Abstract
Hepatitis E virus (HEV) is an important human pathogen. In addition to humans, HEV has also been identified in pig, chicken, mongoose, deer, rat, rabbit and fish. There are four recognized and two putative genotypes of mammalian HEV. Genotypes 1 and 2 are restricted to humans, while genotypes 3 and 4 are zoonotic. The recently identified rabbit HEV is a distant member of genotype 3. Here, we first expressed and purified the recombinant capsid protein of rabbit HEV and showed that the capsid protein of rabbit HEV cross-reacted with antibodies raised against avian, rat, swine and human HEV. Conversely, we showed that antibodies against rabbit HEV cross-reacted with capsid proteins derived from chicken, rat, swine and human HEV. Since pigs are the natural host of genotype 3 HEV, we then determined if rabbit HEV infects pigs. Twenty pigs were divided into five groups of four each and intravenously inoculated with PBS, US rabbit HEV, Chinese rabbit HEV, US rat HEV and swine HEV, respectively. Results showed that only half of the pigs inoculated with rabbit HEV had low levels of viraemia and faecal virus shedding, indicative of active but not robust HEV infection. Infection of pigs by rabbit HEV was further verified by transmission of the virus recovered from pig faeces to naïve rabbits. Pigs inoculated with rat HEV showed no evidence of infection. Preliminary results suggest that rabbit HEV is antigenically related to other HEV strains and infects pigs and that rat HEV failed to infect pigs.
Introduction
Hepatitis E virus (HEV), the causative agent of hepatitis E, is an important human pathogen (Emerson & Purcell, 2007). Sporadic cases of acute hepatitis E have also been reported in many industrialized countries including the United States (Meng, 2010b; Yazaki et al., 2003). HEV is transmitted primarily by the faecal–oral route and causes self-limiting acute hepatitis with a high morbidity in young adults (Emerson & Purcell, 2003, 2007; Ma et al., 2010; Meng, 2010a, b). At least four major genotypes of HEV have been recognized thus far (Ahmad et al., 2011; Meng, 2011; Meng et al., 2012; Purdy & Khudyakov, 2010): genotypes 1 and 2 are restricted to humans, whereas genotypes 3 and 4 have an expanded host range and are zoonotic (Arankalle et al., 2006; Bouquet et al., 2011; Hakze-van der Honing et al., 2011; Meng, 2010a; 2011; Okamoto, 2007). Recently, two potential new genotypes of HEV were identified from rats in Germany and the USA (Johne et al., 2010; Purcell et al., 2011) and from wild boars in Japan (Sato et al., 2011; Takahashi et al., 2011). Avian HEV from chickens is likely to represent a new genus within the family Hepeviridae (Bilic et al., 2009; Meng, 2010a, b). The strain of HEV recently identified from cutthroat trout appears to belong to a new genus as well (Batts et al., 2011).
HEV is a small, non-enveloped virus with a positive-sense RNA genome of approximately 7.2 kb (Aggarwal & Jameel, 2011; Okamoto, 2007). The virus contains three ORFs: ORF1 encodes non-structural proteins, ORF2 encodes the viral capsid protein and ORF3 encodes a cytoskeleton-associated phosphoprotein with multiple functions (Huang et al., 2007; Meng, 2010a; Surjit et al., 2004; Wang et al., 2000; Zafrullah et al., 1997). The recent availability of cell culture systems for HEV will aid future studies of HEV biology (Okamoto, 2011a, b). The first isolation of a non-human animal strain of HEV was from a pig in the USA in 1997, designated swine HEV (Meng et al., 1997), and since then swine HEV has been genetically identified from pigs worldwide (Meng, 2011). Thus far, all strains of HEV identified from pigs belong to either genotypes 3 or 4, although a putative new genotype was recently identified from a wild boar (Takahashi et al., 2011). Genotypes 3 and 4 strains of HEV can infect across species barriers and are zoonotic (Meng, 2010a; 2011; Pavio et al., 2010; Shukla et al., 2011). In addition to pigs and humans, genetically divergent strains of HEV have also been isolated from several other animal species including chicken, rat, mongoose, horse, deer and rabbit (Haqshenas et al., 2001; Johne et al., 2010; Ma et al., 2010; Meng et al., 1998b; Nakamura et al., 2006; Saad et al., 2007; Sonoda et al., 2004; Takahashi et al., 2004; Zhao et al., 2009).
The novel rabbit strain of HEV identified from farmed rabbits in China is distantly related to the strains from genotype 3 (Geng et al., 2011; Zhao et al., 2009). More recently, we genetically identified a novel strain of HEV from rabbits in the USA (Cossaboom et al., 2011) that is also distantly related to strains from genotype 3. We showed that infection of rabbits by genotype 3 HEV is potentially widespread in the USA (Cossaboom et al., 2011). It appears that the rabbit strains of HEV from both China and the USA belong to genotype 3 (Cossaboom et al., 2011; Purdy & Khudyakov, 2010).
A genetically distinct strain of HEV, designated rat HEV, was identified from Norway rats in Germany and shares approximately 60 and 50 % nucleotide sequence identity with human and avian strains of HEV, respectively (Johne et al., 2010). More recently, a rat HEV similar to that from Germany was identified from urban rats in Los Angeles, California (Purcell et al., 2011). Transmission of the US rat HEV to naïve laboratory rats was successful, although the transmission was spotty and did not result in a robust infection. An attempt to transmit the US rat HEV to rhesus monkeys was unsuccessful, suggesting that rat HEV is probably not a source of HEV infection in humans (Purcell et al., 2011).
Since pigs are the natural host of genotype 3 HEV, it is possible that, like swine HEV (Meng et al., 1998b), the rabbit strain of HEV may also have the ability to infect across species. Therefore, the objectives of this study were to determine whether the rabbit and rat strains of HEV can cross species barriers and infect pigs, and whether there is an antigenic cross-reactivity in the capsid protein between the rabbit strain of HEV and other known animal strains of HEV.
Results
Generation of infectious stocks of the Chinese and US strains of rabbit HEV
The two rabbits (#560 and #562) inoculated with the PCR-positive serum sample containing a Chinese strain of rabbit HEV became infected. Faecal shedding of the virus was detected at 10 days post-infection (p.i.). At 22 days p.i., rabbit #560, which had shed virus in its faeces for two consecutive weeks (Table 1), was euthanized. An infectious stock of Chinese rabbit HEV (strain RC39) was then prepared as a 10 % suspension (w/v in PBS) of the intestinal content and bile collected during the necropsy of rabbit #560, and this infectious virus stock has a titre of approximately 2×106 genome equivalent (GE) ml−1. The US strain of rabbit HEV (USRab-14) infectious virus stock was prepared as a 10 % suspension of faeces (w/v in PBS) collected from PCR-positive farmed rabbits in Virginia with an approximate titre of 2×105 GE g−1 of faeces. The semi-quantification of virus GE titres was essentially done as described previously (Kasorndorkbua et al., 2002; Tsarev et al., 1993, 1994).
Table 1. Faecal virus shedding in rabbits experimentally inoculated with Chinese rabbit HEV (RC-39) and US rabbit HEV (USRab-14) collected from rabbit and pig faeces.
| Rabbit HEV inocula | Rabbit ID | Positive (+) or negative (−) of HEV RNA detected in faecal samples at indicated weeks p.i.* | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| RC-39 from rabbit serum | 560 | − | − | + | + | X† | |||||
| 562 | − | − | + | + | + | + | + | + | + | X† | |
| USRab-14 from pig faeces | 1 | − | − | + | − | − | + | + | − | − | − |
| 3 | − | − | − | + | + | + | − | − | − | − | |
| USRab-14 from rabbit faeces | 7 | − | + | + | + | + | + | + | + | + | + |
| 8 | − | + | + | + | + | X† | |||||
The faecal samples were collected every other day and the results are considered positive for that indicated week if at least one of the samples collected during the week tested positive.
X, Indicates that the animal was euthanized during that week.
Cross-species infections of specific-pathogen-free (SPF) pigs by US and Chinese strains of rabbit HEV but not by the US strain of rat HEV
Groups of four pigs were each inoculated with the US rabbit HEV, Chinese rabbit HEV, US rat HEV, genotype 3 swine HEV (positive control) and PBS buffer (negative control). Two pigs, one in the negative control group and one in the rat HEV-inoculated group, died prior to the termination of the project from causes unrelated to the infection. There was no detectable faecal virus shedding in the negative control group. In pigs inoculated with the US rabbit HEV, faecal virus shedding was sporadic and began at 5 week p.i. in only 1/4 inoculated pigs, even though transient viraemia was detected in 2/4 pigs. In pigs inoculated with the Chinese rabbit HEV, faecal virus shedding was apparent in 2/4 pigs beginning at 6 weeks p.i. (Table 2). In pigs inoculated with a genotype 3 swine HEV, faecal virus shedding began as early as 1 week p.i., and 4/4 positive control pigs shed virus in faeces, and 3/4 pigs were viraemic starting at 2 weeks p.i. (Table 2). In pigs inoculated with the US rat HEV, there was no detectable faecal virus shedding or viraemia (Table 2). Seroconversion to IgG anti-HEV was detected in pigs inoculated with swine HEV, but not in pigs from any other group using genotype 1 human HEV, rat HEV or rabbit HEV antigens (data not shown).
Table 2. Faecal virus shedding and viraemia in pigs experimentally inoculated with a US strain of rabbit HEV, a Chinese strain of rabbit HEV, a US strain of rat HEV and a genotype 3 swine HEV.
| Virus inocula | Pig ID | Positive (+) or negative (−) of HEV RNA detected in faecal/serum samples at indicated weeks p.i.* | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | L | B | ||
| PBS buffer | 1 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | X† | |||
| 2 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| 3 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| 4 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| US rabbit HEV | 15 | −/− | −/− | −/+ | −/− | −/− | −/+ | −/− | −/+ | −/− | −/− | −/− | − | − |
| 16 | −/− | −/− | −/− | −/− | −/− | +/− | −/− | −/− | −/− | +/+ | −/− | − | − | |
| 17 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| 18 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| Chinese rabbit HEV | 19 | −/− | −/− | −/− | −/− | −/− | −/− | +/− | +/− | +/− | +/− | +/− | + | + |
| 20 | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− | +/− | +/− | +/− | − | + | |
| 21 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| 22 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| US rat HEV | 23 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − |
| 24 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| 25 | −/− | −/− | −/− | −/− | −/− | X† | ||||||||
| 26 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | − | − | |
| Genotype 3 swine HEV | 27 | −/− | −/− | −/− | +/− | +/− | +/− | +/+ | −/− | −/− | −/− | −/− | + | − |
| 28 | −/− | −/− | -/+ | −/− | −/− | −/− | −/− | +/− | +/+ | +/− | +/− | + | − | |
| 29 | −/− | +/− | +/− | +/+ | +/+ | +/− | +/− | −/− | −/− | +/− | +/− | + | − | |
| 30 | −/− | +/− | −/− | +/− | +/− | +/− | +/− | −/− | −/− | +/− | +/− | + | − | |
L, Liver; B, bile collected during necropsies.
X, Pigs #1 and #25 died prior to the termination of the project due to circumstances unrelated to the study.
All liver and bile samples collected during necropsies at 10 weeks p.i. from pigs in the negative control group and in groups inoculated with US rabbit HEV and rat HEV tested negative for HEV RNA. In the group inoculated with Chinese rabbit HEV, two pigs were positive for HEV RNA in the bile, and one was also positive for HEV RNA in the liver. All four pigs in the positive control group were positive for HEV RNA in the liver, but none in the bile (Table 2).
A semi-quantitative nested RT-PCR was performed on the positive faecal samples from pigs that had been inoculated with US rabbit HEV and Chinese rabbit HEV. The approximate GE titre of the positive faecal samples from pigs infected by the US rabbit HEV ranged from 103 to 105 GE g−1 of faeces, while the approximate GE titre of the faecal samples from pigs infected by the Chinese rabbit HEV ranged from 102 to 106 GE g−1 of faeces, indicating a low level of replication of the rabbit HEV in pigs.
The virus recovered from faeces of pigs experimentally infected with rabbit HEV can infect naïve rabbits under laboratory conditions
To further confirm if pigs are indeed infected by rabbit HEV and excrete infectious virus in faeces, two rabbits (ID#1 and #3) were intravenously inoculated with a 10 % suspension (w/v in PBS) of faeces collected from pigs inoculated with the rabbit HEV USRab-14 that tested positive for HEV RNA by RT-PCR. The two inoculated rabbits began to shed virus sporadically in faeces at 12 days p.i. and the faecal virus shedding continued until week 6 p.i. (Table 1). In addition, as a positive control, we also inoculated two other rabbits (ID#7 and #8) with a 10 % suspension of faeces prepared from farmed rabbits in Virginia that tested positive by RT-PCR for rabbit HEV RNA. These two inoculated rabbits began to shed virus in the faeces at 6 days p.i. and continued to be positive until euthanasia (Table 1). One of these two rabbits, ID#8, was euthanized during the acute stage of infection (third week of the study) in order to prepare a higher titre virus stock of USRab-14 for future studies.
Rabbits ID#7 and #8 also seroconverted to IgG anti-HEV (Fig. 1), although rabbit #8 was euthanized at week 3 p.i. However, the two other rabbits (ID#1 and #3) had no detectable seroconversion (Fig. 1). The bile, intestinal content and liver collected during necropsies from rabbits #7 and #8 tested positive for rabbit HEV RNA. Similarly, the liver and intestinal content from rabbit #1, but not #3, also tested positive for rabbit HEV RNA.
Fig. 1.
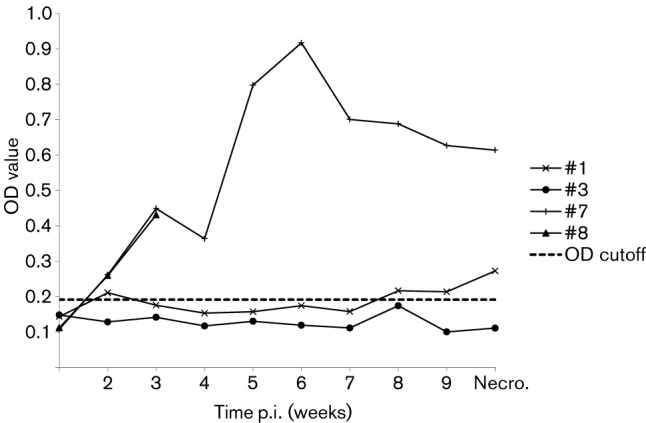
Seroconversion to IgG anti-HEV in rabbits experimentally inoculated with the rabbit HEV (USRab-14 strain) recovered from farmed rabbits in Virginia (ID#7 and #8) and with a 10 % suspension of RT-PCR-positive faeces from a pig experimentally infected with the same strain. The ELISA OD values are plotted over weeks post-infection. The rabbits were necropsied at 10 weeks p.i.
Rabbit HEV is antigenically related to avian, rat, swine and human HEVs
To determine antigenic cross-reactivity, we performed Western blot analyses. The results showed that the rabbit HEV antiserum cross-reacted with truncated recombinant capsid antigens derived from avian, rat, swine and human HEV strains (Fig. 2a). A very strong reactivity was demonstrated between the purified truncated capsid protein of rabbit HEV and rabbit HEV antiserum (Fig. 2a). The truncated genotype 1 human HEV capsid protein (GenWay Biotech) and the truncated genotype 3 swine HEV capsid protein also reacted strongly with the rabbit HEV antiserum, whereas the cross-reactivity between the truncated avian HEV (Haqshenas et al., 2002) and rat HEV capsid proteins with the rabbit HEV antiserum was much weaker. The rabbit HEV recombinant capsid protein did not react with pre-immune serum from rabbits (Fig. 2a). The rabbit HEV capsid protein did not react with pre-immune serum from rabbits (Fig. 2a). The purity of the respective HEV antigens was demonstrated in the Coommassie-staining gel (Fig. 2b). The extraneous bands, especially the lower molecular mass bands that appear in some lanes (Fig. 2a), may be due to protein degradation, which is not uncommon in Western blots.
Fig. 2.
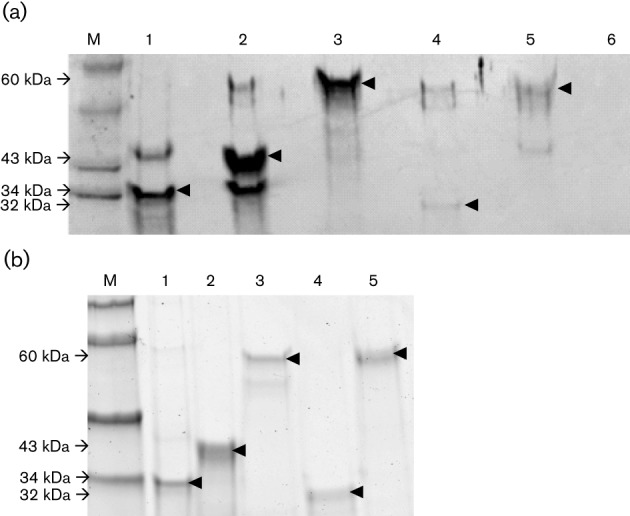
Antigenic cross-reactivity between rabbit HEV antiserum and recombinant capsid antigens from other animal HEV strains by Western blot analysis. (a) Each lane was loaded with an equal amount (1 µg) of truncated recombinant capsid proteins derived from different strains of HEV: (1) US rabbit HEV (34 kDa), (2) genotype 1 human HEV (43 kDa), (3) genotype 3 swine HEV (60 kDa), (4) avian HEV (32 kDa) or (5) rat HEV (60 kDa). The membrane containing each of the recombinant antigens was incubated with a rabbit HEV antiserum. Lanes 6 and M, negative control and molecular marker, respectively. Arrowheads indicate the expected bands of antigen–antibody reaction in the Western blot analysis. (b) Coomassie-staining gel of truncated recombinant capsid proteins derived from different strains of HEV. Each lane was loaded with an equal amount (1 µg) of either (1) US rabbit HEV (34 kDa), (2) genotype 1 human HEV (43 kDa), (3) genotype 3 swine HEV (60 kDa), (4) avian HEV (32 kDa) or (5) rat HEV (60 kDa). Arrowheads indicate the expected sizes of each truncated HEV capsid protein.
Similarly, a truncated purified recombinant capsid protein of rabbit HEV demonstrated some degree of cross-reactivity with the antisera raised against all of the different strains of HEV that were tested in this study (Fig. 3). However, quantification of the degree of antigenic cross-reactivity in this experiment was not possible since the antibody titre of each antiserum was unknown. To estimate the anti-HEV antibody level of these different HEV antisera used in the Western blot analysis, we performed an ELISA on 10-fold serial dilutions of each antiserum. The genotype 3 HEV pig antiserum had the highest level of anti-HEV antibodies compared with the other HEV antisera (data not shown), thus explaining the observed high level of cross-reactivity between the genotype 3 HEV pig antiserum and the rabbit HEV antigen (Fig. 3).
Fig. 3.
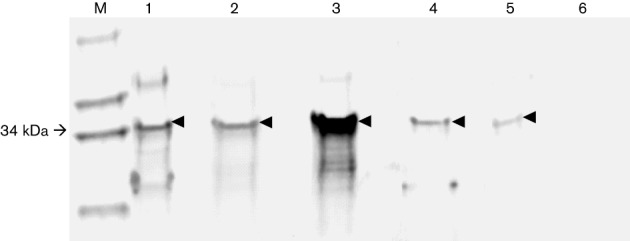
Antigenic cross-reactivity between recombinant rabbit HEV capsid antigen and anti-HEV antisera raised against different animal strains of HEV in a Western blot analysis. Each lane was loaded with an equal amount (1 µg) of the truncated recombinant capsid protein of rabbit HEV (34 kDa). The membrane containing the rabbit HEV capsid antigen was incubated with antisera raised against different strains of HEV: (1) rabbit antiserum against US strain of rabbit HEV, (2) swine hyperimmune antiserum from a pig immunized with the capsid antigen of the genotype 1 human HEV, (3) swine antiserum from a pig experimentally infected with genotype 3 swine HEV, (4) chicken antiserum from a chicken experimentally infected with avian HEV or (5) swine antiserum from a pig immunized with the recombinant rat HEV capsid protein. Lanes 6 and M, negative control and molecular marker, respectively. Arrowheads indicate the expected bands of antigen–antibody reaction in the Western blot analysis.
Discussion
Hepatitis E is a recognized zoonotic disease: pigs and probably other animal species are reservoirs for HEV (Meng, 2010a, b; 2011). Recently, a novel genotype 3 strain of HEV was identified in rabbits, first from China (Zhao et al., 2009) and subsequently in the USA (Cossaboom et al., 2011). More recently, another unique strain of HEV was identified in rats in Germany (Johne et al., 2010) and in the USA (Purcell et al., 2011). Although the rat HEV could not be transmitted to rhesus macaques (Purcell et al., 2011), the biological significance of the rabbit HEV remains unclear. In this study, we aimed to characterize the antigenic relatedness between the rabbit HEV and other known animal strains of HEV and to determine if the rabbit HEV and the US rat HEV have the ability to infect across species.
As expected, the rabbit HEV (strain USRab-14) capsid antigen reacted with the rabbit HEV antiserum as well as with antisera against genotype 1 human HEV and genotype 3 swine HEV, suggesting that the rabbit HEV shares conserved antigenic epitopes with genotypes 1 and 3 HEV. Interestingly, the rabbit HEV capsid antigen also reacted with antisera raised against the distantly related avian HEV and rat HEV, further indicating that there exists only one serotype among mammalian HEV (Emerson & Purcell, 2007). The antigenic cross-reactivity was further verified in a reverse Western blot analysis when the capsid antigens derived from genotypes 1 and 3 HEV were shown to react with rabbit HEV antiserum. Avian and rat HEV antigens also reacted with rabbit HEV antiserum. The results revealed that the rabbit HEV is antigenically related to the known mammalian HEV strains as well as the avian HEV strain.
Since the rabbit HEV is a distant member of genotype 3 (Purdy & Khudyakov, 2010) and since pigs are the natural host of the genotype 3 HEV, we assessed the ability of rabbit HEV to cause cross-species infection in a pig model. We demonstrated that, indeed, both the US strain and the Chinese strain of rabbit HEV were able to infect SPF pigs when inoculated intravenously, as approximately half of the inoculated pigs developed transient viraemia and shed the virus sporadically in faeces. It appears that, when compared to the pigs infected with the genotype 3 swine HEV, the pigs infected with the rabbit HEV have a delayed onset and shorter duration of viraemia and faecal virus shedding. There is no detectable seroconversion in the pigs inoculated with rabbit HEV, even though seroconversion was evident in pigs infected with swine HEV. The lack of seroconversion suggested that infection of pigs by rabbit HEV is not robust, and the replication level of the rabbit HEV in pigs is low since the estimated GE titre of rabbit HEV in the positive-pig faecal samples ranged from 103 to 105 GE g−1 of faeces for the US rabbit HEV group, and 102 to 106 GE g−1 of faeces for the Chinese rabbit HEV group. The transient and spotty nature of viraemia and faecal virus shedding detected in only half of the rabbit HEV-inoculated pigs also indicated an inefficient and low level of rabbit HEV replication in pigs. This is not surprising, considering that this is a cross-species infection and the rabbit HEV will need to adapt to the new host before it can replicate more efficiently. In addition, the relatively low titres of the rabbit HEV stocks used for the pig inoculation (2×105–106 GE ml−1) may also play a role in the observed low level of rabbit HEV replication in pigs since it is well documented that hepatitis E is a dose-dependent disease (Tsarev et al., 1993, 1994). It is possible that the rabbit HEV replication level in pigs is not robust and therefore is insufficient to elicit a detectable level of humoral immune response.
To further verify that the pigs are indeed infected by rabbit HEV and excrete infectious virus in the faeces, we subsequently inoculated two naïve rabbits with a 10 % suspension of PCR-positive pig faeces collected from rabbit HEV-infected pigs. Viraemia and faecal virus shedding were consistently detected in the two inoculated rabbits, indicating that the faeces from the rabbit HEV-infected pig contain infectious virus. Again, seroconversion to rabbit HEV antibody was not detected in the pig faeces-infected rabbits, even though seroconversion was evident in the rabbits experimentally infected with rabbit HEV USRab-14. Nonetheless the successful transmission of rabbit HEV recovered from faeces of inoculated pigs to naïve rabbits further confirmed that rabbit HEV has the ability to infect pigs.
None of the pigs inoculated with a US rat HEV showed any evidence of infection, as there was no viraemia, faecal virus shedding or seroconversion. This is not unexpected as the rat HEV appears to belong to a new genotype (Johne et al., 2010; Purcell et al., 2011). In a recent study, an attempt to infect rhesus monkeys with the rat HEV was also unsuccessful (Purcell et al., 2011). Therefore, the preliminary data from this study suggest that the rat HEV identified from the USA and Germany has a limited host range and is probably not zoonotic; however, future studies are warranted to confirm this.
In conclusion, the results from this study demonstrated that the newly identified rabbit HEV is antigenically related to the other known animal strains of HEV, and that, like other genotype 3 HEV strains, the rabbit HEV also has the ability to infect across the species barrier. This raises potential concern of zoonotic transmission of the rabbit strain of HEV to humans through direct contact with infected rabbits or through the consumption of undercooked rabbit meat. Future studies are warranted to determine the zoonotic potential of the rabbit HEV.
Methods
Sources of viruses.
The USA strain of rabbit HEV (USRab-14) was a 10 % faecal suspension in PBS buffer (w/v) prepared from faeces of farmed rabbits in Virginia that tested positive by RT-PCR for HEV RNA (Cossaboom et al., 2011). The Chinese strain of rabbit HEV was from a serum sample of a rabbit that was experimentally infected with the RC-39 strain of rabbit HEV isolated in China (Ma et al., 2010; Zhao et al., 2009). The US rat strain of HEV was a homogenate of liver from a laboratory rat experimentally infected with the US rat HEV with an infectious titre of 105.9 per 0.5 ml (Purcell et al., 2011). The genotype 3 swine HEV used as the positive control was from an experimentally infected pig with a titre of 104.5 50 % pig infectious dose (PID50) (Feagins et al., 2008; Halbur et al., 2001; Meng et al., 1998b; Sanford et al., 2011).
Animals.
Twenty, 6-week-old, cross-bred SPF pigs were obtained from the Virginia-Maryland Regional College of Veterinary Medicine’s Swine Breeding Facility for the cross-species transmission study. Prior to inoculation, all pigs were confirmed negative for IgG anti-HEV by an ELISA. Six, 8-week-old, New Zealand white rabbits were obtained from Harlan Laboratories for the rabbit infection study. Prior to inoculation, each rabbit was confirmed negative for IgG anti-HEV by an ELISA.
Generation of an infectious stock of the Chinese rabbit strain of HEV.
In order to generate an infectious virus stock of the Chinese rabbit HEV, a serum sample shown to be positive by RT-PCR for a Chinese rabbit HEV (RC-39) was used to inoculate two naïve rabbits (#560 and #562). Briefly, the rabbits were intravenously inoculated with 0.5 ml of an RT-PCR-positive serum. Faeces were collected every other day, and blood was collected once a week from each rabbit. Rabbit #560 was necropsied during the acute stage of infection at 22 days p.i. Intestinal content and bile collected during the necropsy were prepared in a 10 % suspension (w/v in PBS) and used as an infectious stock of the Chinese rabbit HEV. Rabbit #562 was kept until 56 days p.i. to determine seroconversion over time.
Experimental inoculation of pigs with rabbit and rat strains of HEV.
Twenty SPF pigs were divided into five groups of four pigs per group. Each group of pigs was housed separately in a BSL-2 facility and was inoculated intravenously with either PBS (negative control), US rabbit HEV, Chinese rabbit HEV, a US strain of rat HEV and a genotype 3 swine HEV (positive control), respectively. Serum and faecal samples were collected weekly from each pig at 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70 days p.i. Serum samples were tested for the respective viral RNA (viraemia) by RT-PCR (Cooper et al., 2005; Córdoba et al., 2011; Huang et al., 2002) and for IgG anti-HEV by ELISA as described previously (Meng et al., 1997). Faecal samples (10 % faecal suspension in PBS) were also tested for the presence of the respective HEV RNA by RT-PCR. All pigs were necropsied at 10 weeks p.i. At necropsy, samples of serum, intestinal content, bile and liver tissue were collected and stored at −80 °C until use.
Experimental inoculation of rabbits with rabbit HEV recovered from experimentally infected pigs and from farmed rabbits.
To further confirm that pigs experimentally inoculated with the rabbit HEV are indeed infected, we inoculated rabbits with a suspension of an RT-PCR-positive faeces collected from a pig experimentally inoculated with the rabbit HEV. Rabbits were housed in separate cages. Two rabbits (ID #1 and #3) were each inoculated with a 10 % suspension of an RT-PCR-positive faeces collected from pigs experimentally inoculated with the US rabbit strain of HEV (USRab-14). Two other rabbits (ID #7 and #8) were each inoculated with a 10 % faecal suspension containing the USRab-14 prepared from faeces directly collected from farmed rabbits in Virginia (Cossaboom et al., 2011). Faecal samples were collected every other day from each rabbit following inoculation. Serum samples were collected at 0, 14, 21, 28, 35, 42, 49, 56, 63 days p.i. Serum samples were tested for rabbit HEV RNA by RT-PCR essentially as described previously (Cooper et al., 2005; Córdoba et al., 2011; Huang et al., 2002) and for anti-HEV IgG by ELISA as described previously (Meng et al., 1997). Faecal samples (10 % faecal suspension in PBS) were also tested for rabbit HEV RNA by RT-PCR. At each necropsy, samples of serum, intestinal content, bile and liver tissue were collected and stored at −80 °C until use.
Detection and semi-quantitative titration of HEV RNA in samples by nested RT-PCR.
RNA extraction was performed using a standard Trizol Reagent protocol on all pig and rabbit serum and faecal samples. Briefly, 200 µl of serum and 200 µl of a 10 % faecal suspension were used for RNA extraction. Additionally, total RNAs were also extracted from 200 µl of bile, 10 % (w/v) intestinal content in PBS and 10 % suspension of liver homogenates. Reverse transcription was performed on 12.25 µl of each RNA sample for 60 min at 42 °C using 1 µl of the virus strain-specific reverse primer (Table S1, available in JGV Online), 0.25 µl (20 U µl−1) Superscript II reverse transcriptase (Invitrogen), 4 µl of 5× RT buffer, 1 µl of 0.1 M dithiothreitol, 0.50 µl (40U µl−1) RNase inhibitor (Promega) and 1 µl of 10 mM deoxynucleoside triphosphates. Five microlitres of the resulting cDNA was then amplified in a 50 µl PCR with AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR parameters used in this study are essentially the same as described previously (Huang et al., 2002) using virus strain-specific primers that amplify a capsid gene region.
The strain-specific PCR primers for the RT-PCR assays to detect the respective HEV RNA in pigs experimentally inoculated with US rabbit strain, Chinese rabbit strain, rat strain and the genotype 3 swine HEV are listed in Table S1. The strain-specific primers were designed based on the published sequences of US rabbit HEV (Cossaboom et al., 2011), Chinese rabbit HEV (Zhao et al., 2009), US rat HEV (Purcell et al., 2011) and genotype 3 swine HEV (Meng et al., 1997). In addition, degenerate primers that can amplify known genotype 3 strains of HEV were also designed based on a multiple sequence alignment of the two known Chinese rabbit strains of HEV (Geng et al., 2011; Zhao et al., 2009) as well as 75 other genotype 3 HEV strains.
To further confirm the RT-PCR results, all serum and faecal samples that tested positive in pigs experimentally inoculated with US rabbit HEV were subsequently retested with a different nested RT-PCR assay using primers that amplify a different genomic region in ORF1. The rabbit serum and faecal samples were similarly tested with USRabF1, USRabF2, USRabR1 and USRabR2 primers (Table S1).
Additionally, the amount of HEV RNA present in the faecal samples that had tested positive by the nested RT-PCR was estimated using a semi-quantitative nested RT-PCR assay and calculated as GE ml−1 of the sample (Kasorndorkbua et al., 2002; Meng et al., 1998a, b). One GE is defined as the number of viral genomes present in the highest 10-fold dilution that tested positive by RT-PCR. The same species-specific primers described above were used for the semi-quantitative nested RT-PCR assay (Table S1).
ELISA to detect IgG anti-HEV in pigs and rabbits.
Serum samples from experimentally inoculated pigs were tested for IgG anti-HEV using an ELISA essentially as described previously (Meng et al., 1997). A truncated recombinant genotype 1 HEV capsid protein containing the immunodominant 452–617 aa region (GenWay Biotech) was used as the antigen. HRP-conjugated goat anti-swine IgG (KPL) was used as the secondary antibody. Pre-immune and convalescent-phase sera from pigs that were experimentally infected with genotype 3 swine HEV (Córdoba et al., 2011), and a hyperimmune pig antiserum from a pig that was immunized with a recombinant capsid protein of the rat HEV (B. J. Sanford and others, unpublished data) were included as negative and positive controls for pigs inoculated with rabbit HEV and rat HEV, respectively.
To further confirm the serology results in pig sera obtained with the ELISA using the genotype 1 HEV antigen described above, we subsequently performed a separate ELISA using species-specific rat HEV and rabbit HEV antigens on all sera from pigs inoculated with rat HEV and rabbit HEV, respectively. A truncated recombinant rat HEV capsid protein (100–660 aa region) and a truncated recombinant rabbit HEV capsid protein (390–660 aa region) were used as the species-specific HEV antigens, and the ELISA protocol was essentially the same as described previously (Meng et al., 1997).
Sera collected from rabbits were also tested for the presence of IgG anti-HEV by ELISA using HRP-conjugated goat anti-rabbit IgG as the secondary antibody. Pre-immune and convalescent-phase serum samples obtained from a rabbit experimentally infected with rabbit HEV were included as negative and positive controls, respectively. The ELISA cut-off for both assays was calculated as the mean negative control OD value plus three sd.
Expression and purification of the truncated capsid protein of a US strain of rabbit HEV.
The truncated ORF2 gene (765 bp) of the rabbit HEV USRab-14 strain was amplified from a 10 % positive faecal suspension (Cossaboom et al., 2011) by a nested RT-PCR using the following primers: first round forward and reverse: degF2 : 5′-GCTGAYACRCTTCTYGGY-3′, ORF2R: 5′-AAACTCCCGGGTTTTACCCA-3′; second round forward and reverse, ORF2F: 5′-GTCAGGTATTCTACTCC-3′, ORF2R. The amplified PCR product was cloned into the pRSET-A bacterial expression vector (Invitrogen). Escherichia coli cells strain BL21(DE3)pLysS (Novagen) were transformed with the recombinant plasmids. The pRSET-A vector uses a T7 promoter sequence to tag the protein with six histidine residues at the N terminus. The transformed cells were grown in Overnight Express Instant TB Medium (Novagen) containing 30 µg ampicillin ml−1. This medium uses auto-induction as a more efficient means of protein expression (Studier, 2005). The cells were then harvested and the protein was extracted using BugBuster Protein Extraction Reagent (Novagen) and purified using HisPur Ni-NTA spin column kit (Qiagen) following standard protocol.
Western blot analyses to determine antigenic cross-reactivity between the rabbit HEV and other known strains of HEV.
To determine if rabbit HEV is antigenically related to other known animal strains of HEV, Western blot analyses were performed with recombinant capsid antigens derived from different HEV strains and anti-HEV antibodies raised against different strains of HEV. First, to determine if the recombinant capsid proteins derived from different strains of HEV cross-react with rabbit HEV antiserum (Fig. 2a), each lane of a 8–16 % SDS-PAGE gel was loaded with the same amount (1 µg) of recombinant capsid proteins derived from different HEV strains including the US rabbit HEV (34 kDa), the genotype 1 human HEV (43 kDa), the genotype 3 swine HEV (60 kDa), avian HEV (32 kDa) (Haqshenas et al., 2002) and rat HEV (60 kDa) (B. J. Sanford and others, unpublished data). The size variation of these recombinant capsid proteins reflects the size difference of the capsid gene from different HEV strains as well as different sizes of truncation. After separation of the proteins in the gel, the proteins were stained with Bio-Safe Coomassie Stain (Bio-Rad) for Coomassie-staining analysis. The separated protein was transferred to a PVDF membrane, which was subsequently blocked for 1 h at room temperature with Odyssey blocking buffer (LI-COR). The membrane was then cut into two separate pieces, the first membrane containing truncated capsid proteins from rabbit HEV, genotype 1 HEV, genotype 3 HEV, avian HEV and rat HEV was incubated overnight with 1 : 100 dilution of a rabbit HEV antiserum (3 ml Odyssey blocking buffer, 30 µl antiserum, 3 µl Tween-20). The second membrane, containing the truncated capsid protein derived from the rabbit HEV was incubated with 1 : 100 dilution of a rabbit serum known to be negative for HEV antibodies as a negative control. Following incubation with the primary antibodies, the membrane pieces were washed in washing buffer (0.2 % Tween-20 PBS solution) and then incubated with 1 : 5000 dilution of Infrared IRDye 680LT goat anti-rabbit secondary antibody (LI-COR) for 1 h. After washing three times with the washing buffer, the membrane pieces were scanned and analysed using the Odyssey Infrared Imaging System (LI-COR) in the 700 nm channel.
Secondly, to determine if the recombinant rabbit HEV capsid antigen cross-reacts with antibodies raised against different HEV strains (Fig. 2c), each lane of an SDS-PAGE gel was loaded with the same amount (1 µg) of the recombinant truncated capsid protein derived from the US rabbit HEV (USRab-14). After transferring the separated protein to a PVDF membrane, the membrane was subsequently blocked for 1 h in Odyssey blocking buffer. The membrane was then cut into separate pieces with each containing one lane, and each membrane piece was separately incubated with 1 : 100 dilution of the respective primary anti-HEV antiserum against different strains of HEV including a rabbit HEV antiserum from a rabbit experimentally infected with US rabbit HEV as the positive control, a genotype 1 human HEV hyperimmune antiserum from a pig immunized with the capsid protein of genotype 1 human HEV (Meng et al., 1997), a genotype 3 HEV antiserum from a pig experimentally infected with a genotype 3 swine HEV (Córdoba et al., 2011), an avian HEV antiserum from a chicken experimentally infected with avian HEV (Pudupakam et al., 2009), a rat HEV antiserum from a pig immunized with a recombinant truncated capsid protein of rat HEV (B. J. Sanford and others unpublished data), and a pre-immune rabbit serum as the negative control. Following incubation with each of the primary antibodies, the membrane pieces were washed and then incubated for 1 h with 1 : 5000 dilution of the respective infrared secondary antibody. The secondary antibodies were infrared IRDye 680LT goat anti-rabbit, goat anti-swine and goat anti-chicken (LI-COR) depending on the host species of the primary antiserum used. The membrane pieces were subsequently washed three times, and then scanned and analysed using the Odyssey Infrared Imaging System (LI-COR) in the 700 nm channel.
An ELISA was used to estimate the antibody titres of the different HEV antisera used for the Western blot analyses essentially as described previously (Meng et al., 1997) using the truncated recombinant genotype 1 HEV capsid protein (GenWay Biotech) as the antigen since it has been shown to cross-react with all of the strains of HEV tested in the study. Ten-fold serial dilutions (1 : 10, 1 : 102, 1 : 103, 1 : 104 and 1 : 105) of each antiserum were performed. Species-specific HRP-conjugated goat anti-swine IgG, goat anti-rabbit IgG or goat anti-chicken IgG were used as the secondary antibody depending on the source of the antiserum. Pre-immune swine and rabbit sera were included as a negative control (data not shown).
Acknowledgements
This work was supported by grants from the National Institutes of Health (AI074667 and AI050611). We would like to thank Drs Suzanne U. Emerson and Robert H. Purcell of the Laboratory of Infectious Diseases, NIAID, NIH, Bethesda, MD for generously providing us with the US strain of rat HEV and its partial sequence. We also thank Pete Jobst, and the animal care staff at Virginia Tech for their assistance in the animal studies.
Footnotes
A supplementary table is available with the online version of this paper.
References
- Aggarwal R., Jameel S. (2011). Hepatitis E. Hepatology 54, 2218–2226 10.1002/hep.24674 [DOI] [PubMed] [Google Scholar]
- Ahmad I., Holla R. P., Jameel S. (2011). Molecular virology of hepatitis E virus. Virus Res 161, 47–58 10.1016/j.virusres.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle V. A., Chobe L. P., Chadha M. S. (2006). Type-IV Indian swine HEV infects rhesus monkeys. J Viral Hepat 13, 742–745 10.1111/j.1365-2893.2006.00759.x [DOI] [PubMed] [Google Scholar]
- Batts W., Yun S., Hedrick R., Winton J. (2011). A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res 158, 116–123 10.1016/j.virusres.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Bilic I., Jaskulska B., Basic A., Morrow C. J., Hess M. (2009). Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J Gen Virol 90, 863–873 10.1099/vir.0.007179-0 [DOI] [PubMed] [Google Scholar]
- Bouquet J., Tessé S., Lunazzi A., Eloit M., Rose N., Nicand E., Pavio N. (2011). Close similarity between sequences of hepatitis E virus recovered from humans and swine, France, 2008-2009. Emerg Infect Dis 17, 2018–2025 10.3201/eid1711.110616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K., Huang F. F., Batista L., Rayo C. D., Bezanilla J. C., Toth T. E., Meng X. J. (2005). Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. J Clin Microbiol 43, 1684–1688 10.1128/JCM.43.4.1684-1688.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba L., Huang Y. W., Opriessnig T., Harral K. K., Beach N. M., Finkielstein C. V., Emerson S. U., Meng X. J. (2011). Three amino acid mutations (F51L, T59A, and S390L) in the capsid protein of the hepatitis E virus collectively contribute to virus attenuation. J Virol 85, 5338–5349 10.1128/JVI.02278-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossaboom C. M., Córdoba L., Dryman B. A., Meng X. J. (2011). Hepatitis E virus in rabbits, Virginia, USA. Emerg Infect Dis 17, 2047–2049 10.3201/eid1711.110428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Purcell R. H. (2003). Hepatitis E virus. Rev Med Virol 13, 145–154 10.1002/rmv.384 [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Purcell R. H. (2007). Hepatitis E virus. In Fields Virology, 5th edn Edited by Knipe D. M., Howley P. M. Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Feagins A. R., Opriessnig T., Huang Y. W., Halbur P. G., Meng X. J. (2008). Cross-species infection of specific-pathogen-free pigs by a genotype 4 strain of human hepatitis E virus. J Med Virol 80, 1379–1386 10.1002/jmv.21223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J., Wang L., Wang X., Fu H., Bu Q., Zhu Y., Zhuang H. (2011). Study on prevalence and genotype of hepatitis E virus isolated from Rex rabbits in Beijing, China. J Viral Hepat 18, 661–667 10.1111/j.1365-2893.2010.01341.x [DOI] [PubMed] [Google Scholar]
- Hakze-van der Honing R. W., van Coillie E., Antonis A. F., van der Poel W. H. (2011). First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS ONE 6, e22673 10.1371/journal.pone.0022673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur P. G., Kasorndorkbua C., Gilbert C., Guenette D., Potters M. B., Purcell R. H., Emerson S. U., Toth T. E., Meng X. J. (2001). Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol 39, 918–923 10.1128/JCM.39.3.918-923.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqshenas G., Shivaprasad H. L., Woolcock P. R., Read D. H., Meng X. J. (2001). Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol 82, 2449–2462 [DOI] [PubMed] [Google Scholar]
- Haqshenas G., Huang F. F., Fenaux M., Guenette D. K., Pierson F. W., Larsen C. T., Shivaprasad H. L., Toth T. E., Meng X. J. (2002). The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J Gen Virol 83, 2201–2209 [DOI] [PubMed] [Google Scholar]
- Huang F. F., Haqshenas G., Guenette D. K., Halbur P. G., Schommer S. K., Pierson F. W., Toth T. E., Meng X. J. (2002). Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol 40, 1326–1332 10.1128/JCM.40.4.1326-1332.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. W., Opriessnig T., Halbur P. G., Meng X. J. (2007). Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J Virol 81, 3018–3026 10.1128/JVI.02259-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne R., Plenge-Bönig A., Hess M., Ulrich R. G., Reetz J., Schielke A. (2010). Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol 91, 750–758 10.1099/vir.0.016584-0 [DOI] [PubMed] [Google Scholar]
- Kasorndorkbua C., Halbur P. G., Thomas P. J., Guenette D. K., Toth T. E., Meng X. J. (2002). Use of a swine bioassay and a RT-PCR assay to assess the risk of transmission of swine hepatitis E virus in pigs. J Virol Methods 101, 71–78 10.1016/S0166-0934(01)00420-7 [DOI] [PubMed] [Google Scholar]
- Ma H., Zheng L., Liu Y., Zhao C., Harrison T. J., Ma Y., Sun S., Zhang J., Wang Y. (2010). Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS ONE 5, e9160 10.1371/journal.pone.0009160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J. (2010a). Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol 140, 256–265 10.1016/j.vetmic.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J. (2010b). Recent advances in hepatitis E virus. J Viral Hepat 17, 153–161 10.1111/j.1365-2893.2009.01257.x [DOI] [PubMed] [Google Scholar]
- Meng X. J. (2011). From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 161, 23–30 10.1016/j.virusres.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J., Anderson D. A., Arankalle V. A., Emerson S. U., Harrison T. J., Jameel S., Okamoto H. (2012). Hepeviridae. In Virus Taxonomy, 9th Report of the International Committee on Taxonomy of Viruses, pp. 1021–1028 Edited by King A. M. Q., Carstens E., Adams M., Lefkowitz E. London: Elsevier/Academic Press [Google Scholar]
- Meng X. J., Purcell R. H., Halbur P. G., Lehman J. R., Webb D. M., Tsareva T. S., Haynes J. S., Thacker B. J., Emerson S. U. (1997). A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A 94, 9860–9865 10.1073/pnas.94.18.9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J., Halbur P. G., Haynes J. S., Tsareva T. S., Bruna J. D., Royer R. L., Purcell R. H., Emerson S. U. (1998a). Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch Virol 143, 1405–1415 10.1007/s007050050384 [DOI] [PubMed] [Google Scholar]
- Meng X. J., Halbur P. G., Shapiro M. S., Govindarajan S., Bruna J. D., Mushahwar I. K., Purcell R. H., Emerson S. U. (1998b). Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol 72, 9714–9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Takahashi K., Taira K., Taira M., Ohno A., Sakugawa H., Arai M., Mishiro S. (2006). Hepatitis E virus infection in wild mongooses of Okinawa, Japan: demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol Res 34, 137–140 10.1016/j.hepres.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Okamoto H. (2007). Genetic variability and evolution of hepatitis E virus. Virus Res 127, 216–228 10.1016/j.virusres.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Okamoto H. (2011a). Efficient cell culture systems for hepatitis E virus strains in feces and circulating blood. Rev Med Virol 21, 18–31 10.1002/rmv.678 [DOI] [PubMed] [Google Scholar]
- Okamoto H. (2011b). Hepatitis E virus cell culture models. Virus Res 161, 65–77 10.1016/j.virusres.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Pavio N., Meng X. J., Renou C. (2010). Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res 41, 46 10.1051/vetres/2010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudupakam R. S., Huang Y. W., Opriessnig T., Halbur P. G., Pierson F. W., Meng X. J. (2009). Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: evidence for attenuation of HVR deletion mutants in vivo. J Virol 83, 384–395 10.1128/JVI.01854-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Engle R. E., Rood M. P., Kabrane-Lazizi Y., Nguyen H. T., Govindarajan S., St Claire M., Emerson S. U. (2011). Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis 17, 2216–2222 10.3201/eid1712.110482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy M. A., Khudyakov Y. E. (2010). Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE 5, e14376 10.1371/journal.pone.0014376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. D., Hussein H. A., Bashandy M. M., Kamel H. H., Earhart K. C., Fryauff D. J., Younan M., Mohamed A. H. (2007). Hepatitis E virus infection in work horses in Egypt. Infect Genet Evol 7, 368–373 10.1016/j.meegid.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Sanford B. J., Dryman B. A., Huang Y. W., Feagins A. R., Leroith T., Meng X. J. (2011). Prior infection of pigs with a genotype 3 swine hepatitis E virus (HEV) protects against subsequent challenges with homologous and heterologous genotypes 3 and 4 human HEV. Virus Res 159, 17–22 10.1016/j.virusres.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Sato H., Naka K., Furuya S., Tsukiji H., Kitagawa K., Sonoda Y., Usui T., Sakamoto H. & other authors (2011). A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch Virol 156, 1345–1358 10.1007/s00705-011-0988-x [DOI] [PubMed] [Google Scholar]
- Shukla P., Nguyen H. T., Torian U., Engle R. E., Faulk K., Dalton H. R., Bendall R. P., Keane F. E., Purcell R. H., Emerson S. U. (2011). Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc Natl Acad Sci U S A 108, 2438–2443 10.1073/pnas.1018878108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda H., Abe M., Sugimoto T., Sato Y., Bando M., Fukui E., Mizuo H., Takahashi M., Nishizawa T., Okamoto H. (2004). Prevalence of hepatitis E virus (HEV) infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J Clin Microbiol 42, 5371–5374 10.1128/JCM.42.11.5371-5374.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. (2005). Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41, 207–234 10.1016/j.pep.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Surjit M., Jameel S., Lal S. K. (2004). The ORF2 protein of hepatitis E virus binds the 5′ region of viral RNA. J Virol 78, 320–328 10.1128/JVI.78.1.320-328.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Kitajima N., Abe N., Mishiro S. (2004). Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330, 501–505 10.1016/j.virol.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Takahashi M., Nishizawa T., Sato H., Sato Y., Jirintai, Nagashima S., Okamoto H. (2011). Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol 92, 902–908 10.1099/vir.0.029470-0 [DOI] [PubMed] [Google Scholar]
- Tsarev S. A., Emerson S. U., Tsareva T. S., Yarbough P. O., Lewis M., Govindarajan S., Reyes G. R., Shapiro M., Purcell R. H. (1993). Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J Infect Dis 167, 1302–1306 10.1093/infdis/167.6.1302 [DOI] [PubMed] [Google Scholar]
- Tsarev S. A., Tsareva T. S., Emerson S. U., Yarbough P. O., Legters L. J., Moskal T., Purcell R. H. (1994). Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J Med Virol 43, 135–142 10.1002/jmv.1890430207 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Ling R., Li H., Harrison T. J. (2000). The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J Gen Virol 81, 1675–1686 [DOI] [PubMed] [Google Scholar]
- Yazaki Y., Mizuo H., Takahashi M., Nishizawa T., Sasaki N., Gotanda Y., Okamoto H. (2003). Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 84, 2351–2357 10.1099/vir.0.19242-0 [DOI] [PubMed] [Google Scholar]
- Zafrullah M., Ozdener M. H., Panda S. K., Jameel S. (1997). The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol 71, 9045–9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Ma Z., Harrison T. J., Feng R., Zhang C., Qiao Z., Fan J., Ma H., Li M. & other authors (2009). A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol 81, 1371–1379 10.1002/jmv.21536 [DOI] [PubMed] [Google Scholar]


