scholar]
- 21.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 22.Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Ajanga A, Lwambo NJ, Blair L, Nyandindi U, Fenwick A, Brooker S. Schistosoma mansoni in pregnancy and associations with anaemia in northwest Tanzania. Trans R Soc Trop Med Hyg. 2006;100:59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Trans R Soc Trop Med Hyg. 2008;102:130–136. doi: 10.1016/j.trstmh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Muhangi L, Woodburn P, Omara M, Omoding N, Kizito D, Mpairwe H, Nabulime J, Ameke C, Morison LA, Elliott AM. Associations between mild-to-moderate anaemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans R Soc Trop Med Hyg. 2007;101:899–907. doi: 10.1016/j.trstmh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyorkos TW, Gilbert NL, Larocque R, Casapia M. Trichuris and hookworm infections associated with anaemia during pregnancy. Trop Med Int Health. 2011;16:531–537. doi: 10.1111/j.1365-3156.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 27.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott AM, Mawa PA, Webb EL, Nampijja M, Lyadda N, Bukusuba J, Kizza M, Namujju PB, Nabulime J, Ndibazza J, Muwanga M, Whitworth JA. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine. 2010;29:247–255. doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtis JD, Higashi A, Wu HW, Gundogan F, McDonald EA, Sharma S, PondTor S, Jarilla B, Sagliba MJ, Gonzal A, Olveda R, Acosta L, Friedman JF. Maternal schistosomiasis japonica is associated with maternal, placental, and fetal inflammation. Infect Immun. 2011;79:1254–1261. doi: 10.1128/IAI.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra I, Ouma JH, Wamachi A, Kioko J, Mungai P, Njzovu M, Kazura JW, King CL. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect Immun. 2003;71:5231–5237. doi: 10.1128/IAI.71.9.5231-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]