Abstract
From July to September, 2009, an outbreak of eastern equine encephalitis virus (EEEv) occurred in five counties in Maine. The virus was isolated from 15 horses, 1 llama, and pheasants in three separate captive flocks. One wild turkey, screened before translocation, also showed exposure to the virus in January 2010. Two pools of Culiseta melanura (Coquillett) tested positive for EEEv during routine seasonal surveillance in York County in September, but none of the mosquitoes collected during rapid response surveys tested positive. There were more Cs. melanura in July, August, and September 2009 than in preceding (2006–08) and subsequent (2010–11) years. August and September Cs. melanura abundances were correlated with July rainfall, and abundance of all species combined was correlated with total rainfall for the meteorological summer. This outbreak represents a substantial expansion of the range of EEEv activity in northern New England.
Introduction
Eastern equine encephalitis virus (EEEv) is a mosquito-borne Alphavirus that causes disease in domestic livestock, wildlife,1–8 and humans, with case fatality rates as high as 33%.4,9 Birds are the primary reservoirs for the virus, which is transmitted through the bite of infected mosquitoes, though occasional bird-to-bird transmission also occurs. In particular, the enzootic vectors are primarily mosquitoes of the genus Culiseta,10 with accompanying bridge vectors including species in the genera Culex, Aedes, and Coquillettidia.6,11,12 Human cases of the disease, though sporadic, have occurred across much of the Eastern and Midwestern United States in recent years13 with serious morbidity and frequent long-term complications resulting from infection.14–16
The neighboring state of New Hampshire reports sporadic human, avian, and veterinary cases of the disease dating back to the 1970s,13 but EEEv was first detected in southern Maine in 2001 in an American goldfinch (Carduelis tristis) submitted for arbovirus testing as part of surveillance for West Nile virus. Subsequent reports of EEEv in southern Maine are summarized (Table 1); in 2005, two horses and 12 dead birds from York and Cumberland counties submitted to Maine's Health and Environmental Testing Laboratory (HETL) tested positive for the virus.13 A smaller number of dead birds tested positive in 2006. In 2008, EEEv was detected in a horse and in mosquito pools of Culiseta melanura and Culiseta inornata (Willison) from York County. Although other arboviruses have been isolated from Cs. inornata,17–19 this is the first report of EEEv being found in this mammal-biting mosquito. Later that year, a Massachusetts resident was diagnosed with EEEv with time of onset indicating possible exposure in either Maine or New Hampshire while vacationing, although no confirmation of where the virus was acquired was possible.20
Table 1.
Historical eastern equine encephalitis virus (EEEv) activity in Maine, USA, 2001–2008
| Year | Animal | Species | Town | County | Latitude | Longitude |
|---|---|---|---|---|---|---|
| 2005 | Bird | Dark-eye Junco | N. Windham | Cumberland | 43°49″11.33″ | 70°26′01.68″ |
| 2005 | Bird | Common raven | Naples | Cumberland | 43°58′14.37″ | 70°36′40.56″ |
| 2005 | Bird | Dark-eye Junco | Hollis | York | 43°38′08.98″ | 70°35′56.05″ |
| 2005 | Bird | Crow, American | Kennebunk | York | 43°26′17.94″ | 70°35′12.05″ |
| 2005 | Bird | Blue Jay | Limington | York | 43°39′57.65″ | 70°40′35.07″ |
| 2005 | Bird | House Finch | Lyman | York | 43°29′55.72″ | 70°37′55.95″ |
| 2005 | Bird | Dark-eye Junco | N. Berwick | York | 43°20′58.07″ | 70°44′02.10″ |
| 2005 | Bird | Dark-eye Junco | Sanford | York | 43°26′20.88″ | 70°46′27.41″ |
| 2005 | Bird | House Sparrow | Sanford | York | 43°26′37.60″ | 70°46′28.29″ |
| 2005 | Bird | Crow, American | Shapleigh | York | 43°31′57.78″ | 70°52′24.99″ |
| 2005 | Bird | House Finch | Wells | York | 43°19′18.47″ | 70°34′48.44″ |
| 2005 | Bird | Warbler | Wells | York | 43°21′13.53″ | 70°38′46.18″ |
| 2005 | Livestock | Horse | Lebanon | York | 43°22′51.42″ | 70°54′52.78″ |
| 2005 | Livestock | Horse | York | York | 43°09′38.59″ | 70°40′24.44″ |
| 2005 | Mosquito | Culiseta melanura | York | York | 43°09′38.59″ | 70°40′24.44″ |
| 2008 | Livestock | Horse | Lebanon | York | 43°23′40.52″ | 70°51′04.43″ |
| 2008 | Mosquito | Culiseta inornata | Arundel | York | 43°25′48.42″ | 70°30′30.85″ |
An epizootic of EEEv expanded from counties in southern to central Maine from July to October 2009. Maine Department of Agriculture reported 16 potential ungulate livestock deaths (15 horses, 1 llama) caused by EEEv with 13 of the deaths confirmed by serologic testing or examination of the brain tissue. In addition, the Maine Department of Inland Fisheries and Wildlife reported mortality in three ring-necked pheasant Phasianus colchicus L. flocks. By the end of September, EEEv was detected in pools of mosquitoes collected during routine arbovirus surveillance. Finally, a wild turkey (Meleagris gallopavo L.) that was live-captured in January 2010 and translocated within the state was sero-positive for EEEv. No human cases were reported during this period. Interest in mosquito abundance for the season was elevated because of abnormally high rainfall in June and early July.
In this report, we describe the epizootic and consider the possible factors that explain the emergence of EEEv in avian and equine hosts, and vector mosquitoes, in Maine in 2009. The unexpected nature of this outbreak means, however, that much of the data were collected after the appearance of cases. Intensive surveys of vector mosquito populations did not take place until after the epizootic had begun.
Materials and Methods
Livestock.
Reports of equine mortality were submitted to the Maine Department of Agriculture by local veterinarians, with the first case reported from the town of Troy (Waldo County) during the week of August 1. Livestock cases continued to be reported from a substantial area of Maine through the week of October 1, occurring in 5 of 16 counties (Figure 1) (Table 2). Initially, brain tissue and serum samples were submitted to HETL in Augusta, ME (N = 7). Subsequent samples from dead horses were also submitted to the National Veterinary Services Laboratories (NVSL) at Ames, IA (N = 4 horses and 1 llama) and the New Hampshire Public Health Testing Laboratory (NHPL) in Concord, NH (N = 1). Vaccination status for all animals was recorded as part of the investigation.
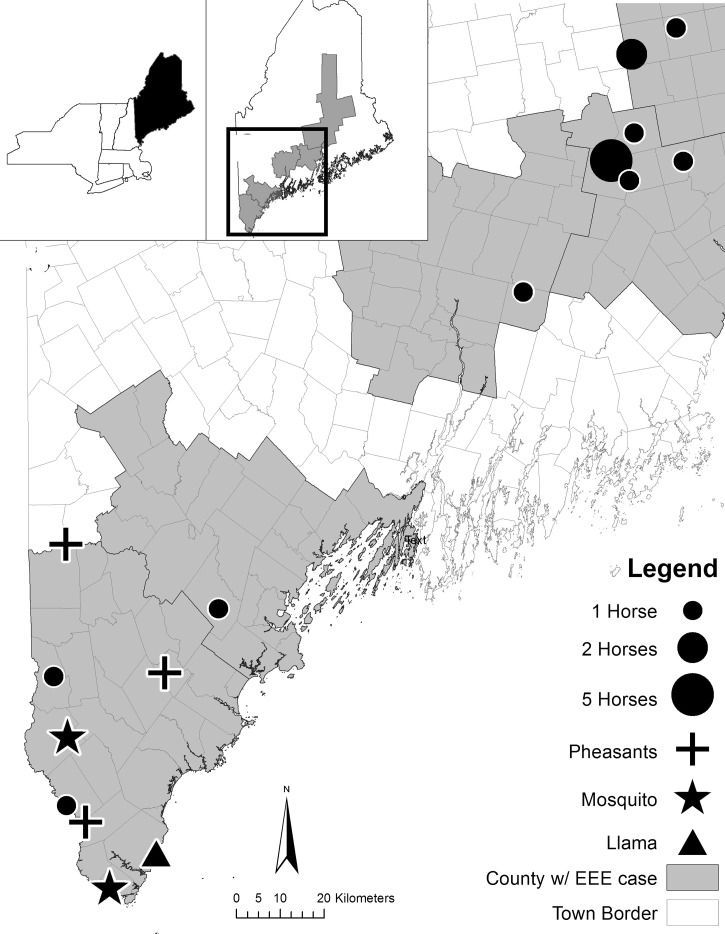
Figure 1.
Eastern equine encephalitis virus (EEEv) activity in Maine, USA in 2009.
Table 2.
Locations of 2009 livestock incidents of eastern equine encephalitis virus (EEEv) in Maine with vaccination status and county of residence
| Animal | County | Onset date | Vaccination |
|---|---|---|---|
| Horse | Waldo | Aug. 3, 2009 | No |
| Horse | Waldo | Aug. 16, 2009 | No |
| Horse | Penobscot | Aug. 23, 2009 | No |
| Horse | York | Aug. 24, 2009 | Yes (incomplete vaccination history) |
| Horse | Cumberland | Aug. 27, 2009 | Yes (incomplete vaccination history) |
| Horse | Waldo | Aug. 27, 2009 | No |
| Horse | Kennebec | Sept. 1, 2009 | No |
| Horse | Waldo | Sept. 4, 2009 | No |
| Horse | Penobscot | Sept. 4, 2009 | Yes (incomplete vaccination history) |
| Horse | York | Sept. 9, 2009 | Yes (incomplete vaccination history) |
| Horse | Waldo | Sept. 14, 2009 | No |
| Horse | York | Oct. 6, 2009 | No |
| Llama | York | Sept. 22, 2009 | N/A |
| Mosquitoes | York | Sept. 3, 2009 | N/A |
| Mosquitoes | York | Sept. 18, 2009 | N/A |
| Pheasants | York | Sept. 18, 2009 | N/A |
| Pheasants | York | Sept. 22, 2009 | N/A |
| Pheasants | York | Oct. 1, 2009 | N/A |
There were two outbreak areas; one was centered on the towns of Berwick (York County [43°24′47.77″N/70°40′12.91″W]), Acton (York County) and Gorham (Cumberland County [43°48′47.87″N/70°23′13.41″W]) in southern Maine. This region is characterized by agricultural land, second growth oak/pine forests, and forested wetlands including red maple (Acer rubrum L.) and Atlantic white cedar (Chamaecyparis thyoides L.) swamps. Elevation ranged from low-lying areas (under 80 m above sea level) to interior foothills (up to 230 m above sea level). Although inland, this area is adjacent to the coastal floodplain, with coastlines only 25 km distant. The second outbreak was centered on the town of Unity (Waldo County [44°30′38.46″N/69°12′28.04″W) and it extended northwards to Stetson (Penobscot County [45°19′23.08″N/69°34′50.42″W]) and southwards to Windsor (Kennebec County [44°26′59.69″N/69°42′13.78″W]). This area is characterized by active agricultural land, with oak/pine or northern hardwood forests as the dominant forest types. Red maple swamps, open water marshes, and lakes are typical in this region of foothills. This region is near to Maine's midcoast area, where the coastal habitats assume a maritime character typified by spruce (Picea spp.).
Vector mosquitoes.
Seasonal surveys.
As part of the state of Maine arbovirus surveillance program, mosquito surveys have been conducted weekly in several communities in southern Maine beginning in 2000, with a concentration in towns with previous arboviral activity. In 2009, towns surveyed from late June through September were Sanford, York, Kittery, Kennebunk, Arundel, Berwick, Lebanon in York County, and Naples in Cumberland County. For all mosquito surveys, investigators used Centers for Disease Control and Prevention (CDC) Miniature Light traps (Model #512, J. W. Hock Co., Gainesville, FL) baited with CO2.
Rapid response surveys.
After the appearance of EEEv in horses, intensive “rapid response” surveys were conducted at and/or near the sites of sick horses, in an effort to find positive mosquitoes, usually within 2 days of notification of positive horses. Towns where rapid response surveys were conducted included Acton (York County), Unity, Troy, and Thorndike (Waldo County), Windsor (Kennebec County), and Gorham and Standish (Cumberland County). As in seasonal surveys, CDC miniature light traps were deployed to collect potentially virus-positive mosquitoes. The number of traps deployed per site varied depending on available habitat (forested wetlands) derived from digital ortho quarto quads and field observations.
All female mosquitoes were cold shocked before identification, identified on a cold surface with a binocular dissecting microscope, and then pooled by site and species. Standard keys were used to identify specimens.19,21,22
Pheasant flocks.
Between the weeks of September 14 and October 12, captive-reared pheasants in three communities in York County (South Berwick, Parsonsfield, and Dayton) (Figure 1) displayed neurologic symptoms characteristic of exposure to EEEv. In total, three flocks were determined to have sick birds. Pheasants were necropsied at the University of Maine Animal Health Laboratory (UMAHL) and a diagnosis of EEEv was confirmed by NVSL. Progression of manifestations in the three flocks varied greatly. Approximately 1 month before the outbreak, a younger flock of captive pheasants had a 13% mortality rate over approximately a week; “gapeworm” (Syngamus tracheae) was diagnosed and the flock responded well to appropriate treatment. Consequently, testing for EEEv was not performed in this case. In the three flocks known to be infected with EEEv, the earliest flock lost ∼ 30% of the birds in the first week, with subsequent losses tapering greatly. In the other two flocks, a gradual loss rate (3–5 birds daily) to virtually no losses (2 of 200 birds reported) appeared to have occurred. Although bird-to-bird transmission of arboviruses is known to occur,23 each flock was housed in a single open-air enclosure limiting the ability to determine this transmission. However, in all cases the birds were seen to be pecking each other violently, with resultant bleeding head wounds at necropsy. Transmission of the virus by this route between birds was certainly possible.
Wild turkeys.
Turkeys are susceptible to infection with EEEv and may become subclinically infected or exhibit mild disease, with low mortality.23,24 Because wild turkeys can become infected but rarely die, do not migrate but inhabit Maine year round, and are associated with farms and human dwellings, U.S. Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS), Wildlife Service's conducted surveillance among turkey flocks along the known frontier where EEEv was discovered in 2009. Turkeys captured by net-gun were sampled from one flock in Kennebec County (N = 10) and from one flock in Penobscot County (N = 7). Blood serum samples were sent to NVSL for testing by plaque reduction neutralization tests (PRNT). Surveillance was planned for other sites, but because of a mild winter, turkeys stopped coming to bait so trapping was halted.
Analysis.
For the seasonal surveys (6/26–9/30/09), we summarized data from Berwick, Kittery, Lebanon, Naples, Sanford, South Berwick, and York. For the rapid response surveys (8/14–9/30/09), we summarized data from Acton, Gorham, Standish, Thorndike, Troy, Unity, and Windsor. The sampling unit was the light trap at each site on each date within a town. By species and survey type, the mean number of mosquitoes per trap night was calculated and compared by non-parametric Wilcoxon rank-sum tests (SAS proc npar1way, 2002–2008 by SAS Institute Inc., Cary, NC). Means were considered significantly different at P ≤ 0.05. The number of pools tested and mosquitoes per pool also was summarized and the number positive for EEEv reported. For a longitudinal analysis using seasonal survey data, species selected were Aedes canadensis, Aedes vexans, Coquillettidia perturbans, Cs. melanura, and Cs. morsitans. Towns with consistent annual data for July through September, 2006–2011, were Lebanon, Sanford, and York. The sampling unit was the light trap at each site on each date within a town. For each species, the mean number of mosquitoes per trap night by month and year was calculated. Mosquito abundance was compared by one-tailed Wilcoxon rank-sum tests for the hypothesis that 2009 > other years for the months of July, August, and September. Monthly precipitation for Portland, Maine, 2006–2011 was obtained from the National Weather Service Forecast Office (http://www.nws.noaa.gov/climate/index.php?wfo=gyx). Spearman rank correlations (ρS) between mosquito abundance in July, August, and September, and rain in the current and previous 3 months were obtained. A post hoc power analysis, with N = 6 (years), α = 0.05, and β (power) = 0.80, determined that ρS ≥ 0.84 and P ≤ 0.05 was required to conclude a significant correlation.25–28
Results
Livestock.
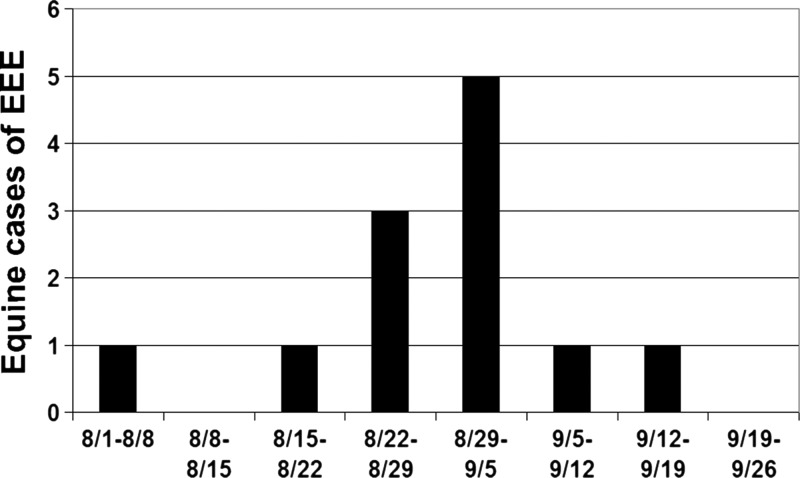
Fifteen horses and one llama were suspected of dying from infection with EEEv. Of those, 13 were tested and exposure to the virus confirmed by either polymerase chain reaction (PCR) (N = 8) or PRNT (N = 5). Two additional horses died at a property in Waldo County where one confirmed horse was found, but both were destroyed before samples could be collected from carcasses. These horses are currently listed as suspect EEE cases. One horse tested negative for the virus by PRNT. The sole llama tested by PRNT also tested positive for the virus. Of the 12 animals that tested positive, four had an incomplete vaccination history, although eight were not vaccinated against EEEv. The llama submitted for testing was also not vaccinated against the virus (Table 2). An epidemiological curve (Figure 2) plotted using date of onset show that the highest number of horse incident cases was reported in September, similar to observations in other northern states like Michigan.29
Figure 2.
Epidemiologic curve of onset of equine cases of eastern equine encephalitis (EEE) virus in Maine in 2009.
Seasonal mosquito surveys.
From late June through September, 272 pools of enzootic EEEV vectors (Culiseta spp. and Culex spp.) and bridge vectors (Cq. perturbans, Ae. vexans, Ae. canadensis, Cx. salinarius, and Ae. sollicitans) (5,930 mosquitoes) were submitted for testing (i.e., collections of 5–50 mosquitoes sorted by species and sex). Mosquitoes were screened for EEEv using forward primer 1858 and reverse primer 1926.30 Over 270 trap nights, 13,395 female mosquitoes were collected including 47 species in the genera Anopheles, Coquillettidia, Culiseta, Culex, Aedes, and Uraenotania. Of the 272 pools of mosquitoes submitted for testing, only two pools containing Cs. melanura tested positive for EEEv by reverse transcription-PCR (RT-PCR). Both pools were collected in York County in the towns of York and Lebanon (Figure 1) as part of the routine seasonal surveillance in September and not collected as part of a rapid response survey to arboviral activity in horses or birds. Positive mosquito pools were collected from red maple swamps in each case.
Rapid response mosquito surveys.
Rapid response surveys occurred from August 13 and the final survey performed on September 29. In total, 2,339 mosquitoes were collected from six such surveys (N = 53 trap nights). Species collected were Ae. canadensis (N = 1,333), Cs. melanura (N = 375), Cs. morsitans (N = 34), Cq. pertubans (N = 403), Ae. vexans (N = 193), and Anopheles punctipennis (N = 136). Despite submission of 109 pools of mosquitoes, no specimens collected during rapid response surveys tested positive for the virus by RT-PCR. With the exception of An. punctipennis, the other primary mosquitoes species are all either enzootic or bridge vectors for EEEv and were considered the targets of the rapid response surveys. Rapid response surveys determined that potential vectors of the virus are present at most of the sites of livestock cases even though low numbers of specimens captured prevented adequate seasonal and spatial analysis.
Birds.
Birds from all three pheasant flocks tested positive for EEEv by RT-PCR (N = 2) and PRNT (N = 1). As a result, all three flocks were euthanized by the Maine Department of Inland Fisheries and Wildlife to prevent further spread of the virus. Of the 17 wild turkeys sampled in January 2010, one tested positive for EEEv antibodies by PRNT. The positive turkey was from the flock from Penobscot County (Figure 1). All turkeys captured were released back into the wild the same day, and thus no further information on the status of the positive bird is available.
Longitudinal analysis.
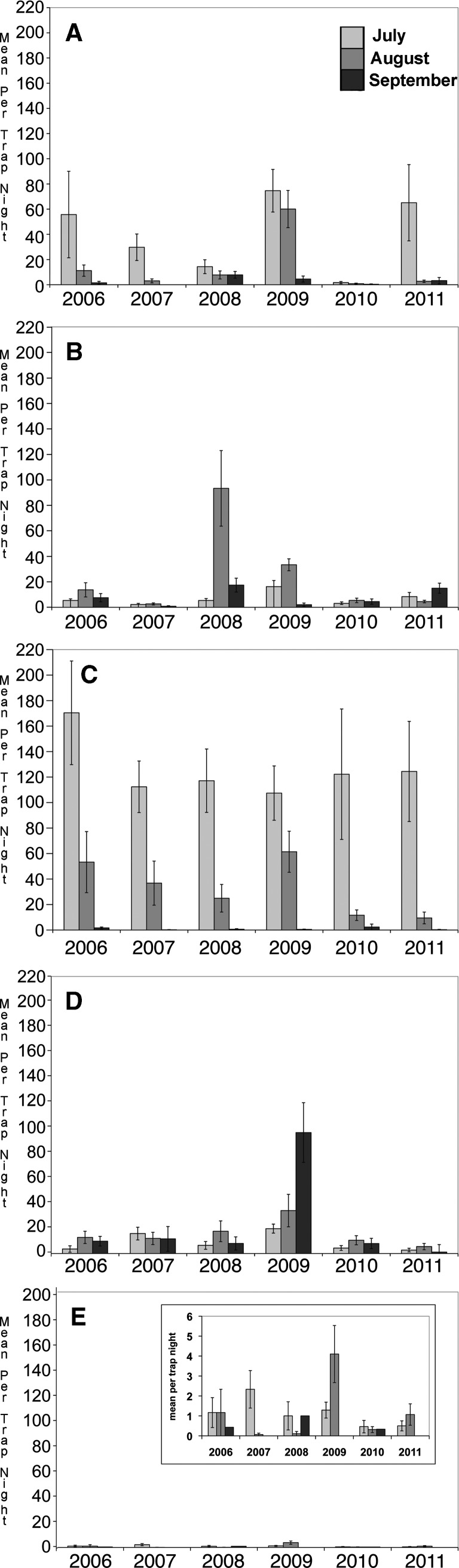
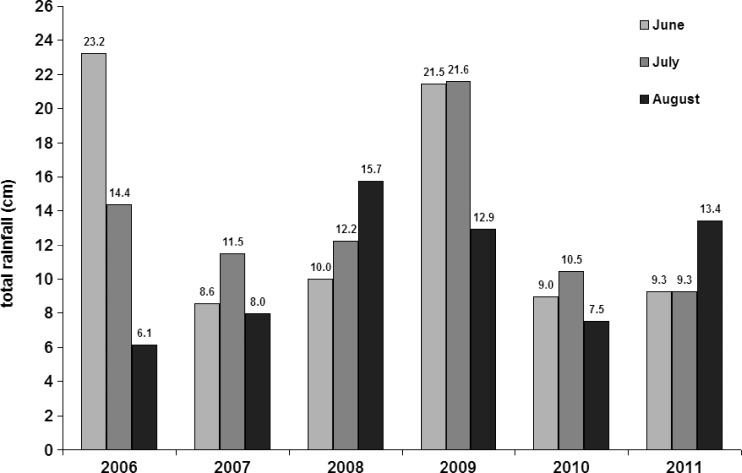
The longitudinal analysis indicated several patterns of note: there were more Cs. melanura in July, August, and September 2009 than in any other year (all P < 0.04, Figure 3 , except July abundance was not greater in 2009 than in 2007 [P = 0.11]). The summer of 2009 was the wettest summer on record in Maine31 in 139 years of recordkeeping, with 56.7 cm of rainfall recorded for the meteorological summer (June through August) and 21.6 cm in July (Figure 4 ) setting the new record for July. Both August and September Cs. melanura abundances were correlated with July rainfall (ρS = 0.95, P = 0.005; ρS = 0.93, P = 0.04, respectively). August Ae. canadensis and Cq. perturbans abundances were correlated with July rainfall (ρS = 0.97, P = 0.005; ρS = 0.87, P = 0.005, respectively). Abundance of all species combined was correlated with total rainfall for the meterological summer (ρS = 0.94, P = 0.005).
Figure 3.
Seasonality of (A) Aedes canadensis, (B) Aedes vexans, (C) Coquillettidia perturbans, (D) Culiseta melanura, and (E) Culiseta morsitans at sites in southern Maine, July–September 2006–2011.
Figure 4.
Rainfall (cm) in Portland, Maine, June–August 2006–2011.
Discussion
This outbreak of EEE developed over a large geographic area in Maine. First identified in Maine in 2001, the virus was previously known only from southwestern regions of the state (Cumberland and York Counties). Despite the abundance of mosquito vectors32,33 and the primary habitat associated with EEEv, forested freshwater wetlands such as red maple or cedar swamps, EEEv activity has historically been rare, and when present, very focal.34 Although the neighboring state of New Hampshire has seen increased EEEv activity in recent years, including several human cases (two of which resulted in death), they have been largely restricted to southern coastal regions.35 Previously, EEEv activity has also been reported from southeastern Canada, in the provinces of Quebec.36 With Maine sandwiched between a regional foci in coastal Massachusetts/New Hampshire and one in Quebec, Canada, it is, therefore, not surprising to find viral activity occurring.
Livestock fatalities occurred over a large area of Maine. Although sampling for vector mosquitoes occurred only after reports of illness were confirmed, habitat at most of the sites of illness were areas near forested wetland or other habitats capable of supporting production of bridge mosquito populations and both epizootic and enzootic transmission. In addition, all of the equine fatalities were either unvaccinated or only partially vaccinated against EEEv and West Nile virus infection (Table 1). In Maine, vaccination of livestock against these two viruses may be performed by livestock owners and is not dependent on veterinarians. Although not a mandated and reportable status, vaccination of these animals is highly advised to prevent such infection, especially in consideration of the fact that an effective and moderately priced combination EEEv/West Nile virus vaccine for horses exists. Even though camelids may be given the vaccine without apparent ill effects,37 to date, there is no evidence of its efficacy in protecting these animals from infection. In keeping with the traditional season for mosquito-borne disease in northern New England, date of onset for livestock cases began August 3 and carried through to October 6 (Table 1, Figure 2), implying that the enzootic cycle amplified in late spring and early summer in avian hosts before spilling over into the mammalian population. Although Maine discontinued dead bird testing for arbovirus surveillance in 2006, data up to that point suggested that bird mortality began in early summer. Despite this, mortality in captive pheasants was not noticed until September. The high number of Cs. melanura found in September 2009 might be the reason for this, as ring-necked pheasants are reported to be highly susceptible to EEEv23 and should be exposed to the same ornithophilic mosquitoes as wild birds. Data from two sites in nearby towns showed that Cs. melanura was abundant late into the season (Table 3). Pheasants may be vaccinated using an equine EEEv vaccine, to protect valuable bloodlines, however the efficacy of the vaccine has been questioned.24 Status of the birds reported here is unknown. The sole EEEv-positive wild turkey was captured in Penobscot county and screened as part of a successful restoration plan for this game species in Maine. The bird was translocated and results were not known until after the cessation of the project but it speaks to the importance of screening for potential pathogens during such a survey. Although the reservoir potential of wild turkeys for EEEv is not known, positive birds have been found among captive flocks.38 Further research into this species' potential role in the cycle of EEEv is warranted.
Table 3.
Eastern equine encephalitis virus (EEEv) enzootic and bridge vector mosquito species captured and tested during seasonal and rapid response surveys, July–September 2009
| Species | Survey | Month | Trap nights | Mosquitoes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total mosquitoes collected | Mean per trap night* | SE | No. mosquito pools tested | Total mosquitoes in pool | EEEv-positive pools | |||||
| Ae. canadensis | Seasonal | July | 69 | 2574 | 37.3 | A | 9.66 | 0 | 0 | |
| August | 105 | 901 | 8.6 | B | 2.77 | 7 | 137 | |||
| September | 96 | 113 | 1.2 | C | 0.39 | 18 | 113 | |||
| Rapid response | August | 21 | 465 | 22.1 | A | 8.97 | 21 | 460 | ||
| September | 31 | 97 | 3.1 | B | 0.98 | 10 | 76 | |||
| Ae. vexans | Seasonal | July | 69 | 326 | 4.7 | A | 1.49 | 0 | 0 | |
| August | 105 | 657 | 6.3 | A | 1.40 | 12 | 315 | |||
| September | 96 | 96 | 1.0 | B | 0.31 | 14 | 96 | |||
| Rapid response | August | 21 | 164 | 7.8 | A | 2.27 | 14 | 160 | ||
| September | 31 | 21 | 0.7 | B | 0.34 | 7 | 19 | |||
| Cq. perturbans | Seasonal | July | 69 | 2632 | 38.1 | A | 7.81 | 0 | 0 | |
| August | 105 | 2439 | 23.2 | B | 3.12 | 40 | 1845 | |||
| September | 96 | 11 | 0.1 | C | 0.05 | 5 | 8 | |||
| Rapid response | August | 21 | 279 | 13.2 | A | 3.78 | 17 | 275 | ||
| September | 31 | 1 | 0.03 | B | 0.03 | 1 | 1 | |||
| Cs. melanura | Seasonal | July | 69 | 1264 | 18.3 | A | 3.25 | 73 | 1212 | |
| August | 105 | 737 | 7.0 | B | 1.40 | 24 | 621 | |||
| September | 96 | 1547 | 15.9 | A | 2.50 | 51 | 1529 | 2 | ||
| Rapid response | August | 21 | 84 | 4.0 | A | 1.22 | 16 | 74 | ||
| September | 31 | 153 | 5.1 | A | 2.64 | 12 | 136 | |||
| Cs. morsitans | Seasonal | July | 69 | 46 | 0.7 | A | 0.19 | 22 | 43 | |
| August | 105 | 45 | 0.4 | AB | 0.18 | 3 | 4 | |||
| September | 96 | 7 | 0.1 | B | 0.05 | 3 | 7 | |||
| Rapid response | August | 21 | 33 | 1.7 | A | 0.76 | 10 | 33 | ||
| September | 31 | 2 | 0.1 | B | 0.06 | 1 | 2 | |||
| Totals | Seasonal | 270 | 13395 | 272 | 5930 | 2 | ||||
| Rapid response | 52 | 1298 | 109 | 1236 | 0 | |||||
| Total | 322 | 14693 | ||||||||
Means with the same letters are not different, non-parametric analysis of variance by species and survey, > 0.05.
Similarly, despite extensive trapping in areas of previous viral activity and recent cases, positive Cs. melanura were not detected until September. Sites for mosquitoes were chosen, in large part, on the abundance of overwintering Cs. melanura larvae crypts. Sites with more productive larval populations of Culiseta were chosen for adult surveys. Since exploratory trapping at sites began during the last week of June, and continued through September, there was an expectation that positive mosquitoes, if present, would have been detected earlier in the season.
Although the collection of EEEv-positive Cs. inornata in 2008 can be considered historical data for this report, this is the first record of this species occurring in Maine and testing positive for the virus. Even though several other species in the genera Culiseta have been reported in the state,32 Cs. inornata is collected locally, in a discreet location, in York County, but is not widely found. Collection sites where it is found are typified by fresh water, forested wetlands containing alders (Alnus rugosa), winterberry holly (Ilex verticillata), and red maple.
There are several possible reasons for the upsurge in EEEv activity in 2009, one being proximity of vector and bridge species to outbreak sites, and the other being the record-high rainfall. Mosquito surveys revealed that at certain sites, the abundance of vector species were quite high in 2009 (Figure 3). Culiseta melanura, Ae. Canadensis, and Cq. perturbans, common mosquito species associated with freshwater wetlands, are ubiquitous during light trap surveys (Figure 3). Aedes canadensis inhabits a variety of wetland habitats, including forested wetlands, whereas Cq. perturbans is commonly associated with cattail (Typha latifolia). Both are efficient bridge vectors for EEEv.39 Recent work by Molaei and others40 examining blood meal analysis of mosquitoes, showed that herbivores form an important component of both species' diets. Although there is a clear link between EEEv cycling and abundance of Cs. melanura, the known bridge vector Ae. canadensis was found consistently at all survey sites, including those sampled during rapid responses (Table 2). The lack of positives from any species except the pools of Cs. melanura collected in September indicate that more extensive and rigorous surveys need to be conducted, employing techniques that will target gravid female enzootic and bridge vectors (i.e., resting boxes). What role, if any, Ae. canadensis played during the outbreak cannot be determined with the small number of specimens collected during the surveys.
In addition to the high precipitation in the fall of 2008 (Figure 3), record-high precipitation in summer 2009 and particularly in July may have created conditions favorable for the EEEv epizootic; rainfall in excess of 20 cm above a 25-year average has been associated with EEE outbreaks in neighboring Massachusetts.16 Summer 2009 rainfall was a 29.1 cm departure from the 30-year average in Portland, Maine.31 By June and July of 2009, 43.1 cm of rain had accumulated when late-season mosquitoes typically emerge, and the correlation analysis suggested a link between July precipitation and the notably high abundance of Cs. melanura. Although speculative, a link between the occurrence of EEEv and precipitation was previously shown.16,41
Although this outbreak of EEEv activity occurred over a large area of Maine, it is uncertain whether such activity will persist; however, increased surveillance for the virus and potential vectors is warranted, especially in emergent areas of the state such as Waldo, Kennebec, and Penobscot counties. Two communities that showed viral activity in 2009 have a recent history of the disease (York and Lebanon), although it is unknown, at this time, whether areas within these two towns can constitute true established foci. Certainly, if constantly present, the virus is active at low levels. Future surveillance activities, however, will concentrate on these areas and will provide data for the development of a more comprehensive surveillance program statewide.
ACKNOWLEDGMENTS
We thank Peter Rand and Eleanor Lacombe of the Maine Medical Center Research Institute, the Maine Department of Inland Fisheries and Wildlife, the Maine Health and Environmental Testing Laboratory, the Maine Department of Forestry, the Maine Vector-borne Working Group for input during the outbreak. Further thanks go to the Maine Organic Farmers and Growers Association, the U.S. Fish and Wildlife Service, various municipal agencies and landowners in York, Cumberland, Waldo, Kennebec, and Penobscot Counties, for access to land for field surveys. Katherine Hayes and Charlene Donahue assisted with field collections. Linda Siddons assisted with data entry.
Footnotes
Financial support: Funding for this project was supplied through the Maine Centers for Disease Control and the Maine Medical Center Research Institute.
Authors' addresses: Charles Lubelczyk, Susan P. Elias, Leticia B. Smith, and Robert P. Smith Jr., Maine Medical Center Research Institute, Vector-borne Disease Laboratory, South Portland, ME, E-mails: lubelc@mmc.org, eliass@mmc.org, leticia.smith@maine.edu, and smithr@mmc.org. John-Paul Mutebi, Centers for Disease Control, Division of Vector-borne Infectious Diseases, Fort Collins, CO, E-mails: jmutebi@cdc.gov and grv0@cdc.gov. Sara Robinson and Stephen Sears, Maine Centers for Disease Control, Infectious Diseases, Augusta, ME, E-mails: sara.robinson@maine.gov and stephen.sears@maine.gov. Sherrie A. Juris, Atlantic Pest Solutions – Surveillance, Arundel, ME, E-mail: sjuris@atlanticpestsolutions.net. Kimberly Foss, SWAMP Inc. – Surveillance, Kittery, ME, E-mail: kfoss81@roadrunner.com. Anne Lichtenwalner, University of Maine Cooperative Extension, Department of Animal and Veterinary Sciences, Orono, ME, E-mail: anne.lichtenwalner@maine.edu. Kirk J. Shively, United States Department of Agriculture, APHIS Wildlife Services, Augusta, ME, E-mail: Kirk.J.Shively@aphis.usda.gov. Donald E. Hoenig, Maine Department of Agriculture, Animal Health, Augusta, ME, E-mail: Donald.E.Hoenig@maine.gov. Lori Webber, Maine Health and Environmental Testing Laboratory – Virology, Augusta, ME, E-mail: lori.webber@maine.gov.
References
- 1.Giltner LT, Shahan MS. The 1933 outbreak of infectious equine encephalomyelitis in the eastern states. North Am Vet. 1933;14:25. [Google Scholar]
- 2.TenBroeck C, Merrill MH. A serological difference between Eastern and Western equine encephalomyelitis virus. Proc Soc Exp Biol Med. 1933;31:217–220. [Google Scholar]
- 3.Tyzzer EE, Sellards AW, Bennett BL. The occurrence in nature of “equine encephalomyelitis” in the ring-necked pheasant. Science. 1938;88:505–506. doi: 10.1126/science.88.2291.505. [DOI] [PubMed] [Google Scholar]
- 4.Fothergill LD, Dingle JH, Fellow JJ. A fatal disease of pigeons caused by the virus of the eastern variety of equine encephalomyelitis. Science. 1938;88:549–550. doi: 10.1126/science.88.2293.549-a. [DOI] [PubMed] [Google Scholar]
- 5.Howitt BF, Dodge HR, Bishop LK, Gorrie RH. Recovery of the virus of Eastern equine encephalomyelitis from mosquitoes (Mansonia perturbans) collected in Georgia. Science. 1949;110:141–142. doi: 10.1126/science.110.2849.141. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain RW, Rubin H, Kissling RE, Eidson ME. Recovery of virus of Eastern equine encephalomyelitis from a mosquito, Culiseta melanura (Coquillett) Proc Soc Exp Biol Med. 1951;77:396–397. doi: 10.3181/00379727-77-18790. [DOI] [PubMed] [Google Scholar]
- 7.Hayes RO, Daniels JB, Anderson KS, Parsons MA, Maxfield HK, Lamotte LC. Detection of eastern encephalitis virus and antibody in wild and domestic birds in Massachusetts. Am J Hyg. 1962;75:183–189. doi: 10.1093/oxfordjournals.aje.a120242. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt SM, Cooley TM, Fitzgerald SD, Bolin SR, Lim A, Schaefer SM, Kiupel M, Maes RK, Hogle SA, O'Brien DJ. An outbreak of Eastern equine encephalitis virus in free-ranging white-tailed deer in Michigan. J Wildl Dis. 2007;43:635–644. doi: 10.7589/0090-3558-43.4.635. [DOI] [PubMed] [Google Scholar]
- 9.Webster LT, Wright FH. Recovery of Eastern equine encephalomyelitis virus from brain tissue of human cases of encephalitis in Massachusetts. Science. 1938;88:305–306. doi: 10.1126/science.88.2283.305. [DOI] [PubMed] [Google Scholar]
- 10.Howard JJ, Wallis RC. Infection and transmission of eastern equine encephalomyelitis virus with colonized Culiseta melanura (Coquillett) Am J Trop Med Hyg. 1974;23:522–525. doi: 10.4269/ajtmh.1974.23.522. [DOI] [PubMed] [Google Scholar]
- 11.Merrill MH, Lacaillade CW, Jr, TenBroeck C. Mosquito transmission of equine encephalomyelitis. Science. 1934;80:251–252. doi: 10.1126/science.80.2072.251. [DOI] [PubMed] [Google Scholar]
- 12.Davis WA. A study of birds and mosquitoes as hosts for the virus of eastern equine encephalomyelitis. Am J Hyg. 1940;32:45–59. [Google Scholar]
- 13.CDC Chart: Confirmed and Probable Eastern Equine Encephalitis Cases, Human, United States, 1964–2009, by State. 2011. http://www.cdc.gov/ncidod/dvbid/Arbor/pdf/EEE_DOC.pdf Available at. Accessed October 18, 2010.
- 14.Ayres JC, Feemster RF. The sequelae of eastern equine encephalomyelitis. N Engl J Med. 1949;240:960–962. doi: 10.1056/NEJM194906162402403. [DOI] [PubMed] [Google Scholar]
- 15.Morris CD. Eastern equine encephalomyelitis. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 1–20. [Google Scholar]
- 16.Przelomski MM, O'Rourke E, Grady GF, Berardi VP, Markley HG. Eastern equine encephalitis in Massachusetts: a report of 16 cases, 1970–1984. Neurology. 1988;38:736–739. doi: 10.1212/wnl.38.5.736. [DOI] [PubMed] [Google Scholar]
- 17.Hammon WM, Reeves WC. Laboratory transmission of St. Louis encephalitis virus by three genera of mosquitoes. J Exp Med. 1943;78:241–253. doi: 10.1084/jem.78.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammon WM, Reeves WC. Laboratory transmission of western equine encephalomyelitis virus by mosquitoes of the genera Culex and Culiseta. J Exp Med. 1943;78:425–434. doi: 10.1084/jem.78.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Means RG. Mosquitoes of New York. Bulletin No. 430b. Albany, NY: New York State Museum; 1987. [Google Scholar]
- 20.MDPH . Arbovirus Surveillance Summary. Boston, MA: Massachusetts Department of Public Health; 2008. [Google Scholar]
- 21.Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 22.Andreadis TG, Thomas MC, Shepard JJ. Identification Guide to the Mosquitoes of Connecticut. New Haven, CT: CAES; 2005. [Google Scholar]
- 23.Hansen W, Docherty DE. Eastern equine encephalomyelitis. In: Friend M, Franson JE, editors. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. U.S. Department of Interior, U.S. Geological Survey; 1999. pp. 171–174. Biological Resources Division, Information and Technology Report 1999–001. [Google Scholar]
- 24.Helm JD. EEE and Emus and Pheasants. Columbia, SC: Clemson University Livestock and Poultry Health Programs; 2003. Animal Health Bulletin. [Google Scholar]
- 25.Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. Third edition. New York: W.H. Freeman; 1995. [Google Scholar]
- 26.Zar JH. Biostatistical Analysis. Fourth edition. Upper Saddle River, NJ; Prentice Hall: 1999. [Google Scholar]
- 27.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 28.McDonald JH. Handbook of Biological Statistics. Second edition. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- 29.McLean RG, Frier G, Parham GL, Francy DB, Monath TP, Campos EG, Therrien A, Kerschner J, Calisher CH. Investigations of the vertebrate hosts of eastern equine encephalitis during an epizootic in Michigan, 1980. Am J Trop Med Hyg. 1985;34:1190–1202. doi: 10.4269/ajtmh.1985.34.1190. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AJ, Martin DA, Lanciotti RS. Detection of North American Eastern and Western equine encephalitis viruses by nucleic acid amplification assays. J Clin Microbiol. 2003;41:379–385. doi: 10.1128/JCM.41.1.379-385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NOAA (National Oceanic and Atmospheric Administration) 2010. http://www.weather.gov/climate/getclimate.php?wfo=gyx Available at. Accessed December 10, 2010.
- 32.Holman MS, Darsi RF, Foss KA. A checklist of the mosquitoes of Maine with new state records. J Am Mosq Control Assoc. 2006;22:327–329. doi: 10.2987/8756-971X(2006)22[327:ACOTMO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Grady GF, Maxfield HK, Hildreth SW, Timperi RJ, Jr, Gilfillan RF, Rosenau BJ, Francy DB, Calisher CH, Marcus LC, Madoff MA. Eastern equine encephalitis in Massachusetts, 1957–1976. A prospective study centered upon analyses of mosquitoes. Am J Epidemiol. 1978;107:170–178. doi: 10.1093/oxfordjournals.aje.a112519. [DOI] [PubMed] [Google Scholar]
- 34.Nasci RS, Berry RL, Restifo RA, Parsons MA, Smith GC, Martin DA. Eastern equine encephalitis virus in Ohio during 1991. J Med Ent. 1993;30:217–222. doi: 10.1093/jmedent/30.1.217. [DOI] [PubMed] [Google Scholar]
- 35.CDC Eastern equine encephalitis–New Hampshire and Massachusetts, August–September 2005. MMWR. 2006;55:697–700. [PubMed] [Google Scholar]
- 36.Chenier S, Cote G, Vanderstock J, Macieira S, Laperle A, Helie P. An eastern equine encephalomyelitis (EEE) outbreak in Quebec in the fall of 2008. Can Vet J. 2010;51:1011–1015. [PMC free article] [PubMed] [Google Scholar]
- 37.Bedenice D, Bright A, Pederson DD, Dibb J. Humoral response to an equine encephalitis vaccine in healthy alpacas. JAVMA. 2009;234:530–534. doi: 10.2460/javma.234.4.530. [DOI] [PubMed] [Google Scholar]
- 38.Guy JS, Siopes TD, Barnes HJ, Smith LG, Emory WH. Experimental transmission of eastern equine encephalitis virus and Highlands J virus via semen of infected tom turkeys. Avian Dis. 1995;39:337–342. [PubMed] [Google Scholar]
- 39.Moore CG, McClean RG, Mitchell CJ, Nasci RS, Tsai TF, Calisher CH, Marfin AA, Moore PS, Gubler DJ. Guidelines for Arbovirus Surveillance Programs in the United States. Fort Collins, CO: Centers for Disease Control and Prevention; 1993. [Google Scholar]
- 40.Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of blood meals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol. 2008;45:1143–1151. doi: 10.1603/0022-2585(2008)45[1143:hpopmv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Ross WA, Kaneene JB. Evaluation of outbreaks of disease attributable to eastern equine encephalitis virus in horses. JAVMA. 1996;208:1988–1997. [PubMed] [Google Scholar]