Abstract
Dengue hemorrhagic fever is characterized by a unique vascular leakage syndrome. The mechanisms of endothelial barrier dysfunction in dengue hemorrhagic fever are not well understood. We examined the modulation of endothelial barrier function in dengue virus type 2 (DENV2) infections using primary human umbilical vein endothelial cells. We demonstrated that the increase in endothelial barrier function within 72 hours after DENV2 infection is mediated by type I interferon–dependent CD73 up-regulation. After 72 hours, DENV2 slowed the recovery of endothelial barrier function in response to tumor necrosis factor-α or vascular endothelial growth factor. This phenomenon was likely caused by type I interferon receptor signaling inhibition and lower CD73 levels in DENV2-infected endothelial cells. Our findings suggest that during DENV2 infection, endothelial barrier homeostasis is maintained by a balance between pro-inflammatory and pro-angiogenic cytokines, and type I interferon–dependent CD73 expression and activity.
Introduction
Dengue is the most prevalent arthropod-borne viral illness in humans, and half of the world's population is at risk for infection. There are up to 50 million cases of dengue estimated each year resulting in 500,000 hospitalizations and 20,000 deaths.1 Dengue viruses (DENVs) are single-stranded, positive-sense, RNA-containing enveloped viruses belonging to the family Flaviviridae and the genus Flavivirus.2 There are four serotypes of DENVs (DENV1–4).
Dengue virus infections produce a wide spectrum of clinical illness. This spectrum ranges from asymptomatic or mild illness to a severe and potentially life-threatening disease, dengue hemorrhagic fever (DHF). The hallmark of DHF is endothelial dysfunction. This dysfunction is manifested by a vascular leakage syndrome and sometimes a hemorrhagic diathesis. The morbidity and mortality of DHF are largely driven by the vascular leakage and its ensuing complications.
The mechanisms of endothelial barrier dysfunction that lead to the unique vascular leakage syndrome of DHF are not well understood. Dengue viruses are not cytopathic to endothelial cells in vitro.3,4 Viral antigen positive endothelial cells have been seen in autopsy tissues with minimal or no endothelial destruction.5,6 Vascular leakage develops abruptly during viral clearance and early symptom recovery, at a time of significant immune activation.7 The initial vascular leakage is focal and occurs predominantly in the pleural and peritoneal spaces.8 The prevailing explanation for DHF vascular leakage is that immune responses to DENV infection produce a cytokine storm that leads to the transient compromise of endothelial barrier function.9 Many pro-inflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α]) and angiogenesis factors (e.g., vascular endothelial growth factor [VEGF]) can decrease endothelial barrier function and thereby increase vascular permeability.10 This hyperpermeability is often measured in vitro by transendothelial cell electrical resistance (TEER) of monolayers.11,12 Circulating levels of many vascular permeability mediators are increased in patients with DHF9 and can be produced by DENV-infected cells in vitro.13,14
Most DENV-infected patients do not show development of a vascular leakage syndrome. Several proteins and signaling pathways are involved in maintenance of endothelial barrier function, and one of these involves CD73 activity. CD73 is a 5′-ectonucleotidase and a glycophosphatidylinositol–anchored protein that is expressed abundantly on endothelial cells. The primary function of CD73 is to convert adenosine monophosphate (5′-AMP) to adenosine.15 Adenosine then signals through endothelial adenosine 2b (A2b) receptors and increases intracellular cyclic AMP levels that leads to strengthened adherens junctions, and augments endothelial barrier function.15,16
We had previously shown that DENV2 ameliorated TNF-α–driven hyperpermeability of human umbilical vein endothelial cell (HUVEC) monolayers at an early time point after infection. This effect was mediated by type I interferon (IFN). At a later time point, DENV2 infection augmented the TNF-α–driven hyperpermeability of HUVEC monolayers.17 In this report, we demonstrate that the increase in HUVEC barrier function within 72 hours after DENV2 infection is mediated by type I IFN-dependent CD73 up-regulation. After 72 hours, DENV2 slowed the recovery of HUVEC barrier function in response to TNF-α or VEGF treatment. Our data suggests that this phenomenon was caused by the blockade of type I IFN receptor (IFNAR) signaling and lower CD73 levels in DENV2 containing endothelial cells.
Methods
Reagents.
Reagents used were recombinant (r)TNF-α (Invitrogen, Carlsbad, CA), rIFN-β (PBL Laboratories, Piscataway, NJ), rVEGF (Invitrogen), rB18R protein (eBioscience, San Diego, CA), anti-IFNAR mouse monoclonal antibody (mAb) (PBL Laboratories), anti-CD73 mouse mAbs (7G2; Invitrogen and 1E9; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse IgG1 isotype control (BD Pharmingen, San Diego, CA). B18R is a soluble-type I IFN receptor analog from vaccinia virus and is used to block type I IFN signaling.
Viruses and cells.
Dengue virus type 2 strain NGC, originally obtained from the American Type Culture Collection (Manassas, VA), was propagated in the mosquito cell line C6/36. Virus stock titers were determined by limiting-dilution plaque assay on Vero cells, and were free of endotoxin and Mycoplasma contamination. Sendai virus (Cantell strain) at a concentration of 4,000 hemagglutinin units/mL was obtained from Charles River (SPAFAS, Wilmington, MA). Primary HUVECs were obtained from Lonza (Basel, Switzerland) and cultured at 37°C in an atmosphere of 5% CO2 in endothelial cell growth medium 2 supplemented with endothelial cell growth medium 2–microvascular endothelial cell medium 2 SingleQuots (Lonza). All HUVEC experiments were performed with subculture passage 2 cells.
DENV infection.
The HUVECs were infected with DENV2 NGC at a multiplicity of infection of 5. Dengue virus type 2 was adsorbed for 2 hours at 37°C, washed with phosphate-buffered saline (Invitrogen), and fresh medium was added to the cells. The HUVECs treated in a similar manner with C6/36 cell supernatants instead of virus were used as uninfected controls.
Tranfection with small interfering RNA.
Validated small interfering RNAs for IFN-β promoter stimulator-1 (IPS-I, gene accession no. NM_0207746), myeloid differentiation factor 88 (MyD88, gene accession no. NM_002468), and a non-silencing control were obtained from QIAGEN (Valencia, CA). Transfection of small interfering RNAs into HUVECs at a concentration of 200 pmol for each sample was performed by using the Amaxa™ electroporation system (Lonza), as per the manufacturer's instructions. Sendai virus was used as a positive control for IPS-1–dependent IFN-β production. Targeted gene knockdown was confirmed by using a quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Trans-endothelial electrical resistance assays.
For measuring TEER, HUVECs were plated at a concentration of 5 × 105 cells/well on collagen-coated transwell inserts (0.4-μm-pore, 6.5-mm-diameter, Transwell-COL, Costar; Corning, Ithaca, NY) and incubated at 37°C in an atmosphere of 5% CO2. After approximately two days, the HUVECs formed a confluent monolayer. Dengue virus type 2, cytokines, or soluble mediators were added at the indicated concentrations and time points to the top chamber. Electrical resistance across HUVEC monolayers was measured by using an EndOhm-6 chamber and EVOM volt-ohmmeter (World Precision Instruments, Sarasota, FL), according to the manufacturer's instructions. Resistance measured by using a transwell insert with media and no endothelial cells was considered a blank reading. TEER was calculated as follows: TEER (Ω.cm2) = (sample-well resistance – blank-well resistance) × area of cell monolayer.
Flow cytometry.
For flow cytometry surface staining of CD73, HUVECs were dissociated from the plate by treatment with mild trypsin-EDTA (Lonza), resuspended in medium, and incubated at 37°C in an atmosphere of 5% CO2 for 1 hour. After two washes, anti-CD73 mouse mAb conjugated to allophycocyanin (BD Biosciences, Franklin Lakes, NJ) was added a concentration of at 50 ng per sample for 30 minutes at 4°C. Cells were then washed, fixed (Cytofix; BD Biosciences), and analyzed by flow cytometry (FACSCalibur; BD Immunocytometry Systems, San Jose, CA). For detection of DENV infection, HUVECs were fixed and permeabilized by using Cytofix/Cytoperm (BD Biosciences), stained with anti-DENV prM 2H2 mouse mAb conjugated to Alexafluor 488 for 30 minutes at 4°C, washed (Perm/Wash buffer; BD Biosciences), and analyzed by flow cytometry. Flow cytometry data were analyzed using FlowJo™ version 6 (TreeStar, Inc., Ashland, OR).
Quantitative reverse transcription polymerase chain reaction.
Total RNA from HUVECs was extracted by using the RNeasy Mini kit (QIAGEN) following the manufacturer's instructions. The qRT-PCR was performed by using the ABI Prism 7300 sequence detection system (Applied Biosystems, Foster City, CA) per the manufacturer's instructions. Primer-probe sets for human IFN-β (Hs00277188_s1), human CD73 (Hs01573922_m1), human A2b receptor (Hs00386497_m1), and human glyceraldehyde 3-phosphate dehydrogenase (Hs02758991_g1) were obtained from Applied Biosystems, and qRT-PCR was performed using TaqMan One-step RT-PCR kit (Applied Biosystems). Primers for human Robo4, Tie-2, and Notch1-4 were obtained from Integrated DNA Technologies (Coralville, IA), and qRT-PCR was performed by using the SYBR Green qRT-PCR kit (Invitrogen). The relative mRNA expression level of each gene was normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase, and relative gene expression levels were then analyzed by using the 2−ΔΔCt method.
Statistical analysis.
Graphpad Prism version 5.0 (Graphpad, San Diego, CA) was used for statistical analysis. For normally distributed variables, comparisons between two groups were performed by using the parametric Student's t test. For non-normally distributed variables, comparisons between two groups were performed by using the non-parametric Mann-Whitney U test. Comparisons among normally distributed multiple groups were performed using one-way or two-way analysis of variance.
Results
Induction of HUVEC IFN-β production by DENV2 in an IPS-1–dependent manner.
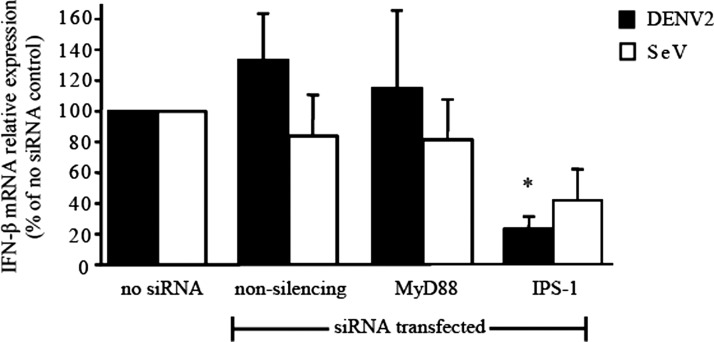
Dengue virus has been reported to induce IFN-β production in cultured fibroblasts using the cytoplasmic RNA-sensing pattern recognition receptors retinoic acid-inducible gene I and melanoma differentiation associated protein 5.18 Retinoic acid-inducible gene I and melanoma differentiation associated protein 5 use the downstream signaling adaptor IPS-1 to stimulate IFN-β production. We examined the role of IPS-1 signaling in DENV2 stimulation of HUVEC IFN-β production. The DENV2 stimulation of HUVEC IFN-β production was nearly completely dependent on IPS-1 signaling, and not on MyD88 signaling (Figure 1).
Figure 1.
Induction of interferon-β (IFN-β) production in human umbilical vein endothelial cells (HUVECs) through an IFN-β promoter stimulator-1 (IPS-1)–dependent mechanism by dengue virus type 2 (DENV2). The HUVECs were transfected with the indicated small interfering RNAs (siRNAs) by electroporation, and 24 h later were stimulated with DENV2 (multiplicity of infection [MOI] = 5) or Sendai virus (SeV, MOI = 0.03 by hemagglutinin units). Total cytoplasmic RNA was extracted from HUVECs and the relative expression of IFN-β mRNA was measured at 48 h by quantitative reverse transcription–polymerase chain reaction. Black bars = DENV2 stimulation; empty bars = SeV stimulation. Values and error bars are mean ± SEM, n = 3 independent experiments. *P = 0.01 compared with no siRNA control.
Induction of up-regulation of CD73 expression in HUVECs by DENV2 in a type I IFN-dependent manner.
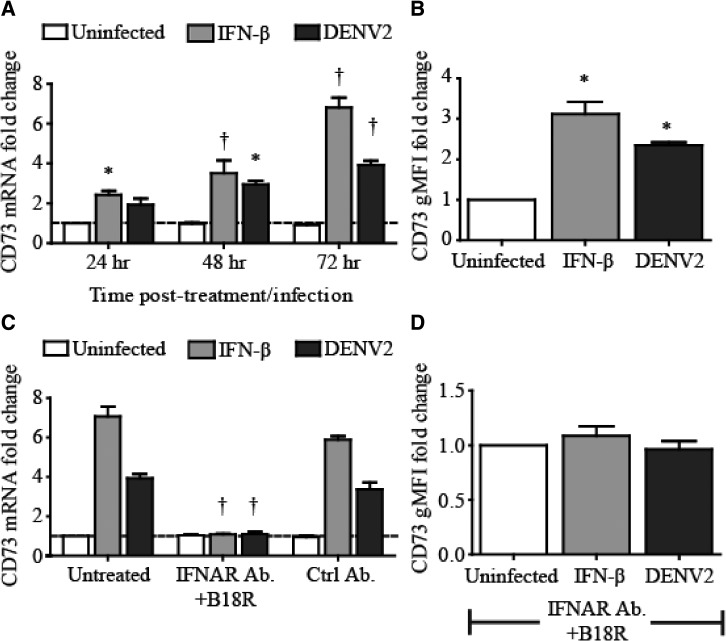
We had previously shown that type I IFN and DENV2 infection (early time point) increase the barrier function of HUVEC monolayers.17 We measured the DENV2-infected HUVEC mRNA levels of genes reported to be involved in the regulation of endothelial barrier function (Robo4, Tie-2, Notch1-4, and CD73). Over the first 72 hours after DENV2 infection, we found that HUVEC CD73 mRNA and protein expression were up-regulated (Figure 2A and B). None of the other genes tested were up-regulated or down-regulated significantly by DENV2 infection in HUVECs. We also found that rIFN-β increased CD73 expression in HUVECs, and DENV2-stimulated HUVEC CD73 up-regulation was dependent on IFNAR signaling (Figure 2). Our data demonstrate that DENV2 induces type I IFN production in HUVECs and IFNAR signaling leads to the up-regulation of CD73 in these cells.
Figure 2.
Up-regulation of CD73 mRNA and surface protein expression in human umbilical vein endothelial cells (HUVECs) in a type I interferon (IFN)–dependent manner by dengue virus type 2 (DENV2). For A and B, HUVECs were stimulated with recombinant (r) interferon-β (IFN-β) (500 U/mL) or DENV2 (multiplicity of infection [MOI] = 5). A, Total cytoplasmic RNA was extracted from HUVECs and the relative expression of CD73 mRNA was measured at the indicated time-points by quantitative reverse transcription polymerase chain reaction (qRT-PCR). B, HUVECs were stained at 72 hours with anti-CD73 conjugated with allophycocyanin (APC), and the geometric mean fluorescence intensity (gMFI) was measured by fluorescence-activated cell sorting (FACS). For C and D, HUVECs were pre-treated with either a combination of a blocking IFN-α receptor monoclonal antibody (IFNAR mAb) and rB18R or a control (ctrl) antibody (Ab) before stimulation with rIFN-β (500 U/mL) or DENV2 (MOI = 5). C, Total cytoplasmic RNA was extracted from HUVECs and the relative expression of CD73 mRNA was measured at 72 hours by qRT-PCR. D, HUVECs were stained at 72 hours with anti-CD73-APC Ab, and the gMFI was measured by FACS. Shown are the gMFIs for samples pre-treated with blocking IFNAR mAb and rB18R. Dark gray bars = DENV2 stimulation; light gray bars = rIFN-β stimulation; empty bars = uninfected cells. Values and error bars are mean ± SEM, n = 3 independent experiments. *P < 0.05, †P < 0.01 compared with uninfected control.
Mediation by CD73 of DENV2-stimulated increase in barrier function and amelioration of TNF-α–driven hyperpermeability in HUVECs.
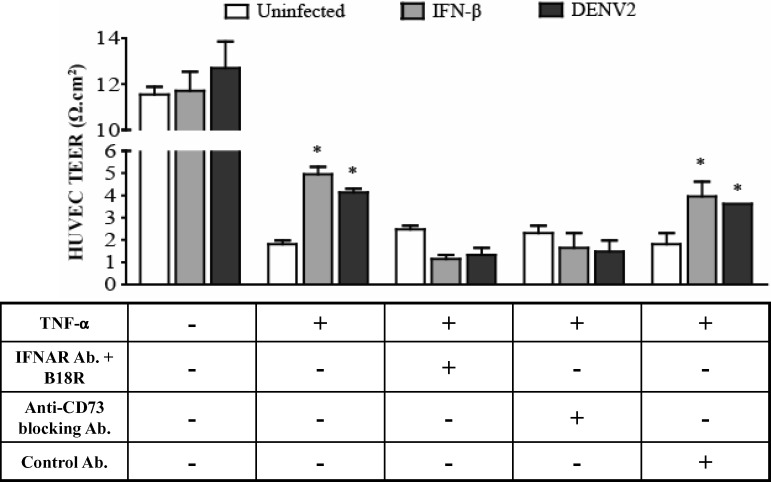
At an early time point after infection, we had previously shown that DENV2 infection of HUVECs ameliorated TNF-α–driven hyperpermeability, and this effect was dependent on type I IFN production and signaling.17 To investigate whether the amelioration of TNF-α–driven hyperpermeability hyperpermeability in HUVECs was mediated by CD73, we blocked CD73 activity and analyzed the TEER of DENV2-infected HUVEC monolayers. The HUVECs expressed A2b receptor mRNA. Therefore, CD73 activity could potentially increase barrier function. In accordance with our previous report, we observed that TNF-α–driven hyperpermeability was ameliorated at 72 hours in HUVECs treated with rIFN-β or infected with DENV2. When HUVECs were pre-treated with a blocking IFNAR mAb and rB18R, or with a blocking anti-CD73 mAb (7G2), TNF-α–driven hyperpermeability was no longer ameliorated by DENV2 infection (Figure 3). Similar results were obtained using a second anti-CD73 blocking mAb (1E9). Our data demonstrate that the increase in HUVEC barrier function within 72 hours after DENV2 infection is mediated by type I IFN signaling and CD73.
Figure 3.
Dengue virus type 2 (DENV2) amelioration of tumor necrosis factor-α (TNF-α)–driven hyperpermeability of human umbilical vein endothelial cells (HUVECs) mediated by type I interferon (IFN) and CD73. HUVECs were grown to confluency in collagen-coated transwells and in some cases pre-treated with a combination of a blocking interferon-α (IFN-α) receptor monoclonal antibody (IFNAR mAb) and rB18R, an anti-CD73 blocking mAb (7G2), or a control Ab before stimulation with recombinant (r)IFN-β (500 U/mL) or DENV2 (multiplicity of infection [MOI] = 5). HUVEC monolayers were then treated with rTNF-α (2 ng/mL), and the transendothelial electrical resistance (TEER) was measured 72 h later. Dark gray bars = DENV2 stimulation; light gray bars = rIFN-β stimulation; empty bars = uninfected cells. Values and error bars are mean ± SEM, n = 3 independent experiments. *P < 0.05 compared with control HUVECs treated with rTNF-α.
Slowing of late recovery in barrier function of TNF-α– or VEGF-treated HUVECs by DENV2 infection.
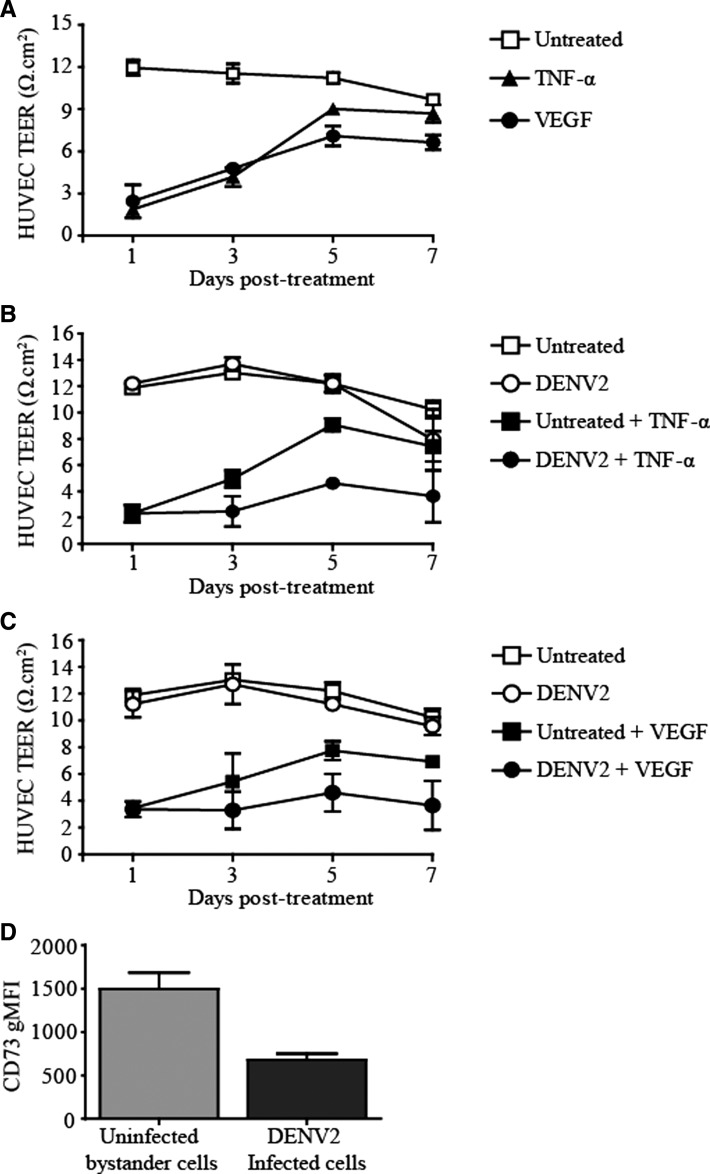
The HUVEC hyperpermeability induced by TNF-α, a pro-inflammatory cytokine, or VEGF, a pro-angiogenic cytokine, is reversible.10 The HUVEC monolayers grown on collagen-coated transwell membranes became permeable upon treatment with rTNF-α or rVEGF. After day 3, TEER began to recover to near normal (untreated) levels in rTNF-α– or rVEGF–treated HUVEC monolayers (Figure 4A). Dengue virus type 2 did not alter the HUVEC TEER nadir produced by TNF-α or VEGF (when Type I IFN receptor signaling was inhibited), but DENV2 infection slowed the TEER recovery (Figure 4B and C).
Figure 4.
Slowing of recovery of endothelial barrier function in human umbilical vein endothelial cells (HUVECs) treated with recombinant tumor necrosis factor-α (rTNF-α) or vascular endothelial growth factor (rVEGF) by dengue virus type 2 (DENV2). HUVECs were grown to confluency in collagen-coated transwells and pre-treated with a combination of a blocking interferon-α (IFN-α) receptor monoclonal antibody (IFNAR mAb) and rB18R. A, HUVECs were treated with rTNF-α (2 ng/mL) or rVEGF (40 ng/mL) and the transendothelial electrical resistance (TEER) was measured over the indicated time points. B, HUVECs were stimulated with DENV2 (multiplicity of infection [MOI] = 5) and then treated with rTNF-α (2 ng/mL) or C, recombinant vascular endothelial growth factor (rVEGF) (40 ng/mL). TEER was measured at the indicated time points. D, CD73 expression on the cell surface of DENV2-infected and DENV2-uninfected HUVECs was analyzed by flow cytometry. The geometric mean fluorescence intensity (gMFI) for CD73 surface expression is shown. Values and error bars are mean ± SEM, n = 2 independent experiments.
Lower CD73 expression in DENV2-infected HUVECs than in uninfected bystander HUVECs.
By day 3 after infection, approximately 20% of the HUVEC monolayer contained DENV2 (multiplicity of infection = 5). The IFN-β production in HUVECs continues to increase up to 5 days after DENV2 infection.17 The DENV-infected cells produce type I IFN but are known to cause potent inhibition of IFNAR signaling.19,20 As such, we observed that CD73 surface protein expression was lower in DENV2-infected HUVECs compared with uninfected bystander HUVECs (Figure 4D), presumably because of the inhibition of IFNAR signaling in these DENV2-infected HUVECs. Expression of CD73 is higher in nearby uninfected bystander HUVECs because type I IFN signaling can occur unimpeded in these cells.
Discussion
We have shown that DENV2 infection of HUVECs induces type I IFN production in an IPS-1-dependent manner. Type I IFN signaling up-regulates CD73 expression, and CD73 is largely responsible for the DENV2 augmentation of HUVEC monolayer barrier function. At later time points, DENV2 slows the recovery of barrier function in HUVEC monolayers treated with TNF-α or VEGF. Our data suggests that the slow recovery of barrier function is largely caused by impaired IFNAR signaling and CD73 expression in endothelial cells containing DENV2.
In essentially all DENV-infected patients, a vascular leakage syndrome is not seen over the first several days after infection. Absence of this syndrome occurs despite a robust innate immune response that produces pro-inflammatory (e.g., TNF-α) and pro-angiogenic (e.g., VEGF) cytokines.9,21 We postulate that endothelial hyperpermeability driven by pro-inflammatory and pro-angiogenic innate immune cytokines is counteracted by DENV-induced type I IFN production, signaling, and CD73 up-regulation in endothelial cells. The DENV stimulated type I IFN production at early time points may occur directly in endothelial cells, as shown here, or in other non-endothelial cells.22,23 Activity of CD73 has been shown to enhance endothelial monolayer barrier function through altered intracellular cyclic AMP levels, cell surface adhesion molecule expression, and actin cytoskeletal reorganization.16
A second heterologous DENV infection remains the most significant relative risk factor for the development of DHF.24 However, the vascular leakage syndrome develops in only a few persons with secondary DENV infections. We postulate that host factors that promote pro-inflammatory or pro-angiogenic cytokine production by cross-reactive adaptive immune responses and inhibit type I IFN signaling and endothelial CD73 activity would favor DHF development. Dengue virus infection of endothelial cells in organ-specific vascular beds increases with time. Dengue virus infection and several non-structural proteins are potent inhibitors of IFNAR signaling.19,20 This IFNAR signaling inhibition could lead to lower CD73 expression and activity in specific vascular beds, and promote prolonged hyperpermeability in response to pro-inflammatory or pro-angiogenic adaptive immune cytokines.
We used low-passaged primary HUVECs to develop a mechanistic model of vascular leakage in DHF.25 The vascular endothelium within and among different organs is heterogeneous.26,27 However, the organ-specific endothelial tropism of DENVs has not been well studied. The relatively undifferentiated HUVECs formed tight inter-endothelial junctions. However, inter-endothelial junction regulation may differ among heterogeneous organ-specific endothelia and is a potential limitation of our study. We also established a dominant role for type I IFN-dependent CD73 expression in the DENV2-induced regulation of endothelial barrier function. Our findings do not exclude other mechanisms participating in DENV modulation of endothelial barrier function.
In conclusion, our findings suggest that during DENV infections endothelial barrier homeostasis is maintained by a dynamic balance between pro-inflammatory/pro-angiogenic cytokines, and type I IFN-dependent CD73 expression and activity. Additional studies of this model using tissue-specific endothelial cells isolated from pleura and peritoneum are planned.
ACKNOWLEDGMENTS
We thank Jurand Janus for technical assistance and Jennifer Wang and Rachel Madera for suggestions and assistance with the experiments.
Footnotes
Financial support: This study was supported by a grant from National Institutes of Allergy and Infectious Diseases/National Institutes of Health (U19 AI057319).
Authors' addresses: Chinmay Patkar, Kris Giaya, and Daniel H. Libraty, Division of Infectious Disease and Immunology, Department of Medicine, University of Massachusetts Medical School, Worcester, MA, E-mails: chinmay.patkar@umassmed.edu, krisanthi.giaya@umassmed.edu, and daniel.libraty@umassmed.edu.
References
- 1.Initiative DV. Disease Burden. 2012. http://www.denguevaccines.org/disease-burden Available at. Accessed May 7, 2012.
- 2.Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewi BE, Takasaki T, Kurane I. In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J Virol Methods. 2004;121:171–180. doi: 10.1016/j.jviromet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Talavera D, Castillo AM, Dominguez MC, Gutierrez AE, Meza I. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J Gen Virol. 2004;85:1801–1813. doi: 10.1099/vir.0.19652-0. [DOI] [PubMed] [Google Scholar]
- 5.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 6.Gubler DJ, Zaki SR. Dengue and other viral hemorrhagic fevers. In: Nelson AM, Horsburgh CR Jr, editors. Pathology of Emerging Infections. Washington, DC: American Society for Microbiology Press; 1998. pp. 2pp. 43–67. [Google Scholar]
- 7.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 8.World Health Oganization . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 1997. [Google Scholar]
- 9.Rothman AL, Ennis FA. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 10.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 11.Shasby DM, Roberts RL. Transendothelial transfer of macromolecules in vitro. Fed Proc. 1987;46:2506–2510. [PubMed] [Google Scholar]
- 12.Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luplertlop N, Misse D, Bray D, Deleuze V, Gonzalez JP, Leardkamolkarn V, Yssel H, Veas F. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006;7:1176–1181. doi: 10.1038/sj.embor.7400814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol. 2001;75:3501–3508. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 16.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol. 2000;165:5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Woda M, Ennis FA, Libraty DH. Dengue virus infection differentially regulates endothelial barrier function over time through type I interferon effects. J Infect Dis. 2009;200:191–201. doi: 10.1086/599795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Madoz JR, Belicha-Villanueva A, Bernal-Rubio D, Ashour J, Ayllon J, Fernandez-Sesma A. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. J Virol. 2010;84:9760–9774. doi: 10.1128/JVI.01051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol. 2007;81:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JP, Liu P, Latz E, Golenbock DT, Finberg RW, Libraty DH. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006;177:7114–7121. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- 23.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 24.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 25.Nooteboom A, Hendriks T, Otteholler I, van der Linden CJ. Permeability characteristics of human endothelial monolayers seeded on different extracellular matrix proteins. Mediators Inflamm. 2000;9:235–241. doi: 10.1080/09629350020025755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- 27.Renkonen J, Tynninen O, Hayry P, Paavonen T, Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]